Abstract
Suppressive oligodeoxynucleotides (Sup ODN) express repetitive TTAGGG motifs that have proven useful in the treatment/prevention of numerous inflammatory and autoimmune diseases. The mechanism underlying the immunosuppressive activity of Sup ODN is incompletely understood. Regulatory T cells (Treg) play a key role in are generated from controlling a variety of pathologic autoimmune responses. Tregs activated CD4+ T cells in a process that involves the phosphorylation of STAT family members. Current studies demonstrate that Sup ODN promote the differentiation of CD4+CD25− T cells into functionally active iTreg in vitro. When administered in vivo, Sup in response to peptide challenge. Central to this ODN promote the generation of iTreg effect is the ability of Sup ODN to block the phosphorylation of STAT1. These findings clarify the mechanism underlying the therapeutic activity of Sup ODN and support their use in Treg-based immunotherapy.
Keywords: suppressive oligonucleotides, regulatory T cells, STAT transcription factors
1 Introduction
DNA has multiple and complex effects on the immune system. Microbial DNA elicits a potent inflammatory response that helps protect the host from infection but can exacerbate autoimmune and inflammatory diseases [1]. Self DNA, in contrast, is anti-inflammatory and can down-regulate pathologic inflammatory responses [2]. The latter effect is mediated, at least in part, by repetitive TTAGGG motifs present at high frequency in mammalian telomeres. Synthetic single-stranded phosphorothioate oligodeoxynucleotides (ODN) expressing TTAGGG motifs mimic the ability of telomeric DNA to prevent/treat a variety of inflammatory and autoimmune diseases [3]. This was established in murine models of lupus, arthritis, encephalomyelitis, iritis and shock [4–7].
The mechanism underlying the anti-inflammatory properties of Sup ODN were initially attributed to their ability to inhibit the maturation of CD4+ T cells into Th1 effectors [7;8]. This was associated with the inhibition of STAT1 and STAT4 phosphorylation and the resultant reduction in IFNg production - a cytokine critical to the generation and maintenance of Th1 immunity. Yet those findings pre-dated the discovery of regulatory T cells (Treg) that are now known to play a key role in restrain the host’s suppressing autoimmune and inflammatory diseases [9]. Treg response to self Ags and thus are of enormous importance in the therapy of inflammatory/autoimmune diseases.
There are two broad categories of Tregs. nTreg arise naturally in the thymus while iTreg are induced in the periphery from CD4+CD25− T cells [10,11]. Forkhead transcription factor (FoxP) 3 acts as the master transcriptional regulator of Treg development. Deficiency in FoxP3 results in fatal multi-organ autoimmune disease as observed in the scurfy mouse and in patients with immunodysregulation polyendocrinopathy enteropathy X-linked syndrome [10–15]. In vitro studies show that CD4+CD25− T cells up-regulate FoxP3 and differentiate into functional iTreg when stimulated via their TCR in the presence of TGFß [16;17]. In vivo, exposure to Ag generates FoxP3+ iTreg in the periphery. For example, large numbers of FoxP3+ iTreg accumulate in the draining lymph nodes of mice carrying OVA-specific CD4 T cells following challenge with the OVA323-339 peptide [18;19].
Treg development is regulated by members of the STAT family. STAT5 binds to the FoxP3 promoter and drives FoxP3 transcription, thereby playing a critical role in Treg generation [20;21]. In contrast, activation of STAT1-dependent pathways prevents FoxP3 expression and blocks Treg development [21,22]. In murine models, unrestrained STAT1 activation results in the breakdown of immunological tolerance and culminates in Th1-mediated pathology [24]. In contrast, deletion of STAT1 promotes the Treg expansion and reduces graft-versus-host disease [25]. These findings suggest that targeting STAT1 represents a promising strategy for therapies designed to improve Treg generation.
Recognizing the critical contribution of Treg to the prevention and treatment of diseases that respond to Sup ODN therapy, we evaluated whether Sup ODN might promote Treg development. Results show that Sup ODN enhance the generation of functional FoxP3+ iTreg from naïve CD4+ precursors in vitro. When administered in vivo, Sup ODN promote the generation of FoxP3+ iTreg in response to peptide challenge. These effects are linked to the ability of Sup ODN to block STAT1 phosphorylation following the activation of CD4+ T cells. These findings clarify the mechanism underlying the therapeutic activity of Sup ODN in autoimmune disease and provide a rationale for the use of Sup ODN in Treg-based immunotherapy.
2 Materials and Methods
2.1 Mice
Female BALB/c and C57BL/6 mice were bred at the National Cancer Institute (Frederick, MD), FoxP3 eGFP reporter mice were obtained from Dr. Y. Belkaid (National Institute of Allergy and Infectious Diseases, Bethesda, MD), STAT1−/− mice from Dr. D. Levy (NYU School of Medicine, New York, NY) and Rag2−/− DO11.10 mice from Dr. M. Leonardo (National Institute of Allergy and Infectious Diseases, Bethesda, MD)[26;27]. All mice were studied at 6 – 10 wk of age and all experiments were approved by the Institutional Animal Care and Use Committee of the National Cancer Institute at Frederick.
2.2 Oligonucleotides
Phosphorothioate ODN were synthesized at the Core Facility of the Center for Biologics Evaluation and Research facility, Food and Drug Administration (Bethesda, MD). The following ODN were used: suppressive ODN A151 (5′ TTAGGGTTAGGGTTAGGGTTAGGG 3′) and control ODN 1612 (5′ GCTAGAGCTTAGGCT 3′). All ODN were free of detectable protein and endotoxin contamination.
2.3 Isolation of murine CD4+CD25− T cells
CD4+ T cells were purified from single spleen cell suspensions by negative selection using magnetic beads. These CD4+ cells were then incubated with PE anti-CD25 and anti-PE beads used to isolate CD4+CD25− T cells. The purity of these T cells typically exceeded 95% and contained fewer than 2% FoxP3+ cells. All reagents were obtained from Miltenyi Biotec, Auburn, CA.
2.4 In vitro generation of murine iTreg
CD4+CD25− T cells (106/ml) were pre-incubated with 1 uM ODN for 2 h and then transferred to a 96 well plate coated with 3 ug/ml anti-CD3 (2C11; eBioscience, San Diego, CA). Cells were cultured in complete medium (RPMI 1640 supplemented with 10% FCS (both from Lonza, Walkersville, MD), 2 mM glutamine, 100 IU/ml penicillin, 100 ug/ml streptomycin, 25 mM HEPES buffer (all from Invitrogen, Carlsbad, CA), 0.0035% 2 ME (Sigma Aldrich, St. Louis, MO) and stimulated with 2 ug/ml soluble anti-CD28 (37.51; eBioscience) plus 20 ng/ml human TGFb1 (R&D Systems). 20 ng/ml of IL-2 (R&D Systems) was included to support the proliferation of Tregs from C57Bl/6 mice. This supplementation was not needed for T cells from BALB/c mice which intrinsically produce sufficient IL-2 when stimulated [28]. In experiments examining whether Sup ODN influenced the differentiation of iTreg, only 5 ng of TGFβ1 was added during culture. At the indicated time points, cells were analyzed for FoxP3 expression by flow cytometry or used in functional assays.
2.5 In vitro generation of human Treg
PBMC were isolated by density gradient centrifugation of buffy coats obtained from normal donors through an IRB approved protocol (NIH, Bethesda, MD). CD4+CD25− T cells were isolated by negative selection over MACS using the naive CD4+ T cell isolation kit II (Miltenyi Biotec, Auburn, CA). FACS analysis showed the purity of these cells was >98%. These cells were then stimulated with anti-CD3/28 coated beads plus 2.5 ng/ml TGFβ1 and 500 IU/ml IL-2 (both from eBioscience, San Diego, CA) in the presence or absence of 3 uM suppressive ODN for 5 days. FoxP3 expression was monitored using a Treg detection kit (Miltenyi Biotec (Auburn, CA).
2.6 Flow cytometry
After blocking FcR with 2.4G2 Ab, cells were stained with PerCP–Cy5.5–anti-CD4 (RM4 5), PE–anti-CD25 (PC61), PE anti-DO11.10 TCR (KJ1-26, all from BD Biosciences, San Jose, CA) and/or APC anti-FoxP3 (FJK 16s, eBioscience). Fluorescence was monitored using a LSRFortessa cell analyzer (BD Biosciences). Intracellular staining was performed with the FoxP3 staining buffer kit, according to the manufacturer’s protocol (eBioscience). Events were collected and analyzed using FlowJo software (Tree Star, Ashland, OR).
2.7 RNA Isolation and Quantitative Real time PCR
Total RNA was isolated from T cells using the RNeasy Mini Kit (Quiagen, Valencia, CA). cDNA was synthesized with a QuantiTect Reverse Transcription kit according to the manufacturer’s instructions (Applied Biosystems, Carlsbad, CA). Gene expression levels (normalized to GAPDH) were analyzed using the StepOnePlus RT PCR system and all reagents were from(Applied Biosystems).
2.8 Treg suppression assay
CD4+ T cells from FoxP3 eGFP reporter mice were isolated using a FACSAria II (BD Biosciences) and cultured in the presence of Sup ODN under Treg polarizing conditions as described above. These FACS sorted CD4+CD25− T cells were >97% pure. On day 3, Treg that had matured in vitro were isolated by FACS based on their up-regulation of GFP. Simultaneously, naïve CD4+CD25− responders (Tresp) were isolated from congenic C57BL/6 spleens and stained with 2.5 μM cell trace violet (Invitrogen, Carslbad, CA). 105 Tresp were stimulated with 0.25 μg/mL soluble anti-CD3 Ab and then mixed with mitomycin C inactivated syngeneic T cell depleted splenocytes (5 × 104) in 96 well round bottom plates for 3 days. Treg generated in the presence of Sup ODN were added at the indicated ratios. Proliferation was measured by monitoring cell trace violet dilution by flow cytometry. The proliferation of stimulated Tresp was set to 100% and the percent suppression observed following the addition of Treg calculated.
2.9 In vivo generation of iTreg
CD4+CD25− T cells were isolated from the spleens of Rag2−/− DO11.10 mice and stained with 5 μM CFSE (Invitrogen, Carslbad, CA) for 5 min at RT. 3 × 106 cells were injected i.v. into BALB/c mice. 24 hr later, these mice were immunized i.v. with 5 μg of OVA323-339 peptide (Gift from Dr. A. Hurwitz, National Cancer Institute, Frederick, MD). Sup ODN (300 ug/mouse) was injected i.p. 3 h before OVA administration. Four days later, cells were isolated from axillary, brachial and inguinal lymph nodes, stained for expression of CD4, FoxP3, and the DO11.10 TCR and analyzed by flow cytometry as described above.
2.10 Flow cytometric analysis of phospho-STAT expression
CD4+CD25− T cells were cultured under Treg polarizing conditions ± 1 uM Sup ODN. Cells were fixed with BD Lyse/Fix Buffer for 10 min at 37° C, washed, permeabilized in ice cold BD Perm Buffer III for 30 min and then stained with PE anti-STAT1 (pY701) or PE anti-STAT4 (pY693) Ab (all reagents from BD Biosciences) for 30 min at RT. Flow cytometric analysis was performed on a LSRFortessa cell analyzer.
2.11 Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Student’s t test was used to examine all results. p values <0.05 were considered to be statistically significant.
3. Results
3.1 Suppressive ODN enhance the generation of murine and human Treg in vitro
The ability of Sup ODN to reduce the duration and strength of inflammatory and autoimmune diseases [3] led us to examine their effect on the generation of Treg. To enable the detection of either a positive or negative effect of Sup ODN on Treg differentiation, splenic CD4+CD25− precursors were incubated in vitro under conditions that induced a significant but suboptimal increase in iTreg frequency. These ‘suboptimal’ conditions involved conventional stimulation with anti-CD3 plus anti-CD28 Abs but lower concentrations of TGFβ (see section 2.8).
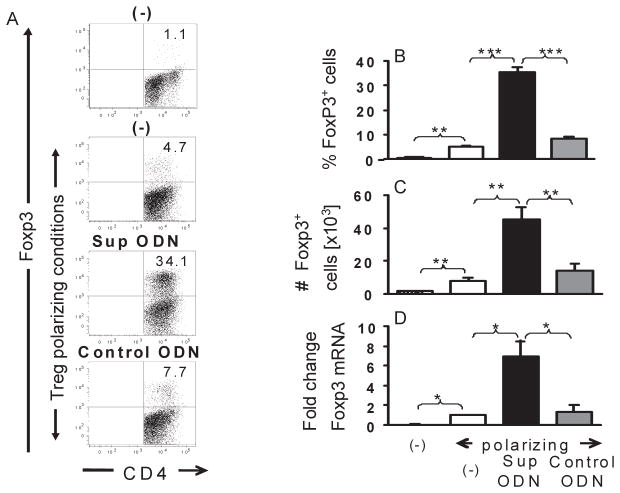
As seen in Fig 1, ≈1% of CD4+CD25− T cells differentiated into iTreg when incubated for 3 – 5 days in medium alone. The frequency of iTreg increased 5-fold when conditions supporting the suboptimal generation of Treg were used during culture (p < 0.01). Of importance, >35% of cultured cells differentiated into CD4+CD25+FoxP3+ T cells under the same conditions when supplemented with Sup ODN (p < 0.001). This effect was sequence specific, as control ODN lacking suppressive TTAGGG motifs had no significant effect on iTreg generation (Fig 1). Sup ODN increased both the percentage and absolute number of iTreg generated in vitro (Fig 1C), a finding confirmed by the increase in FoxP3 mRNA levels detected by RT PCR (Fig 1D; p < 0.05). To determine whether Sup ODN had an effect on nTregs, CFSE-labeled CD4+CD25+ T cells were incubated with IL-2 and the proliferation of FoxP3+ cells monitored by flow cytometry. The inclusion of Sup ODN had no effect on the proliferation of CD4+FoxP3+ Tregs (Supplemental Fig 1). These data indicate that Sup ODN selectively facilitate the differentiation of naive T cells into iTregs.
Figure 1. Suppressive ODN promote the generation of iTreg in vitro.
CD4+CD25− T cells were isolated from female BALB/c mice by negative selection. They were incubated for 2 h with 1 uM of suppressive or control ODN and then cultured under Treg polarizing conditions (3 ug/ml of anti-CD3, 2 ug/ml of anti-CD28 and 5 ng/ml of TGFβ) for 5 days. iTreg were identified based on their expression of CD4 and FoxP3 by flow cytometry. B, C) Combined results from 4 independent experiments showing the frequency and absolute number of CD4+ FoxP3+ Treg (mean + SE) generated by each type of treatment over 3–5 days. (D) mRNA was isolated after 5 days of culture and analyzed for FoxP3 gene expression by RT PCR. Relative mRNA levels were calculated by comparison to cells cultured under the same conditions in the absence of ODN. Results from each group were normalized to GAPDH mRNA levels. Each bar represents the mean + SE of 3 independent experiments.
*, p<0.05; **, p<0.01; ***, p<0.001
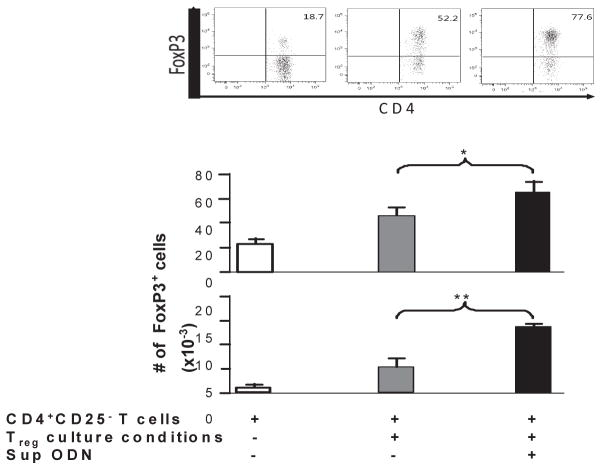
To examine whether results from these murine studies were relevant to humans, the effect of Sup ODN on the generation of human iTregs was examined. CD4+CD25− T cells were isolated from the peripheral blood of normal healthy volunteers. These were stimulated with IL-2, anti-CD3 and anti-CD28 plus a suboptimal amount of TGFβ1 (identified in preliminary studies). After 5 days, cells were analyzed for FoxP3 expression by FACS. As seen in Fig 2, the inclusion of Sup ODN led to a significant increase in both the percentage and absolute number of human CD4+CD25+FoxP3+ T cells generated in vitro (p < 0.05 and p < 0.01 respectively)
Figure 2. Suppressive ODN promote the generation of human iTreg in vitro.
CD4+CD25− T cells were isolated from normal healthy volunteers (N=3) and incubated for 2 h with 3 uM of suppressive ODN in medium supplemented with IL-2, anti-CD3/CD28 and TGFβ1 as described in the methods section. The cells were stained to detect expression of CD4 and FoxP3 and analyzed by FACS on day 5. Each donor was analyzed independently on a different day and results show the mean + SD of all samples.
*, p<0.05; **, p<0.01
3.2 iTreg functional activity
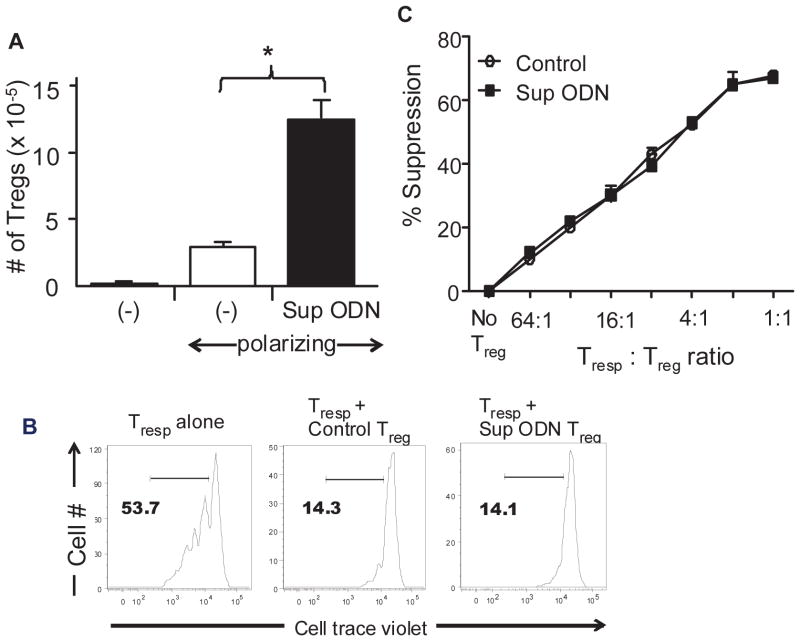
Further study focused on the activity of the murine iTregs generated in the presence of Sup ODN. To eliminate any possibility that mature Treg were contaminating the CD4+CD25− T cell pool, FoxP3 GFP knock-in mice were used as cell donors [26]. All Treg from these animals are GFP+, so FACS isolation of CD4+GFP− T cells insures that no Treg are present in the starting population. The cells were then cultured under the Treg polarizing conditions described above. Consistent with results obtained above using cells from WT mice (Fig 1), the addition of Sup ODN resulted in a >4-fold increase the frequency of CD4+GFP+FoxP3+ Treg generated (from 3.1 ± 0.4 ×105 to 12.8 ± 1.3 ×105).
The functional activity of these iTreg was evaluated based their ability to inhibit the proliferation of activated T cells. Consistent with previous reports, Treg suppressed the proliferation of syngeneic T cells in a dose dependent manner (Fig 3 and [29]). The suppressive activity of the Treg generated in the presence of Sup ODN was nearly identical to that of control Treg on a per cell basis (Fig 3). These findings indicate that Sup ODN promote the generation of functionally active iTreg.
Figure 3. Suppressive ODN enhance the generation of functional iTreg.
CD4+FoxP3− T cells were FACS isolated from the spleen of FoxP3 GFP knock-in mice and induced to differentiate under Treg polarizing conditions for 3 days as described in Fig 1. The CD4+ FoxP3+ Treg generated in vitro were purified and mixed with CD4+CD25− responders (Tresp) labeled with cell trace violet and stimulated with anti-CD3 plus mitomycin C treated APCs. A) Absolute number of iTreg generated. B) Effect of Treg on the proliferation of activated Tresp at a 1 : 1 ratio. C) Effect of Treg frequency on the proliferation of Tresp. All data represent the mean of two independent experiments using Treg generated in the presence or absence of Sup ODN. *, p<0.05.
3.3 Suppressive ODN promote the generation of Treg in vivo
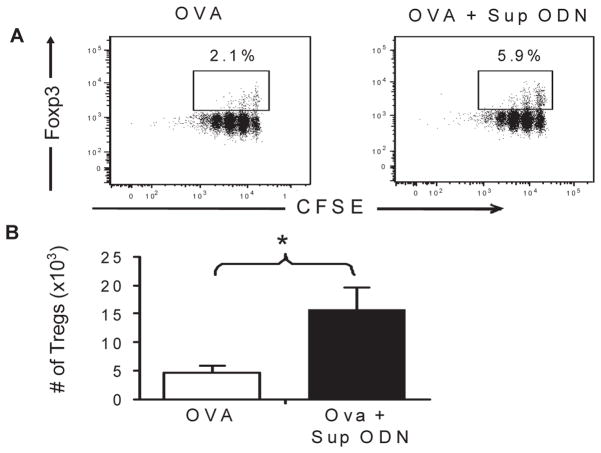
To determine whether Sup ODN support the generation of Treg under physiologically relevant conditions, a well established murine model of peripheral tolerance was used [18]. CD4+CD25− T cells were isolated from Rag2 KO donors expressing the DO11.10 TCR (such T cells are specific for OVA). These T cells were labeled with CSFE and transferred into WT recipients. The recipients were then injected with soluble OVA323-339 peptide and the CFSE-labeled FoxP3+ cells monitored. Consistent with previous findings, CD4+CD25− T cells from Rag2 KO / DO11.10 donors differentiated into iTreg when exposed to OVA peptide in vivo (Fig 4 [19;30]). When the recipient mice were treated with Sup ODN, the number of Treg generated rose by >3-fold (from 4.8 ± 1.1 ×103 to 15.7 ± 3.9 ×103, p < 0.05).
Figure 4. Suppressive ODN increase the number of Ag-specific iTreg generated in vivo.
FoxP3− T cells were isolated from Rag2−/− / DO11.10 mice, labeled with CSFE, and injected i.v. into syngeneic BALB/c mice. Recipients were treated on day 1 with 5 ug of OVA323-339 peptide ± 300 ug of Sup ODN. On day 5, peripheral lymph nodes were isolated and FACS analyzed for the presence of CFSE+ donor cells. A) The percent of transferred cells maturing into FoxP3+ Treg in vivo and B) the absolute number of Treg (defined as CD4+ FoxP3+CFSE+KJ1-26+ cells). Data represent the mean + SD of three independent experiments involving 4 independently studied mice/group.
*, p<0.05
3.4 Role of STAT1 in the generation of iTreg by Sup ODN
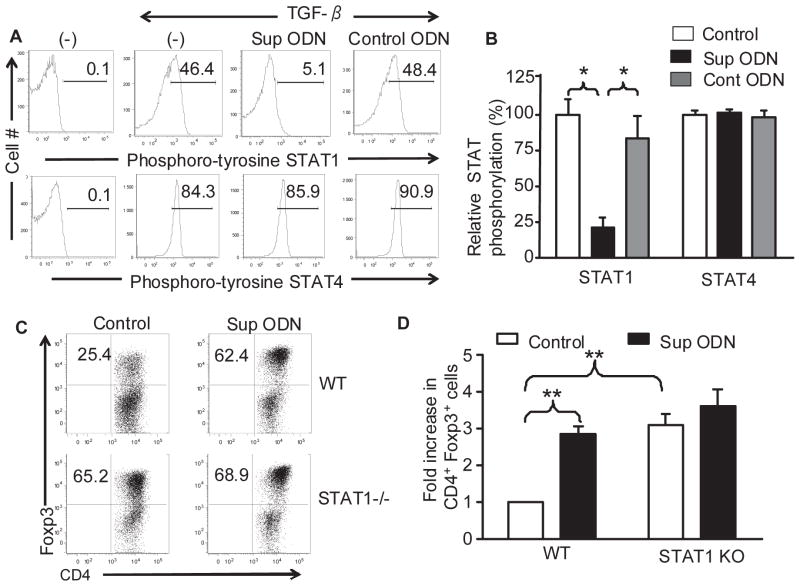
Previous studies established that i) Sup ODN can bind to and prevent the phosphorylation of STAT1 and STAT4 in Th1 cells [8] and ii) the phosphorylation of STAT1 and STAT4 can inhibit Treg generation [22]. These observations led us to examine the effect of Sup ODN on STAT phosphorylation in CD4+CD25− T cells. In the absence of stimulation, STAT phosphorylation was nearly undetectable (Fig 5A). When CD4+CD25− T cells were cultured under suboptimal Treg polarizing conditions, the phosphorylation of STAT1 and STAT4 rose rapidly to 45% and 85%, respectively (Fig 5A, B). The addition of Sup ODN reduced the phosphorylation of STAT1 in these cells by >75% (p <0.05) but had no effect on the phosphorylation of STAT4 (Fig 4A, B). This effect of Sup ODN was sequence specific as control ODN did not significantly alter STAT phosphorylation.
Figure 5. Effect of Suppressive ODN on STAT 1 phosphorylation and iTreg generation.
A,B) CD4+CD25− T cells from BALB/c mice were induced to differentiate under Treg polarizing conditions as described in Fig 1. The level of STAT1 and STAT4 phosphorylation was determined by flow cytometry using phospho-specific Abs at 4 h (A). The mean + SE level of STAT phosphorylation from 4 independent experiments (B). C,D) CD4+CD25− T cells from C57Bl/6 or STAT1-deficient C57Bl/6 mice were induced to differentiate under Treg polarizing conditions. Treg were identified on day 3 on the basis of CD4 and FoxP3 expression (C). The fold increase in CD4+ FoxP3+ Treg frequency was calculated by comparison to cells from WT mice cultured under optimized conditions but in the absence of Sup ODN (D). Data represent the mean + SE of 3 independent experiments. **, p<0.01
To explore whether the inhibition of STAT1 phosphorylation represented the mechanism by which Sup ODN promoted the generation of iTreg, the response of T cells from WT and STAT1-deficient mice was compared [27]. When CD4+CD25− T cells were cultured under identical conditions, ≈ 2.5-fold more iTreg were generated from STAT1-deficient vs WT donors (Fig 5C,D; p < 0.01). Adding Sup ODN to these cultures increased the frequency of Treg generated from WT precursors to that observed in the STAT1 KOs (Fig 5C,D; p < 0.01). Consistent with the inhibition of STAT1 phosphorylation being the mechanism by which Sup ODN support the generation of Treg, Sup ODN did not further boost the generation of Treg from targets deficient in STAT1.
4. Conclusions
One attractive strategy for treating autoimmune and inflammatory diseases is to capable of down-regulating these pathological immune responses. This provide Treg study examines the effect of synthetic ODN expressing immunosuppressive TTAGGG motifs on iTreg generation. Results indicate that Sup ODN promote the differentiation of both human and murine CD4+CD25− T cells into functionally active iTreg under conditions in which either TGFβ or IL-2 is limiting (Figs 1–4). Evidence that Sup ODN inhibits the STAT1 phosphorylation of activated T cells provides insight into the mechanism underlying this effect (Fig 5).
Sup ODN contain multiple TTAGGG motifs identical to those found at high frequency in mammalian telomeres and mimic the ability of telomeric DNA to reduce inflammation and autoimmune reactions [5;6;31–33]. Previous studies found that Sup ODN inhibited the differentiation of naive CD4+ cells into Th1 effectors [7;8]. That effect was postulated to explain their therapeutic utility [8] as the importance of Treg in modulating immune activation and suppressing pathological immune responses had not yet been discovered. Current findings establish that in addition to altering the balance between Th1, Th2 and Th17 [7;8;34] cells that Sup ODN also promote the differentiation of CD4+CD25− T cells into iTreg.
Naive CD4+ T cells can differentiate into a diverse array of effector phenotypes under appropriate conditions [35]. For example, CD4 T cells in a pro-inflammatory environment differentiate into Th17 cells when exposed to TGFβ while the same cells exposed to TGFβ in the absence of inflammatory cytokines differentiate into Treg [36]. Our group recently showed that the addition of Sup ODN to CD4 T cells facilitated their maturation into Th17 cells under pro-inflammatory Th17 polarizing conditions [34]. Results from the current work show that adding Sup ODN to the same cells in the absence of pro-inflammatory cytokines induces them to differentiate into Treg. From this we conclude that similar to TGFβ, the effect of Sup ODN on CD4 T cell generation is context-dependent.
Previous work established that Sup ODN were useful in the treatment of various autoimmune diseases [4–7]. To date, efforts to determine whether that effect was mediated by Sup ODN increasing the frequency of Treg have been unrevealing. As previously shown, successful treatment of murine autoimmunity requires that Sup ODN be administered repeatedly over many weeks. This prolonged intervention has many effects on the immune milieu, preventing us from establishing a cause-effect relationship with Treg generation. To circumvent this problem, we used a well established model of in vivo tolerance that allowed precise analysis of the effect of a single treatment with Sup ODN on Treg generation (Fig 4).
Sup ODN are fabricated from nuclease-resistant phosphorothioate nucleotides to improve their in vivo half life and activity. Bouladoux et al reported that Sup ODN could prevent CpG ODN from inhibiting Treg generation but did not examine whether Sup ODN directly promoted Treg generation [37]. Kim et al reported that phosophorthioate ODN could stablilize the expression of FoxP3 in a sequence non-specific manner but did not examine whether Sup ODN promoted the generation of Treg from CD4+ precursors [38]. While phosphorothioate ODN can exert sequence non-specific immune effects at the high concentrations used by Kim et al [38;39], the control ODN used in our studies and by other groups uniformly failed to increase Treg development and/or function [37;40–42]. Similarly, studies performed by multiple groups show that suppressive but not control ODN down regulate inflammatory and autoimmune responses in vivo [5;6;31–33].
Current findings are consistent with recent reports that Treg generation is inhibited by STAT1 phosphorylation and that the deletion of STAT1 promotes Treg differentiation [21–23,28]. The precise mechanism by which a decrease in STAT1 activation promotes the generation of Tregs is unclear. STAT1 may interfere with STAT5 driven Treg production or it may trigger pathways that inhibit Treg development. Specifically, we find that i) CD4+ T cells from STAT1-deficient mice generate significantly more iTreg than those from WT donors, ii) Sup ODN block STAT1 phosphorylation and simultaneously enhance iTreg generation and iii) Sup ODN do not enhance the generation of iTreg from mice deficient in STAT1 (Fig 5). These findings suggest that the mechanism by which Sup ODN promote the generation of iTreg is by inhibiting STAT1 phosphorylation. Consistent with that conclusion, we previously demonstrated that Sup ODN inhibited the differentiation of naive CD4 T cells into Th1 effectors by reducing STAT1 phosphorylation [8]. Other members of the STAT family also play a role in Treg generation. FoxP3 expression and Treg differentiation are enhanced by STAT5 phosphorylation [20;21] but inhibited by activation of the STAT4 and STAT6 signaling pathways [22]. We find that Sup ODN inhibit the phosphorylation of STAT1 but have no effect on STATS 3, 4 or 5 under culture conditions conducive to the generation of iTreg (Fig 5 and data not shown).
The therapeutic benefit of increasing Treg frequencies in patients with autoimmune and inflammatory disorders is being examined clinically [43]. Such therapy currently requires the adoptive transfer of Treg, a strategy that is difficult, time consuming, and expensive [44]. The same outcome might be achieved by administering Sup ODN. Our studies of CD4+CD25− T cells from normal human donors shows that Sup ODN induces them to differentiate into iTreg. To study the effect of Sup ODN in vivo, the well defined DO11.10 Tg / Rag2 KO mouse model was employed [18]. This model has been used to identify other agents capable of eliciting Ag-specific Treg responses [19;45]. Our results establish that Sup ODN significantly increase the number of CD4+CD25− T cells that differentiate into Treg in vivo (Fig 4). Other agents designed to increase Treg production caused moderate-severe adverse events in clinical trials [43;46;47]. By comparison, pre-clinical studies found that Sup ODN are safe even when administered repeatedly and at high doses [48]. Taken together, these findings suggest that Sup ODN may be a promising tool to augment the generation of iTreg in humans.
Supplementary Material
Highlights.
Supressive oligonucleotides (Sup ODN) enhance the generation of functionally active iTreg in vitro
Sup ODN promote the generation of iTregs in response to peptide challenge in vivo 3) The inhibition of STAT1 activation by Sup ODN increases the generation of iTreg
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the National Cancer Institute at large.
Abbreviations
- ODN
phosphorothiate oligodeoxynucleotide
- Sup
suppressive
Footnotes
Conflict of Interest
Members of Dr. Klinman’s lab have patents related to the use of suppressive oligonucleotides. All rights to such patents have been assigned to the Federal Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atianand MK, Fitzgerald KA. Molecular basis of DNA recognition in the immune system. J Immunol. 2013;190:1911. doi: 10.4049/jimmunol.1203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 3.Klinman DM, Tross D, Klaschik S, Shirota H, Sato T. Therapeutic applications and mechanisms underlying the activity of immunosuppressive oligonucleotides. Ann NY Acad Sci. 2009;1175:80. doi: 10.1111/j.1749-6632.2009.04970.x. [DOI] [PubMed] [Google Scholar]
- 4.Dong I, Ito S, Ishii K, Klinman D. Suppressive Oligodeoxynucleotides Delay the Onset of Glomerulonephritis and Prolong the Survival of Lupus-prone NZB/W Mice. Arthritis Rheum. 2004;52:651. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 5.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum. 2004;50:1686. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto C, Klinman DM, Shi G, yin H, Vistica BP, Lovaas JD, Wawrousek EF, Igarashi T, Chan CC, Gery I. A suppressive oligodeoxynucleotide inhibits ocular inflammation. Clin Exp Immunol. 2009;156:528. doi: 10.1111/j.1365-2249.2009.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 8.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173:5002. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+ J Immunol. 2004;172:5149. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 18.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 19.Hackl D, Loschko J, Sparwasser T, Reindl W, Krug AB. Activation of dendritic cells via TLR7 reduces Foxp3 expression and suppressive function in induced Tregs. Eur J Immunol. 2011;41:1334. doi: 10.1002/eji.201041014. [DOI] [PubMed] [Google Scholar]
- 20.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 21.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma H, Lu C, Ziegler J, Liu A, Sepulveda A, Okada H, Lentzsch S, Mapara MY. Absence of Stat1 in donor CD4(+) T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest. 2011;121:2554. doi: 10.1172/JCI43706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Oppenheim JJ, Howard OM. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+ J Leukoc Biol. 2005;78:114. doi: 10.1189/jlb.0604341. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 30.Chang JH, Kim YJ, Han SH, Kang CY. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 31.Kaminski JJ, Schattgen SA, Tzeng TC, Bode C, Klinman DM, Fitzgerald KA. Synthetic Oligodeoxynucleotides Containing Suppressive TTAGGG Motifs Inhibit AIM2 Inflammasome Activation. J Immunol. 2013;191:3876. doi: 10.4049/jimmunol.1300530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagci FC, Aslan O, Gursel M, Tincer G, Ozdamar Y, Karatepe K, Akcali KC, Gursel I. Mammalian telomeric DNA suppresses endotoxin-induced uveitis. J Biol Chem. 2010;285:28806. doi: 10.1074/jbc.M110.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeuchi H, Kinjo T, Klinman DM. Effect of suppressive oligodeoxynucleotides on the development of inflammation-induced papillomas. Cancer Prev Res(Phila) 2011;4:752. doi: 10.1158/1940-6207.CAPR-10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bode C, Yang XP, Kiu H, Klinman DM. Suppressive oligodeoxynucleotides promote the development of Th17 cells. PLoS One. 2013;8:e67991. doi: 10.1371/journal.pone.0067991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Bouladoux N, Hall JA, Grainger JR, dos Santos LM, Kann MG, Nagarajan V, Verthelyi D, Belkaid Y. Regulatory role of suppressive motifs from commensal DNA. Mucosal Immunol. 2012;5:623. doi: 10.1038/mi.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisetsky DS, RCF Inhibition of murine macrophage IL-12 production by natural and synthetic DNA. Clin Immunol. 2000;96:198. doi: 10.1006/clim.2000.4897. [DOI] [PubMed] [Google Scholar]
- 40.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaRosa DF, Gelman AE, Rahman AH, Zhang J, Turka LA, Walsh PT. CpG DNA inhibits CD4+CD25+ Treg suppression through direct MyD88-dependent costimulation of effector CD4+ T cells. Immunol Lett. 2007;108:183. doi: 10.1016/j.imlet.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivier A, Sainz-Perez A, Dong H, Sparwasser T, Majlessi L, Leclerc C. The adjuvant effect of TLR agonists on CD4(+) effector T cells is under the indirect control of regulatory T cells. Eur J Immunol. 2011;41:2303. doi: 10.1002/eji.201041387. [DOI] [PubMed] [Google Scholar]
- 43.von BH, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12:51. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 44.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr Opin Organ Transplant. 2012;17:349. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 45.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost. 2009;7:1523. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham PT, Pham PC, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, Singer J, Shah T, Wilkinson AH. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77:1215. doi: 10.1097/01.tp.0000118413.92211.b6. [DOI] [PubMed] [Google Scholar]
- 47.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharm. 1997;37:117. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 48.Klinman DM, Gursel I, Klaschik S, Dong L, Currie D, Shirota H. Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann NY Acad Sci. 2005;1058:87. doi: 10.1196/annals.1359.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.