Abstract
The signaling pathways that govern the lactotrope-specific differentiated phenotype, and those that control lactotrope proliferation in both physiological and pathological lactotrope expansion, are poorly understood. Moreover, the specific role of MAPK signaling in lactotrope proliferation vs differentiation, whether activated phosphorylated MAPK is sufficient for prolactinoma tumor formation remain unknown. Given that oncogenic Ras mutations and persistently activated phosphorylated MAPK are found in human tumors, including prolactinomas and other pituitary tumors, a better understanding of the role of MAPK in lactotrope biology is required. Here we directly examined the role of persistent Ras/MAPK signaling in differentiation, proliferation, and tumorigenesis of rat pituitary somatolactotrope GH4 cells. We stimulated Ras/MAPK signaling in a persistent, long-term manner (over 6 d) in GH4 cells using two distinct approaches: 1) a doxycycline-inducible, oncogenic V12Ras expression system; and 2) continuous addition of exogenous epidermal growth factor. We find that long-term activation of the Ras/MAPK pathway over 6 days promotes differentiation of the bihormonal somatolactotrope GH4 precursor cell into a prolactin-secreting, lactotrope cell phenotype in vitro and in vivo with GH4 cell xenograft tumors. Furthermore, we show that persistent activation of the Ras/MAPK pathway not only fails to promote cell proliferation, but also diminishes tumorigenic characteristics in GH4 cells in vitro and in vivo. These data demonstrate that activated MAPK promotes differentiation and is not sufficient to drive tumorigenesis, suggesting that pituitary lactotrope tumor cells have the ability to evade the tumorigenic fate that is often associated with Ras/MAPK activation.
Prolactin-secreting lactotrope cells and GH-secreting somatotrope cells are derived from the common pituitary-specific transcription factor-1 (Pit-1) lineage. In some species, including rat, a bifunctional somatolactotrope precursor cell gives rise to lactotropes and somatotropes (1–3). Somatolactotropes retain plasticity, allowing for rapid cell differentiation and expansion in response to physiological demands. Somatolactotropes differentiate into lactotropes during pregnancy and lactation, and into somatotropes in response to exercise (1, 4). Despite the species-specific differences in lactotrope cell origin, conserved signaling pathways are critical for controlling lactotrope biology from humans to rodents. Pituitary lactotropes are under tonic inhibition by hypothalamic dopamine, which acts via the dopamine D2 receptor (D2R) to inhibit cAMP/protein kinase A and MAPK signaling pathways to limit prolactin (PRL) production and secretion, lactotrope proliferation, and growth of prolactin-secreting adenomas (5–9). Dopamine also increases autocrine TGFβ-1 signaling to inhibit lactotrope proliferation (10, 11). During pregnancy and lactation, dopaminergic inhibition is diminished by estradiol, allowing local growth factors from folliculostellate support cells to stimulate lactotropes, promoting lactotrope hyperplasia and a doubling in pituitary size (5, 12–14). Dysregulation of these pathways contributes to the tumorigenesis of prolactin-secreting adenomas, or prolactinomas (14–16). Prolactinomas cause hypogonadism, infertility, osteoporosis, and tumor mass effects, and are the most common type of neuroendocrine tumor (17, 18).
The specific signaling pathways that govern the lactotrope-specific phenotype and those that control lactotrope proliferation in both physiological and pathological lactotrope expansion are poorly understood. Uncontrolled activation of growth factor signaling pathways, such as the Ras/MAPK pathway, results in lactotrope hyperplasia with delayed but eventual benign adenoma formation in transgenic mice (15, 19). Ras proteins are frequently mutated in human cancers, including the naturally occurring valine 12 (V12) mutation, which results in constitutive activation of Ras signaling (20, 21). Ras is a critical effector of MAPK activation (19), and key regulators of lactotrope biology, such as TRH and vasoactive intestinal peptide, act via Ras to activate MAPK in somatolactotrope cells (22–24).
The precise role of MAPK signaling in lactotrope proliferation vs differentiation has been somewhat controversial. In vitro studies using rat pituitary somatolactotrope (GH3) or lactotrope (PR1) cell lines have shown that short-term (24–96 h) MAPK pathway activation mediates cellular proliferation (12, 25, 26). By contrast, long-term treatment of GH3 or GH4 rat pituitary somatolactotrope tumor cells over 4–7 days with epidermal growth factor (EGF), fibroblast growth factor (FGF)-4, or TRH result in a decreased GH4 cell proliferation and enhanced differentiation to the lactotrope phenotype (27–31). However, neither the pattern nor the peak of MAPK activation with long-term growth factor treatment has been reported. One study examined the differential effects of short-term vs long-term phosphorylated MAPK (pMAPK) activation, reporting that GH4 cells treated with FGF4 resulted in short-term pMAPK activation (<15 min) and increased cell proliferation, whereas GH4 cells treated with FGF2 resulted in prolonged pMAPK activation (>30 min) and little change in the cell number (32). Importantly, a persistent pattern of pMAPK activation has been shown to play a pivotal role in cellular differentiation in other endocrine tumors including thyroid carcinoma and pheochromocytoma (33, 34). The specific role of MAPK signaling in durable lactotrope proliferation and differentiation, and whether activated pMAPK is sufficient for lactotrope proliferation and tumor formation remain unknown. Given that Ras mutations and persistently activated pMAPK are found in human tumors (35, 36), including prolactinomas and other pituitary tumors (16, 37, 38), a better understanding of the causative role of MAPK in prolactinoma tumorigenesis is required.
In this study, we aimed to directly examine the role of persistent Ras/MAPK signaling in differentiation, proliferation, and tumorigenesis of rat pituitary somatolactotrope GH4 cells. We stimulated Ras/MAPK signaling in a persistent, long-term manner (over 6 d) in GH4 cells using two distinct approaches: 1) a doxycycline-inducible, oncogenic V12Ras expression system; and 2) the addition of exogenous EGF. We found that long-term activation of the Ras/MAPK pathway over 6 days promotes differentiation of the bihormonal somatolactotrope GH4 precursor cell into a prolactin-secreting, lactotrope cell phenotype. Furthermore, we show that persistent activation of the Ras/MAPK pathway not only fails to promote cell proliferation, but also diminishes tumorigenic characteristics in GH4 cells.
Materials and Methods
Plasmid construction
pcDNA3.1+ human H-Ras G12V 3× hemagglutinin (HA)-tagged (N terminus) plasmid was purchased from UMR cDNA Resource Center (University of Missouri-Rolla, Rolla, Missouri). Plasmid was cut with HindIII and XbaI to remove the insert. pTRE-Tight plasmid was purchased from CLONTECH Laboratories Inc and cut with HindIII, XbaI and then calf intestinal alkaline phosphatase (Fisher Scientific). Vector and cut inserts were run on 1% agarose gel, bands were cut out, and gel purified using QIAquick gel extraction kit (QIAGEN). The insert and vector were ligated (3:1 ratio) using T4 DNA ligase (Invitrogen) and then transformed in DH5α competent cells. Single colonies were picked and sequenced.
Cell culture
GH4T2 cells were maintained in DMEM (Gibco Life Technologies, Inc) supplemented with 15% horse serum and 2.5% fetal calf serum (Gibco Life Technologies). Cells were grown in a humidified tissue culture incubator at 37°C in 5% CO2. For the doxycycline (dox)-inducible cells, 2.5% fetal bovine serum (FBS) was replaced with 2.5% tetracycline (Tet) system-approved FBS (CLONTECH Laboratories Inc).
Dox-inducible clones
BS/IRES-M2 clone number 13 was established by cotransfecting GH4 cells with BS/IRES-M2 plasmid (a kind gift from Dr Stefania Lamartina, Istituto Di Richerche Di Biologia Molecolare, Rome, Italy) and empty pEGFP-C3 vector (Invitrogen; 10:1 ratio). Aliquots of 3 × 106 cells in 200 μL of medium was added to plasmid DNA and transfected by electroporation at 220 V and 500 μF using a GeneZapper 450/2200 (IBI/Kodak) with 4-mm gap cuvettes. After transfection, cells were plated on 60-mm tissue culture plates in culture media and incubated for 24 hours. Changed to DMEM with 300 μg/mL G418 (Corning Cellgro) for selection. Clones were picked and maintained in DMEM with 100 μg/mL G418.
To create dox-inducible clones, BS/IRES-M2 clone number 13 was cotransfected with pTRE-H-Ras G12V 3× HA-tagged plasmid (constructed as described above) and empty pQCXIP-puro plasmid (CLONTECH; 5:1 ratio). Changed to DMEM with 100 μg/mL G418 and 4 μg/mL puromycin (Sigma). Clones were picked and maintained in DMEM with 100 μg/mL G418 and 2 μg/mL puromycin. We performed all experiments in HA-V12Ras clones number 10 and number 20 because these clones required dox for 3HA-V12Ras expression. The figures in this paper show experimental results from clone number 10. Comparable results were obtained from clone number 20 for all experiments (data not shown).
Protein sample preparation, SDS-PAGE, and Western blotting
Cells were plated on 60-mm tissue culture dishes with serum-starved media (0.05% Tet approved FBS) for dox-inducible clones or with complete media (15% horse serum, 2.5% FBS) for EGF experiments. Changed every 2 days with or without 2 μg/mL doxycycline (Sigma) or with or without 30 ng/mL EGF (Sigma). Cells were washed and harvested with extraction buffer: 10 mM Tris, pH 7.4; 5 mM EDTA; 50 mM NaCl; 50 mM NaF; 0.1% BSA; 1% Triton X-100; 2 mM Na3VO4; 1 mM phenylmethylsulfonyl fluoride; and 1 mM dithiothreitol. Protein was quantitated using a Bio-Rad DC protein assay (Bio-Rad Laboratories). Twenty-five to 50 μg of protein sample were run on a 10% PAGE gel. Primary antibodies, anti-HA (1:5000; Covance), anti-pMAPK (1:1000; Cell Signaling), anti-MAPK (1:7500; Upstate Biotechnology), anti-PRL [PRL; 1:5000; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)], anti-GH (1:5000; NIDDK), anti-Pit-1 (1:5000; BAbCo), anti-cMyc (1:1000; Abcam), and anti-p21 (1:7500; Sigma) were incubated overnight at 4°C. Antitubulin (1:10,000; Calbiochem) and anti-MAPK phosphatase 2 (MKP-2) (1:200; Santa Cruz Biotechnology) primary antibodies were incubated at room temperature for 1 hour. Polyclonal goat horseradish peroxidase-conjugated secondary antibodies against mouse, rabbit, or rat (1:5000; Bio-Rad Laboratories Inc), or polyclonal donkey horseradish peroxidase-conjugated secondary antibody against chicken (1:2000; Bio-Rad Laboratories), were incubated on membranes for 1 hour at room temperature. Blots were stripped using Chemicon strong reblot reagent (Chemicon Inc) prior to reprobing with additional primary antibodies. Film was imaged using a scanner. To quantify PRL and GH protein expression, film was imaged using an AlphaImager gel documentation system, and densitometry of bands was measured using AlphaImager software (Alpha Innotech). PRL and GH protein expression was normalized to tubulin loading control and graphed as a percentage of total (PRL + GH) protein expression for each time point.
Proliferation assay
Cells were harvested with 0.05% trypsin-EDTA and resuspended in DMEM culture medium. Culture medium is as described above but with 15% horse serum and 2.5% FBS or Tet-approved FBS (for dox inducible clones). Dox-inducible clones were maintained in serum-complete media for proliferation assays because cells failed to proliferate in serum-starved conditions, independent of dox addition (data not shown). For dox-inducible GH4 clones, 12 500 cells/well in 100 μL medium was plated in 96-well plates. Fresh medium with or without 2 μg/mL doxycycline was added to culture medium on days 1 and 3. To fix time points, cells were washed with 1× PBS, fixed with 4% paraformaldehyde (PFA) for 15 minutes, and washed twice more with 1× PBS on days 1, 2, 3, and 4. For EGF experiments, 9000 cells/well in 100 μL media were plated in 96-well plates. Fresh medium with or without 30 ng/mL EGF were added to culture medium on days 1 and 3. Cells were fixed with 4% PFA, as described above, each day for 6 days. All proliferation assays were incubated in a humidified tissue culture incubator at 37°C in 5% CO2. For analysis, cells were stained with 0.1% crystal violet (Sigma) in 25% methanol for 1 hour at room temperature. Cells were washed four times with double-distilled H2O (ddH2O) and then lysed with 10% acetic acid for 15 minutes. Cell lysates (100 μL/sample) were transferred to a new 96-well plate and read at an OD of 570 nm using a Synergy HT microplate reader (BioTek Instruments, Inc). For a subset of experiments, cells were stained with trypan blue and manually counted, revealing comparable results (data not shown).
Clonogenicity assay
Clonogenicity assays were performed in assay medium consisting of DMEM supplemented with 15% horse serum and 2.5% FBS or Tet-approved FBS (for dox inducible clones). Cells were harvested with 0.05% trypsin-EDTA, and 3000 cells/well were plated in six-well tissue culture plates. All clonogenicity assays were incubated in a humidified tissue culture incubator at 37°C in 5% CO2. Medium with or without 2 μg/mL doxycycline, or with or without 30 ng/mL EGF, was changed every other day for a total experiment length of 11 days. On day 11, cells were washed with 1× PBS, fixed with 4% PFA for 15 minutes, and washed twice more with 1× PBS. Cells were stained with 0.1% crystal violet in 25% methanol for 1 hour at room temperature and then washed four times with ddH2O. Colonies were photographed using a digital camera, and the acquired images were analyzed using ImageJ software (National Institutes of Health, Bethesda, Maryland). Colonies larger than 150 μm in diameter were counted.
Soft agar assay
Soft agar assays were performed in six-well tissue culture plates in assay medium consisting of DMEM (Gibco BRL Life Technologies, Inc) supplemented with 15% horse serum and 2.5% Tet-approved FBS. Individual wells were covered with 1.5 mL of base layer (0.6% agar), composed of 60% agar stock (1% Difco TM noble agar in ddH2O) and 40% assay medium with or without 2 μg/mL dox or 30 ng/mL EGF. Base layers were allowed to solidify for 30 minutes at room temperature. The cells were grown in complete media, then harvested with trypsin and suspended in assay media. Subsequently, cells were counted and resuspended at 25 000 cells/well. All cell suspensions were mixed with agar-containing assay media such that the final concentration of agar for the top layer was 0.3%. The top layer was plated on the solidified base layer (1.5 mL/well) and left to solidify at room temperature for 30 minutes. All soft agar cultures were incubated in a humidified tissue culture incubator at 37°C in 5% CO2 for a total experiment length of 17 days, and 150 μL of complete medium with or without 2 μg/mL dox or 30 ng/mL EGF, was added to each well every other day. Cell colonies were treated with 200 μL Nitroblue reagent (1 mg/mL; Amresco) and incubated 37°C overnight to develop a blue stain. Colonies were photographed with a digital camera, and the images were analyzed using ImageJ software (National Institutes of Health). Colonies larger than 150 μm in diameter were counted.
Nude mouse xenografts
Xenograft experiments were conducted in nude female mice, aged 7–8 weeks, purchased from the National Cancer Institute. During the experiment, mice were supplemented with estrogen released from a pellet (mixture containing 1 g 17 β-estradiol and 4 g cellulose) placed sc 1 week prior to injection of cells. Mice were provided with water in amber bottles containing 5% sucrose with or without 2 mg/mL dox for 3 days prior to injection of cells. The dox-inducible clones were harvested with trypsin, washed twice with 1× PBS, and resuspended in DMEM at 1 × 107 cells/mL. Mice were anesthetized with isoflurane, and 200 μL of the cell suspension (2 × 106 cells) was injected sc into each flank. Animals were examined for tumor formation every 2 days, and tumor size was measured using a caliper. Tumor volume was calculated using the following formula: (0.52 × length × width2). Mice were euthanized 45 days after cell injection. Nude mouse xenograft experiments were performed under an animal protocol approved by the Animal Care and Use Committee of the University of Colorado AMC.
Tissue processing
Tumors were extracted 45 days after cell injection and were immediately fixed in 4% PFA in PBS overnight at room temperature. Fixed tumors were then rinsed with 70% EtOH. Tumors were embedded in paraffin, cut into sections, and mounted on glass slides by the University of Colorado AMC Research Histology Shared Resource facility.
Immunohistochemistry
Slides were deparaffinized in xylene and rehydrated in graded ethanol. For antigen retrieval, slides were heated in sodium citrate (10 mM solution in Tween 20, pH 6.0; Fisher Scientific) in a decloaker (Biocare Medical). Slides were treated with 0.3% hydrogen peroxide (Fisherbrand) for 30 minutes to block endogenous peroxidase activity and were then blocked in 5% BSA in Tween 20. For anti-HA staining, slides were incubated with primary anti-HA antibody (1:25; Roche) overnight. Diaminobenzidine (DAB)+ peroxidase substrate (Dako) was used per the manufacturer's instructions. Nuclei were counterstained using Mayer's hematoxylin (diluted 1:10) for 30 seconds. The sections were dehydrated in graded ethanol and mounted using Permount (Fisher). For anti-phospho-H3 and anti-PRL staining, slides were incubated overnight with primary antibodies antiphosphorylated histone H3 (pH3) (1:400; Upstate Signaling Solutions) or anti-PRL (1:1000; NIDDK). Slides were incubated for 1 hour with biotin-conjugated goat antirabbit IgG secondary antibody (1:200; Vector Laboratories), followed by washes and DAB staining as explained above. For pH3, 10 fields per slide were randomly selected and an individual that was blinded to the identity of the samples counted cells positive for pH3 staining. The number of positive cells per field was averaged for each sample and plotted. For anti-GH staining, slides were incubated overnight in primary anti-GH antibody (1:1000; NIDDK) and were then incubated for 1 hour with biotin-conjugated goat antimonkey IgG secondary antibody (1:500; Santa Cruz Biotechnology), followed by washes and DAB staining as explained above. For PRL and GH scoring, a pathologist blinded to the identity of the sections scored PRL and GH staining. At least 200 cells in a minimum of three fields of view were scored for each tumor. Necrotic tumor regions were excluded from analysis. Cells with weak to strong DAB staining were scored as positive and reported as a percentage of total cells counted per tumor. To calculate PRL or GH staining as a percentage of total staining, the percentage of total cells positive for PRL or GH was divided by the sum of cells positive for PRL and cells positive for GH.
Results
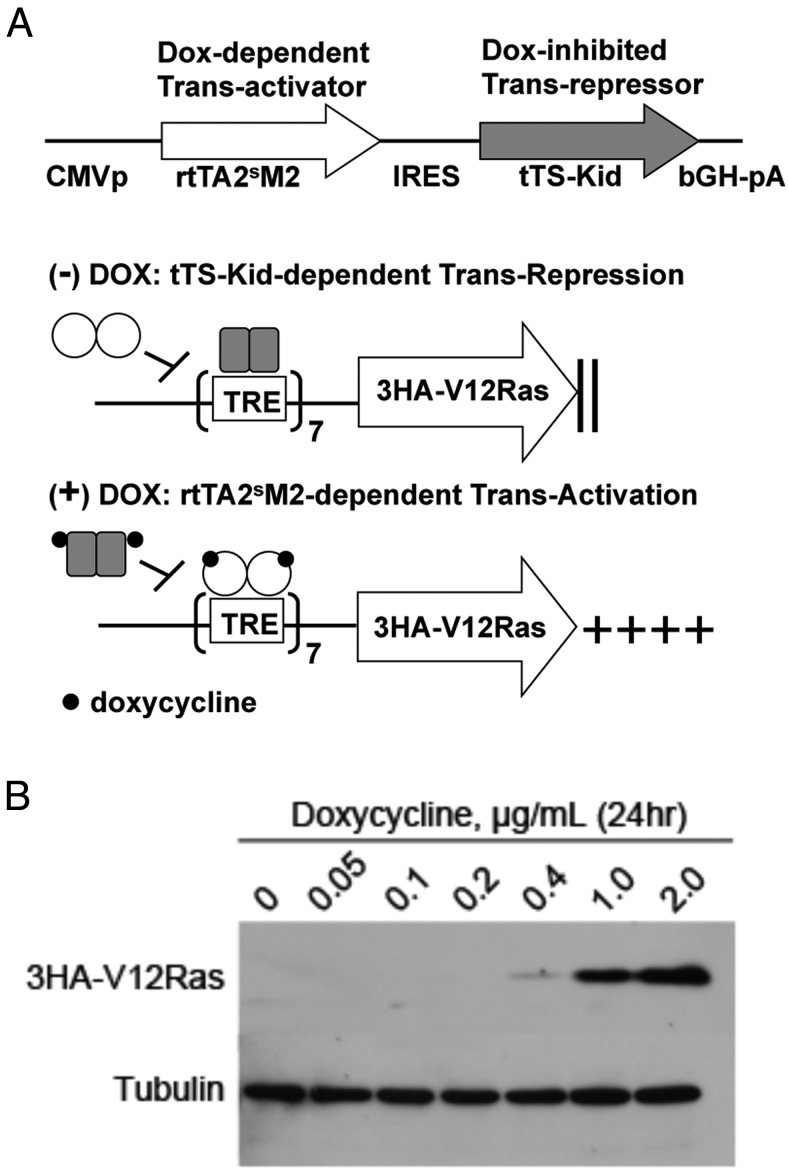
To determine the role of persistent activation of MAPK in the proliferation and differentiation of GH4 pituitary somatolactotrope cells, we sought to achieve tight, reversible control of pMAPK activation. We established and selected optimal GH4 clonal cell lines (number 10 and number 20) stably expressing HA-tagged, constitutively active V12Ras under the control of a precisely regulated dox-inducible system (Figure 1A) (39). A dox-dependent transcriptional activator, rtTA2sM2, and the dox-inhibited transcriptional repressor, tTS-Kid, were expressed from a single cytomegaolovirus promoter via an internal ribosome entry site (IRES). In the absence of dox, tTS-Kid binds and strongly represses the TetO7 operator that is expressed upstream of 3HA-V12Ras in the pTRE-Tight effector plasmid. In the presence of dox, DNA-binding of the tTS-Kid silencer is destabilized, whereas that of the rtTA2sM2 transactivator is stabilized, thus switching from repression to activation of 3HA-V12Ras transcription in a dox dose-dependent manner. We performed all experiments in 3HA-V12Ras clones number 10 and number 20 because these clones required dox for 3HA-V12Ras expression. Here we show experimental results for clone number 10, and comparable results were obtained for clone number 20 for all experiments (data not shown). GH4 clone number 10 cells were serum starved (0.05% FBS) overnight, and then treated with concentrations of dox ranging from 0 to 2 μg/mL. Addition of dox to GH4 cells resulted in stable expression of 3HA-V12Ras within 24 hours that was tightly regulated in a dose-dependent manner (Figure 1B). Expression of V12Ras peaked at a dox concentration of 2 μg/mL, and was similar to 3 μg/mL and 10 μg/mL dox (data not shown). Thus, 2 μg/mL dox was used for subsequent in vitro studies. Expression of 3HA-V12Ras protein was not detectable at dox concentrations of 0, 0.05, or 0.1 μg/mL, even with prolonged membrane exposure (data not shown), demonstrating that dox-induced expression of 3HA-V12Ras was tightly regulated and was not leaky.
Figure 1.
Doxycycline-dependent 3HA-V12Ras expression is stable and tightly regulated. A, Model of dox-dependent 3HA-V12Ras expression. The dox-dependent transcriptional activator, rtTA2sM2, and the dox-inhibited transcriptional repressor, tTS-Kid, are expressed from a single cytomegalovirus promoter via an IRES. In the absence of dox, tTS-Kid (gray rectangles) binds and strongly represses the TetO7 operator that is expressed upstream of 3HA-V12Ras from the pTRE-Tight effector plasmid. In the presence of dox (black dots), DNA binding of the tTS-Kid silencer is destabilized, whereas DNA binding of the rtTA2sM2 transactivator (white circles) is stabilized, thus switching from repression to activation of 3HA-V12Ras transcription in a dox dose-dependent manner. B, Western blot analysis of dox-induced 3HA-V12Ras expression. GH4 cells were cotransfected with a pTRE vector containing 3HA-V12Ras and an empty pQCXIP-puro plasmid. Clones were picked and maintained in media with 100 μg/mL G418 and 2 μg/mL puromycin. Clone number 10 (shown here) was serum starved (0.05% FBS) overnight and treated ±dox with varying concentrations for 24 hours. Whole-cell extracts (50 μg) were separated by SDS-PAGE and probed with anti-HA and antitubulin antibodies.
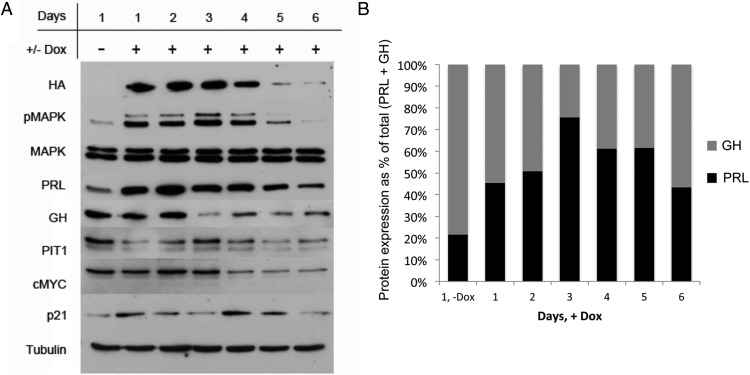
To determine the effect of V12Ras expression on pMAPK activation in GH4 cells, cells were serum starved overnight. Serum-starved medium (0.05% FBS) containing 2 μg/mL doxycycline was added on days 1, 3, and 5, and cells were harvested daily for 6 days. A Western blot for HA shows increased expression of 3HA-V12Ras on days 1–4 of doxycycline treatment, with reduced expression beginning on day 4 and apparent loss of HA expression occurring by day 5 (Figure 2A). Activation of pMAPK reflected the expression pattern of 3HA-V12Ras, with increased activation on days 1–4 that was diminished by days 5 and 6 (Figure 2A). Levels of total MAPK protein in GH4 cells were not altered by dox treatment (Figure 2A). The loss of HA and pMAPK protein, despite continued dox treatment, also occurred with a separate dox-inducible GH4 clone (clone number 20; data not shown). MKP-2, a dual-specificity phosphatase that dephosphorylates both threonine and tyrosine residues on MAPK, shows increased expression on days 2 and 4 subsequent to the addition of dox on days 1 and 3, but no expression was detected on days 5 or 6 (Supplemental Figure 1A). The expression of MKP-1 did not change with the addition of dox (data not shown).
Figure 2.
Stable expression of V12Ras increases PRL and reduces GH expression. A, Western blot analysis of GH4 clone number 10 treated ±dox. GH4 clone number 10 cells expressing dox-inducible 3HA-V12Ras (from Figure 1) were serum starved (0.05% FBS) overnight. Serum-starved medium containing 2 μg/mL dox was added on days 1, 3, and 5, and cells were harvested daily for 6 days. For day 1, (−)Dox cells were harvested after overnight serum starvation and were not treated with dox. Whole-cell extracts (50 μg) were separated by SDS-PAGE and probed with the antibodies listed (n = 3 independent experiments; a representative experiment is shown). B, Quantification of PRL and GH expression ±dox. PRL (black bars) and GH (gray bars) protein expression from panel A was quantified by densitometric analysis using AlphaImager software (Alpha Innotech), normalized to PRL expression on day 1 (−)Dox, and graphed as a percentage of total (PRL + GH) expression for each day.
Upon addition of dox, PRL expression is strongly increased for 4 days, with diminished expression beginning on day 5 (Figure 2A). GH expression is decreased by day 3, and remains low for the duration of doxycycline treatment (Figure 2A). This increase in the PRL to GH ratio (Figure 2B) suggests differentiation from a bihormonal somatolactotrope precursor cell into a lactotrope phenotype, and reiterates our previous PRL and GH reporter work showing that PRL, but not GH, is Ras/MAPK responsive (40, 41). Furthermore, Pit-1, a transcription factor responsible for cell-specific expression of PRL and GH, and cMyc, a transcription factor that governs cell proliferation, both show decreased expression after day 3 of dox treatment, essentially following the GH response pattern (Figure 2A). These data indicate that persistent V12Ras/pMAPK activation in GH4 cells does not impart a growth response. The cyclin-dependent kinase inhibitor p21 exhibits small fluctuations in expression that do not correspond to the pattern of pMAPK activation (Figure 2A).
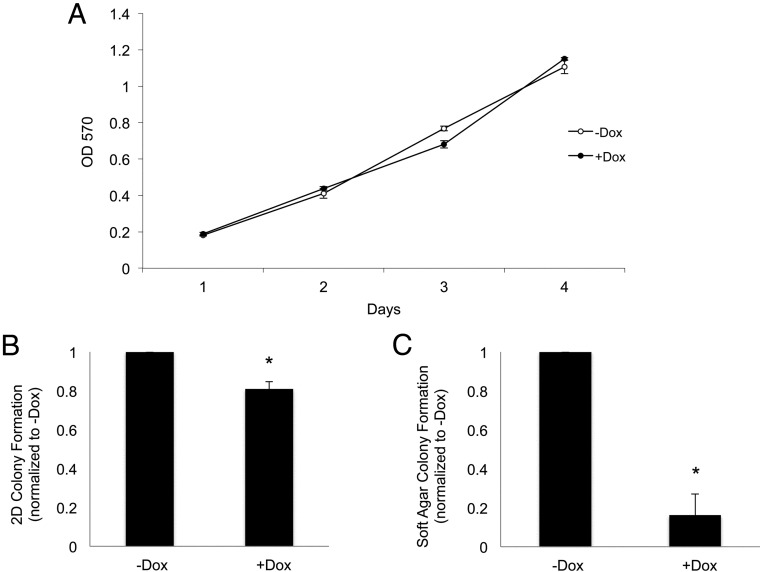
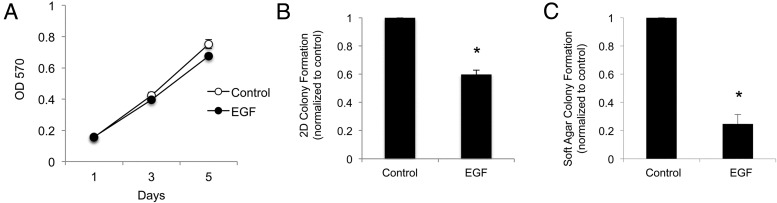
We next sought to determine how alterations in Ras/MAPK signaling affect GH4 cell proliferation and transformation. GH4 clone number 10 cells expressing doxycycline-inducible 3HA-V12Ras were maintained in complete media (15% horse serum, 2.5% FBS), because cells failed to proliferate in serum-starved conditions (data not shown). Cells were treated with or without 2 μg/mL dox on days 1 and 3, and were fixed daily for analysis. Crystal violet staining revealed that V12Ras has no effect on GH4 cell proliferation over a 4-day period (Figure 3A), or after 6 days (data not shown). To determine the effect of persistent Ras/MAPK signaling on GH4 cell tumorigenicity, GH4 clone number 10 cells were maintained in complete media with or without 2 μg/mL dox and allowed to form colonies in two dimensions (2D) on poly-L-lysine-coated tissue culture plates or in three dimensions (3D) in soft agar. Cell colonies were stained with crystal violet for 2D colony formation assays or overnight with nitroblue for 3D soft agar anchorage-independent colony formation assays. Colonies were photographed and counted using ImageJ software (National Institutes of Health). GH4 cells with activated V12Ras exhibited a 20% reduction in colony formation (Figure 3B) as well as an 80% reduction in anchorage-independent colony number in soft agar (Figure 3C). Colony size was not noticeably different for dox-treated cells (data not shown). These data indicate that V12Ras-mediated activation of the MAPK pathway is insufficient to induce GH4 cell proliferation or tumorigenesis. Thus, contrary to studies of short-term pMAPK activation, expression of V12Ras and persistent pMAPK activation reduces tumorigenic phenotype in GH4 pituitary somatolactotrope cells.
Figure 3.
V12Ras expression does not increase GH4 cell proliferation but decreases tumorigenic characteristics. A, Analysis of GH4 clone number 10 cell proliferation ±dox. GH4 clone number 10 cells stably expressing dox-inducible 3HA-V12Ras (from Figure 1) were maintained in complete media (15% horse serum, 2.5% FBS) because cells failed to proliferate in serum-starved conditions (data not shown). In 96-well plates, 12 500 cells/well were plated in 100 μL of complete media with or without 2 μg/mL dox. On day 3, 100 μL of complete medium with our without 2 μg/mL dox was added. Cells were fixed on days 1, 2, 3, and 4 with 4% PFA, stained with crystal violet (0.05% in 25% methanol), and lysed with 10% acetic acid. Absorbance was read at OD 570 (n = 3 independent experiments; a representative experiment is shown). B, Analysis of 2D GH4 clone number 10 cell colony formation. GH4 clone number 10 cells were maintained in complete media. In six-well plates, 3000 cells/well were plated in 2 mL of complete media with or without 2 μg/mL dox. Complete medium with or without 2 μg/mL dox was added every other day, and cells were fixed with 4% PFA after 11 days. Cell colonies were stained with crystal violet, wells were photographed, and colonies were counted with ImageJ software (National Institutes of Health; n = 3). *, P < .05. C, Analysis of GH4 clone number 10 colony formation in soft agar. GH4 clone number 10 cells were maintained in complete media. In six-well plates, 25 000 cells/well were resuspended in a 0.3% agar solution with or without 2 μg/mL dox and plated on top of a base layer containing 0.6% agar with or without 2 μg/mL dox. Complete medium (150 μL) with or without 2 μg/mL dox was added every other day. On day 17, colonies were stained overnight with Nitroblue. Colonies were photographed and counted with ImageJ software (National Institutes of Health; n = 3). *, P < .05.
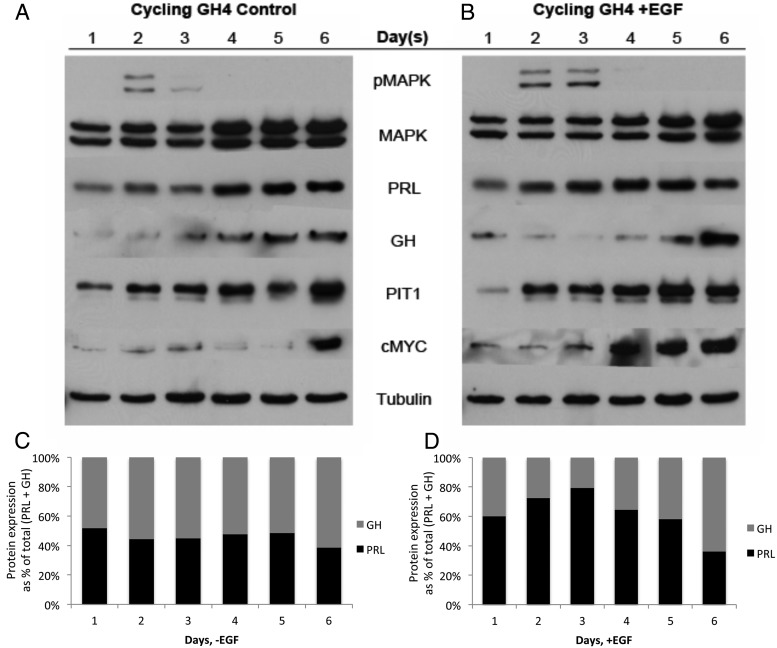
As a distinct approach to stimulate the Ras/MAPK pathway, nontransfected, cycling GH4 cells in serum-complete media were treated with exogenous EGF on days 1, 3, and 5, and harvested daily for 6 days. In control-treated cells, slight activation of pMAPK was observed on day 2, was decreased on day 3, and was absent on days 4–6 (Figure 4A). Treatment with EGF resulted in moderate activation of pMAPK that persisted for 2 days (Figure 4B). However, pMAPK did not stay activated despite prolonged EGF treatment (Figure 4B). Total MAPK protein expression was increased on days 4, 5, and 6 in control-treated cells, and on days 5 and 6 in EGF-treated cells (Figure 4, A and B). MKP-2 expression was not greater in cells treated with EGF than in cells treated without EGF at any point during the 6-day experiment, and MKP-1 expression did not change with EGF treatment (Supplemental Figure 1B and data not shown). The expression of PRL was increased on day 2 of EGF treatment, whereas PRL expression did not increase until day 4 in control-treated cells (Figure 4, A and B). Growth hormone expression was decreased on days 2–4 of EGF treatment and then increased on days 5 and 6 (Figure 4B). Conversely, GH expression steadily increased over days 1–6 in control-treated cells (Figure 4A). The PRL to GH ratio was initially increased with EGF stimulation of pMAPK and then returned to a level comparable with cycling, control-treated GH4 cells as pMAPK expression faded (Figure 4, B and D). The PRL to GH ratio remained relatively constant in control-treated cells (Figure 4C). These data support the interpretation that activation of Ras/MAPK signaling promotes differentiation of GH4 cells from a somatolactotrope precursor to a predominantly lactotrope phenotype.
Figure 4.
EGF increases PRL to GH expression ratio. A and B, Western blot analysis of GH4 cells treated (−)EGF (A) and (+)EGF (B). GH4 cells were maintained in serum-complete media. Complete medium with or without 30 ng/mL EGF was added on days 1, 3, and 5, and cells were harvested daily for 6 days. Whole-cell extracts (50 μg) were separated by SDS-PAGE and probed with the antibodies listed. C and D, Quantification of PRL and GH expression in GH4 cells treated (−)EGF (C) and (+)EGF (D). PRL and GH protein expression from panels A and B was quantified by densitometric analysis using AlphaImager software (Alpha Innotech), normalized to PRL expression on day 1 (−)EGF (A) or day 1 (+)EGF (B), and graphed as a percentage of total (PRL + GH) expression for each day (n = 3 independent experiments; a representative experiment is shown).
The expression of Pit-1 was increased over 6 days in EGF-treated cells, and was not different from that in cycling, control-treated GH4 cells (Figure 4, A and B). The expression of cMyc increased on days 4 and 5 of EGF treatment, 2 days earlier than the increase in cMyc expression observed in control-treated cells (Figure 4, A and B). However, GH4 cell proliferation after 5 days of EGF treatment was not significantly different compared with control-treated cells (Figure 5A). Furthermore, EGF treatment resulted in a 40% reduction in GH4 cell 2D colony formation and a 70% reduction in 3D anchorage-independent colony formation (Figure 5, B and C). Individual colony size was not noticeably different for EGF-treated cells (data not shown). Together these data demonstrate that persistent activation of the Ras/MAPK signaling pathway, whether via activation of V12Ras or with exogenous EGF, drives differentiation of GH4 somatolactotrope cells to a lactotrope phenotype, does not influence cell proliferation, and reduces tumorigenic phenotype.
Figure 5.
EGF does not influence proliferation but reduces 2D colony formation and anchorage-independent growth of GH4 cells. A, Analysis of GH4 cell proliferation ± EGF. GH4 cells were maintained in complete media. In 96-well plates, 5000 cells/well were plated in 100 μL complete media with or without 30 ng/mL EGF. Complete medium (100 μL) with or without EGF was added every other day. Cells were fixed on days 1, 3, and 5 with 4% PFA, stained with crystal violet (0.05% in 25% methanol), and lysed with 10% acetic acid. Absorbance was read at OD 570 (n = 3; a representative experiment is shown). B, Analysis of 2D GH4 cell colony formation ±EGF. GH4 cells were maintained in complete media. In six-well plates, 3000 cells/well were plated in 2 mL of complete media with or without 30 ng/mL EGF. Complete medium with or without EGF was added every other day, and cells were fixed with 4% PFA after 11 days. Colonies were stained with crystal violet, wells were photographed, and colonies were counted with ImageJ software (National Institutes of Health; n = 3). *, P < .05. C, Analysis of GH4 colony formation in soft agar ± EGF. GH4 cells were maintained in complete media. In six-well plates, 25 000 cells/well were resuspended in a 0.3% agar solution with or without 30 ng/mL EGF and plated on top of a base layer containing 0.6% agar with or without 30 ng/mL EGF. Complete medium (150 μL) with or without 30 ng/mL EGF was added every other day. On day 17, cells were stained overnight with nitroblue. Colonies were photographed and counted with ImageJ software (National Institutes of Health; n = 3). *, P < .05.
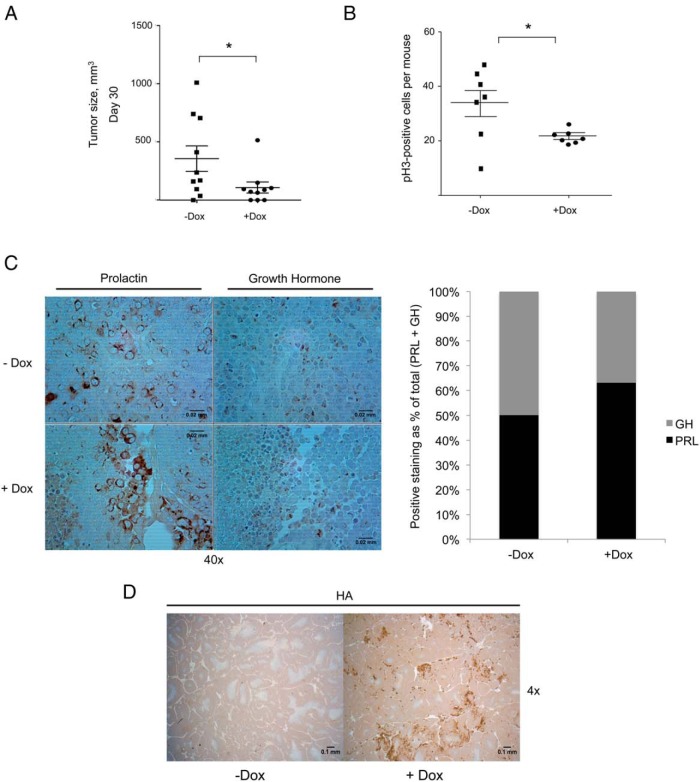
To further validate our in vitro findings, GH4 clone number 10 cells were injected into flanks of female nude mice. These studies have the advantage of studying a single clone (clone number 10), with the only experimental variable being treatment with or without dox. Mice were supplemented with estrogen pellets 1 week prior to flank injections because tumor formation with GH4 cells is estrogen dependent. Dox was administered via drinking water to turn on expression of V12Ras in the flank-injected cells in a subset of mice. Tumor size was measured every other day using a digital caliper. After 30 days, mice that received dox (+Dox) had significantly lower average xenograft tumor size (109 ± 48 mm3) compared with control mice (−Dox) (357 ± 110 mm3) that received flank injections but no dox (Figure 6A). Tumors were extracted and analyzed by immunohistochemistry for pH3, PRL, GH, and HA. There were significantly fewer cells positive for pH3, a marker of cell proliferation, in (+)Dox mice compared with (−)Dox mice (Figure 6B). Quantitative scoring of tumor sections revealed a 13% increase in the PRL to GH ratio in xenograft tumors from (+)Dox mice compared with (−)Dox mice (Figure 6C). GH4 clone number 10 xenograft tumors from (+)Dox mice expressed 3HA-V12Ras and tumors from (−)Dox mice did not (Figure 6D). Together, these in vivo data illustrate the role of Ras/MAPK signaling in promoting differentiation of somatolactotrope precursor cells to a lactotrope phenotype. Moreover, our findings show a reduced in vivo tumorigenic phenotype of GH4 cells with activated V12Ras, which suggests that pituitary lactotrope tumor cells may have the ability to evade the tumorigenic fate that is often associated with Ras/MAPK activation.
Figure 6.
Dox-induced 3HA-V12Ras expression diminishes GH4 cell xenograft tumor growth and promotes a lactotrope phenotype. A, GH4 clone number 10 xenograft tumor growth in nude mice. Female nude mice were supplemented with estrogen pellets for 1 week. Mice were provided with water containing 5% sucrose with or without 2 mg/mL doxycycline for 3 days prior to cell injection. GH4 clone number 10 cells were injected bilaterally into flanks of nude mice using 2 × 106 cells suspended in 200 μL DMEM for each injection. Animals were monitored for tumor formation every other day, and tumor size was measured with a digital caliper. Tumor volume was calculated using the following formula: (0.52 × length × width2). Tumor volume on day 30 after cell injection was plotted (n = 10). *, P < .05. B, Quantification of pH3-positive cells in GH4 clone number 10 xenograft tumors by immunohistochemical analysis. Sections of paraffin-embedded xenograft tumors from panel A were incubated with an anti-pH3 primary antibody and then stained with a biotin-conjugated secondary antibody. Cells that were positively stained for pH3 were manually counted from 10 fields per slide. The average number of positive cells per field is plotted for each tumor section (n = 7). *, P < .05. C, Immunohistochemical (IHC) analysis and scoring of PRL and GH staining in GH4 clone number 10 xenograft tumors. Sections of paraffin-embedded xenograft tumors from panel A were incubated with an anti-PRL primary antibody or an anti-GH primary antibody and then stained with a biotin-conjugated secondary antibody. Cells with weak to strong PRL or GH DAB staining were scored as positive, reported as percentage positive of total cells scored, and are graphed as a percentage of the total positive cells (PRL+ GH) (n = 3). D, IHC analysis of HA-tagged V12Ras expression in GH4 clone number 10 xenograft tumors. Sections of paraffin-embedded xenograft tumors from panel A were incubated with an anti-HA primary antibody, as detailed in Materials and Methods (n = 3).
Discussion
The timing, extent, and duration of MAPK activation appears to be critical in regulating cell differentiation vs proliferation, and this often occurs in a tissue-specific manner. Previous studies addressing the functional role of MAPK in GH4 rat pituitary somatolactotrope cells have been controversial; some report the role of activated pMAPK in proliferation (12, 25, 26), whereas others show pMAPK promotes differentiation (27–31). However, the duration and extent of pMAPK activation in these studies varies widely. Here we show that the duration of MAPK activation is critical in dictating the biological response. We demonstrate the effect of persistent pMAPK to drive differentiation toward a lactotrope phenotype. Based on our data, as well as data from others, Ras/MAPK-driven differentiation of pituitary precursor cells to a lactotrope phenotype may represent the mechanism for physiological lactotrope differentiation as well as a pathological mechanism for amplified lactotrope differentiation and secretion of prolactin in prolactinomas. As our findings illustrate, activation of Ras/MAPK signaling is not sufficient to drive lactotrope proliferation. In pituitary cells, oncogenic mutations in Ras or Raf alone drive hyperplasia and delayed, benign adenoma formation, but are not sufficient for transformation (19).
Transgenic mice studies targeting growth factors (nerve growth factor, TGFα, FGF-R4) to pituitary lactotropes resulted in early hyperplasia, occurring within approximately 4 months, followed by delayed adenoma formation at approximately 10 months, but these pituitary cells were resistant to true carcinogenesis (42–45). Activating mutations in an additional pathway, often phosphatidylinositol 3-kinase (PI3K), must also occur to promote tumorigenesis (19, 46–48). The PI3K/AKT signaling pathway is also downstream of Ras and governs cell survival, proliferation, and differentiation (19). Studies targeting oncogenic Ras to thyroid and ovarian endocrine cells in transgenic mice show that activated MAPK is necessary, but not sufficient, to mediate proliferative and tumorigenic responses and that the PI3K pathway is essential (49–52). Furthermore, the D2R isoforms D2L and D2S, when activated by dopamine, function to inhibit downstream PI3K and stimulate downstream MAPK signaling, respectively (9, 53). Therefore, dopamine, the neurotransmitter responsible for tonic inhibition of lactotrope proliferation and prolactin secretion, is capable of simultaneously altering both the MAPK and PI3K signaling pathways, further suggesting that both of these pathways are important to lactotrope homeostasis in normal physiological conditions. Indeed, we speculate that it may be the balance of MAPK and PI3K signaling that is particularly important in establishing an outcome of differentiation vs proliferation. Moreover, concurrent mutations in these pathways may act synergistically to drive prolactinoma tumorigenesis. A recent study comparing D2S- and D2L-null mice reported that expression of one dopamine receptor isoform is sufficient to maintain lactotrope homeostasis in physiological conditions, but signaling from both is required to prevent pathological lactotrope hyperplasia when challenged with estrogen (53). These findings further support the notion that the MAPK and PI3K signaling pathways work in unison to drive lactotrope differentiation and proliferation during pregnancy or prolactinoma formation.
In the present study, we used an experimental model in which we established persistent activation of MAPK up to 6 days using a dox-inducible V12Ras transgene, or with exogenous EGF stimulation. We demonstrate that the activation of pMAPK leads to GH4 pituitary somatolactotrope differentiation to a lactotrope phenotype. Upon dox-induced V12Ras expression, or with addition of exogenous EGF, the Ras/MAPK signaling pathway is stimulated as evidenced by activated pMAPK, and is persistently activated for at least 2 days (Figures 2A and 4B). Furthermore, the increase in the PRL to GH ratio observed both in vitro (Figures 2 and 4), and in vivo (Figure 6C) suggests the differentiation of GH4 somatolactotrope cells into a lactotrope phenotype. The lack of cell proliferation in the presence of MAPK pathway activation suggests that cells may be exiting the cell cycle. Moreover, phosphorylation of histone 3 is associated with chromosome condensation at mitosis (54), and xenograft tumors expressing V12Ras show a reduction in histone 3 phosphorylation (Figure 6B). However, cell cycle analysis shows that the expression of p21 (Figure 2A), p27, cyclin D3, and cyclin-dependent kinase-4 (data not shown) is not significantly changed with V12Ras expression or EGF treatment (data not shown). Further analysis of cell cycle regulators may reveal that GH4 cells exit the cell cycle in response to persistent activation of MAPK signaling. Nevertheless, the precise mechanism by which MAPK signaling promotes endocrine cell differentiation remains unclear.
The MAPK pathway also has implications unrelated to proliferation in other endocrine systems. ERK1 and ERK2 signaling is required for expression of LHβ from pituitary gonadotrope cells, and ablation of ERK signaling in the pituitary of female mice impairs ovulation and fertility (55). Likewise, female mice that lack ERK1 and ERK2 in ovarian granulosa cells fail to ovulate (56). The role of MAPK activation in the differentiation of pituitary gonadotrope cells and in the ovarian endocrine system is implicit rather than explicit. ERK5, a downstream target of Ras, is known to play a role in neural differentiation and cardiovascular development (57). Investigation into ERK5 activation with stimulated Ras/MAPK signaling in GH4 cells may unveil a mechanism by which MAPK signaling regulates the differentiation of somatolactotrope cells.
An interesting finding in this report has been the discovery that GH4 cells do not tolerate the persistent activation of MAPK beyond 4 days in either the dox-inducible V12Ras clones or with EGF stimulation. By the fourth day of activated Ras/MAPK signaling, the expression of HA-V12Ras and pMAPK fade, despite a continued addition of dox to turn on the transcription of HA-V12Ras (Figure 2A), noted in both clone number 10 and clone number 20. Likewise, pMAPK expression was diminished by the fourth day of EGF treatment, even with continuous addition of EGF (Figure 4B). These findings suggest that GH4 cells do not tolerate prolonged activation of the Ras/MAPK signaling pathway, and evolve mechanisms to silence the V12Ras transgene and diminish responsiveness to EGF.
In the early stages of our experiments, we used a first-generation Tet-Off dox-inducible system to express V12Ras in GH4 cells. However, upon analysis by Western blot, we discovered that this system was leaky, and expression of 3HA-V12Ras and activation of pMAPK occurred without the addition of dox (data not shown). We were unable to maintain stable clones due to poor cell growth, most likely due to GH4 cell differentiation and a reduction in proliferation, as shown here. Indeed, GH4 cells stably overexpressing V12Ras also display slow cell growth (data not shown). These findings, together with data reported here, reveal that persistent Ras/MAPK signaling is a negative regulator of GH4 cell proliferation.
The mechanism by which Ras/MAPK signaling is turned off in our 3HA-V12Ras dox-inducible GH4 clones, and in GH4 cells stimulated with exogenous EGF, is not immediately clear. There are multiple levels of negative feedback within the Ras/MAPK signal transduction pathway (58–61) that may allow cells to elude prolonged activation of pMAPK despite pathway stimulation by V12Ras or EGF. However, no detailed mechanism for this has been reported in lactotrope cells. MKP-1 and MKP-2 are dual-specificity MAPK phosphatases that have been implicated in the regulation of ERK signaling in the anterior pituitary (62). MKP-2 expression was increased on days 2 and 4 in GH4 cells, subsequent to the addition of dox on days 1 and 3, but no expression was detected on days 5 and 6 (Supplemental Figure 1A). Although MKP-2 is initially increased in response to pMAPK activation, MKP-2 is not expressed on days 5 and 6 and therefore does not account for the loss of pMAPK expression after day 4. Furthermore, the loss of expression of the HA-V12Ras transgene, despite the continued addition of dox, cannot be explained by the altered expression of a MAPK phosphatase. MKP-2 expression is not increased with EGF treatment (Supplemental Figure 1B), and MKP-1 expression does not change with the addition of dox or with EGF treatment (data not shown). Further investigation into MAPK effectors that are downstream of Ras is necessary to determine how GH4 somatolactotrope cells are capable of turning off the Ras/MAPK signaling pathway in the presence of exogenous stimulation.
Distinct cell types have been shown to respond to activated Ras signaling in a cell-specific manner. Oncogenic Ras drives tumorigenesis and/or metastasis in some human epithelial cancers, including pancreatic, colon, and lung cancers (63–65). Conversely, oncogenic Ras triggers differentiation of PC12 pheochromocytoma cells and TT medullary thyroid carcinoma cells (33, 34). Our findings demonstrate that in pituitary somatolactotropes, activated Ras promotes differentiation and is not sufficient to drive tumorigenesis. Indeed, other groups have reported that activated Ras is insufficient for transformation of pituitary lactotropes (42–45). The ability of pituitary lactotropes to evade tumorigenic responses to V12Ras suggests that there may be a differential expression of inhibitory signaling molecules or negative cell cycle regulators that are capable of acting as a brake to prevent the tumorigenic effects of sustained Ras/MAPK signaling. Investigation into GH4, TT, and PC12, and other cell types that do not respond to Ras in a tumorigenic manner has the potential to unveil a protective mechanism present in these cells, but absent in pancreatic, colon, and lung cancer cells that have a tumorigenic response to oncogenic Ras. A better understanding of the cell-specific responses to Ras gained by studies in neuroendocrine systems could shed light on the oncogenic mechanism of Ras mutations, and provide a better approach to counteract tumorigenesis, even in the most malignant human cancers.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We acknowledge and thank Drs Andy Bradford, Rebecca Schweppe, and Dawn Duval for critical reading and discussions of this manuscript. We also acknowledge the University of Colorado Research Histology Shared Resource, supported by the University of Colorado Cancer Center Support Grant P30CA046934.
This work was supported by National Institutes of Health Grants R01 DK46868 and R01 CA141201.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- D2R
- dopamine D2 receptor
- dox
- doxycycline
- EGF
- epidermal growth factor
- FBS
- fetal bovine serum
- FGF
- fibroblast growth factor
- HA
- hemagglutinin
- IRES
- internal ribosome entry site
- MKP-2
- MAPK phosphatase-2
- pH3
- phosphorylated histone H3
- PI3K
- phosphatidylinositol 3-kinase
- Pit-1
- pituitary-specific transcription factor-1
- pMAPK
- phosphorylated MAPK
- PRL
- prolactin
- Tet
- tetracycline
- V12
- valine 12.
References
- 1. Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355. [DOI] [PubMed] [Google Scholar]
- 2. Cuny T, Gerard C, Saveanu A, Barlier A, Enjalbert A. Physiopathology of somatolactotroph cells: from transduction mechanisms to cotargeting therapy. Ann NY Acad Sci. 2011;1220:60–70. [DOI] [PubMed] [Google Scholar]
- 3. Kineman RD, Faught WJ, Frawley LS. The ontogenic and functional relationships between growth hormone- and prolactin-releasing cells during the development of the bovine pituitary. J Endocrinol. 1992;134:91–96. [DOI] [PubMed] [Google Scholar]
- 4. Kineman RD, Henricks DM, Faught WJ, Frawley LS. Fluctuations in the proportions of growth hormone- and prolactin-secreting cells during the bovine estrous cycle. Endocrinology. 1991;129:1221–1225. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. [DOI] [PubMed] [Google Scholar]
- 6. Albert PR, Neve KA, Bunzow JR, Civelli O. Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. J Biol Chem. 1990;265:2098–2104. [PubMed] [Google Scholar]
- 7. Lew AM, Yao H, Elsholtz HP. G(i)α2- and G(o)α-mediated signaling in the Pit-1-dependent inhibition of the prolactin gene promoter. Control of transcription by dopamine D2 receptors. J Biol Chem. 1994;269:12007–12013. [PubMed] [Google Scholar]
- 8. Liu JC, Baker RE, Sun C, Sundmark VC, Elsholtz HP. Activation of Go-coupled dopamine D2 receptors inhibits ERK1/ERK2 in pituitary cells. A key step in the transcriptional suppression of the prolactin gene. J Biol Chem. 2002;277:35819–35825. [DOI] [PubMed] [Google Scholar]
- 9. Banihashemi B, Albert PR. Dopamine-D2S receptor inhibition of calcium influx, adenylyl cyclase, and mitogen-activated protein kinase in pituitary cells: distinct Gα and Gβγ requirements. Mol Endocrinol. 2002;16:2393–2404. [DOI] [PubMed] [Google Scholar]
- 10. Minami S, Sarkar DK. Transforming growth factor-β1 inhibits prolactin secretion and lactotropic cell proliferation in the pituitary of oestrogen-treated Fischer 344 rats. Neurochem Int. 1997;30:499–506. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar DK, Chaturvedi K, Oomizu S, Boyadjieva NI, Chen CP. Dopamine, dopamine D2 receptor short isoform, transforming growth factor (TGF)-βI, and TGF-β type II receptor interact to inhibit the growth of pituitary lactotropes. Endocrinology. 2005;146:4179–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaturvedi K, Sarkar DK. Mediation of basic fibroblast growth factor-induced lactotropic cell proliferation by Src-Ras-mitogen-activated protein kinase p44/42 signaling. Endocrinology. 2005;146:1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hentges S, Boyadjieva N, Sarkar DK. Transforming growth factor-β3 stimulates lactotrope cell growth by increasing basic fibroblast growth factor from folliculo-stellate cells. Endocrinology. 2000;141:859–867. [DOI] [PubMed] [Google Scholar]
- 14. Hentges S, Sarkar DK. Transforming growth factor-β regulation of estradiol-induced prolactinomas. Front Neuroendocrinol. 2001;22:340–363. [DOI] [PubMed] [Google Scholar]
- 15. Sarkar DK. Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res. 2006;35:32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spada A, Mantovani G, Lania A. Pathogenesis of prolactinomas. Pituitary. 2005;8:7–15. [DOI] [PubMed] [Google Scholar]
- 17. Beshay VE, Beshay JE, Halvorson LM. Pituitary tumors: diagnosis, management, and implications for reproduction. Semin Reprod Med. 2007;25:388–401. [DOI] [PubMed] [Google Scholar]
- 18. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. [DOI] [PubMed] [Google Scholar]
- 19. Karreth FA, Tuveson DA. Modelling oncogenic Ras/Raf signalling in the mouse. Curr Opin Genet Dev. 2009;19:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. [DOI] [PubMed] [Google Scholar]
- 21. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pertuit M, Romano D, Zeiller C, Barlier A, Enjalbert A, Gerard C. The gsp oncogene disrupts Ras/ERK-dependent prolactin gene regulation in gsp inducible somatotroph cell line. Endocrinology. 2011;152:1234–1243. [DOI] [PubMed] [Google Scholar]
- 23. Wang YH, Maurer RA. A role for the mitogen-activated protein kinase in mediating the ability of thyrotropin-releasing hormone to stimulate the prolactin promoter. Mol Endocrinol. 1999;13:1094–1104. [DOI] [PubMed] [Google Scholar]
- 24. Romano D, Magalon K, Ciampini A, Talet C, Enjalbert A, Gerard C. Differential involvement of the Ras and Rap1 small GTPases in vasoactive intestinal and pituitary adenylyl cyclase activating polypeptides control of the prolactin gene. J Biol Chem. 2003;278:51386–51394. [DOI] [PubMed] [Google Scholar]
- 25. Oomizu S, Chaturvedi K, Sarkar DK. Folliculostellate cells determine the susceptibility of lactotropes to estradiol's mitogenic action. Endocrinology. 2004;145:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hubina E, Nanzer AM, Hanson MR, et al. Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur J Endocrinol. 2006;155:371–379. [DOI] [PubMed] [Google Scholar]
- 27. Schonbrunn A, Krasnoff M, Westendorf JM, Tashjian AH. Epidermal growth factor and thyrotropin-releasing hormone act similarly on a clonal pituitary cell strain. Modulation of hormone production and inhibition of cell proliferation. J Cell Biol. 1980;85:786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson LK, Baxter JD, Vlodavsky I, Gospodarowicz D. Epidermal growth factor and expression of specific genes: effects on cultured rat pituitary cells are dissociable from the mitogenic response. Proc Natl Acad Sci USA. 1980;77:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsdell JS. Transforming growth factor-α and -β are potent and effective inhibitors of GH4 pituitary tumor cell proliferation. Endocrinology. 1991;128:1981–1990. [DOI] [PubMed] [Google Scholar]
- 30. Ramsdell JS. Thyrotropin-releasing hormone inhibits GH4 pituitary cell proliferation by blocking entry into S phase. Endocrinology. 1990;126:472–479. [DOI] [PubMed] [Google Scholar]
- 31. Felix R, Meza U, Cota G. Induction of classical lactotropes by epidermal growth factor in rat pituitary cell cultures. Endocrinology. 1995;136:939–946. [DOI] [PubMed] [Google Scholar]
- 32. Jackson TA, Koterwas DM, Bradford AP. Differential regulation of cell growth and gene expression by FGF-2 and FGF-4 in pituitary lactotroph GH4 cells. Mol Cell Endocrinol. 2006;247:183–191. [DOI] [PubMed] [Google Scholar]
- 33. Nakagawa T, Mabry M, de Bustros A, Ihle JN, Nelkin BD, Baylin SB. Introduction of v-Ha-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc Natl Acad Sci USA. 1987;84:5923–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. [DOI] [PubMed] [Google Scholar]
- 35. McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. [DOI] [PubMed] [Google Scholar]
- 36. Yajima I, Kumasaka MY, Thang ND, et al. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant melanoma progression and therapy. Dermatol Res Pract. 2012;2012:354191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dworakowska D, Wlodek E, Leontiou CA, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer. 2009;16:1329–1338. [DOI] [PubMed] [Google Scholar]
- 38. Rubinfeld H, Shimon I. PI3K/Akt/mTOR and Raf/MEK/ERK signaling pathways perturbations in non-functioning pituitary adenomas. Endocrine. 2012;42:285–291. [DOI] [PubMed] [Google Scholar]
- 39. Lai J-F, Cheng H-Y, Cheng T-L, et al. Doxycycline- and tetracycline-regulated transcriptional silencer enhance the expression level and transactivating performance of rtTA. J Gene Med. 2004;6:1403–1413. [DOI] [PubMed] [Google Scholar]
- 40. Bradford AP, Conrad KE, Tran PH, Ostrowski MC, Gutierrez-Hartmann A. GHF-1/Pit-1 functions as a cell-specific integrator of Ras signaling by targeting the Ras pathway to a composite Ets-1/GHF-1 response element. J Biol Chem. 1996;271:24639–24648. [DOI] [PubMed] [Google Scholar]
- 41. Gutierrez-Hartmann A, Duval DL, Bradford AP. ETS transcription factors in endocrine systems. Trends Endocrinol Metab. 2007;18:150–158. [DOI] [PubMed] [Google Scholar]
- 42. Borrelli E, Sawchenko PE, Evans RM. Pituitary hyperplasia induced by ectopic expression of nerve growth factor. Proc Natl Acad Sci USA. 1992;89:2764–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roh M, Paterson AJ, Asa SL, Chin E, Kudlow JE. Stage-sensitive blockade of pituitary somatomammotrope development by targeted expression of a dominant negative epidermal growth factor receptor in transgenic mice. Mol Endocrinol. 2001;15:600–613. [DOI] [PubMed] [Google Scholar]
- 44. McAndrew J, Paterson AJ, Asa SL, McCarthy KJ, Kudlow JE. Targeting of transforming growth factor-α expression to pituitary lactotrophs in transgenic mice results in selective lactotroph proliferation and adenomas. Endocrinology. 1995;136:4479–4488. [DOI] [PubMed] [Google Scholar]
- 45. Ezzat S, Zheng L, Zhu X, Wu GE, Asa SL. Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis. J Clin Invest. 2002;109(1):69–78 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. [DOI] [PubMed] [Google Scholar]
- 47. Daikoku T, Hirota Y, Tranguch S, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwanaga K, Yang Y, Raso MG, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gire V, Marshall CJ, Wynford-Thomas D. Activation of mitogen-activated protein kinase is necessary but not sufficient for proliferation of human thyroid epithelial cells induced by mutant Ras. Oncogene. 1999;18:4819–4832. [DOI] [PubMed] [Google Scholar]
- 50. Gire V, Marshall C, Wynford-Thomas D. PI-3-kinase is an essential anti-apoptotic effector in the proliferative response of primary human epithelial cells to mutant RAS. Oncogene. 2000;19:2269–2276. [DOI] [PubMed] [Google Scholar]
- 51. Fan H-Y, Liu Z, Paquet M, et al. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69:6463–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fan H-Y, Richards JS. Minireview: physiological and pathological actions of RAS in the ovary. Mol Endocrinol. 2010;24:286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Radl D, De Mei C, Chen E, Lee H, Borrelli E. Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol Endocrinol. 2013;27:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. [DOI] [PubMed] [Google Scholar]
- 55. Bliss SP, Miller A, Navratil AM, et al. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fan H-Y, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fritsche-Guenther R, Witzel F, Sieber A, et al. Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol. 2011;7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sturm OE, Orton R, Grindlay J, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Sci Signal. 2010;3:ra90. [DOI] [PubMed] [Google Scholar]
- 60. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. [DOI] [PubMed] [Google Scholar]
- 61. Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. [DOI] [PubMed] [Google Scholar]
- 62. Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJG, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LβT2 gonadotropes. Endocrinology. 2010;151:4882–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Collins MA, Brisset J-C, Zhang Y, et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLoS One. 2012;7:e49707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Makrodouli E, Oikonomou E, Koc M, et al. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer. 2011;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.