Abstract
Placebo effects can act as powerful pain relievers. While the ethics of therapeutic placebo use is highly controversial, recent evidence suggests that medical providers frequently utilize placebo treatments, and patients may be open to these interventions under certain contexts. This investigation used a patient-centered approach to answer essential questions about placebo treatment acceptability. People with chronic musculoskeletal pain completed a placebo survey where they: 1) rated their knowledge of placebo and its efficacy for alleviating pain; 2) evaluated the acceptability of a placebo analgesic interventions across several unique medical contexts; and 3) responded to six different patient-physician treatment scenarios to assess the role of deception and placebo effectiveness on mood and provider trust. Results showed that participants had limited knowledge of placebo and it’s efficacy for alleviating pain. Placebo acceptability was highly dependent on the context of the intervention, as placebo treatments were considered acceptable when used as complementary/adjunct treatments and when no other established treatments were available. Also, an analgesic placebo response mitigated the negative consequences of deception by improving provider trust and decreasing negative mood. These findings suggest that patients may be rather pragmatic in their appraisals of placebo treatment acceptability and may consider a variety of treatments/contexts as permissible for managing their pain. This is the first study of its kind to quantify perceptions of placebo analgesia knowledge and efficacy among individuals with chronic pain, and to assess the role of different medical contexts in treatment acceptability.
1. INTRODUCTION
The effectiveness of most medical interventions is derived partially from contextual or “non-specific” factors – commonly referred to as placebo effects [12]. These effects have demonstrated remarkable potency for the alleviation of pain, and under certain circumstances, placebos have produced effect sizes indistinguishable from established medications [24,42], surgeries [30], and other analgesic treatments [25,40,43]. With clearly defined neurobiological [2,31] and psychological underpinnings [7,32,37], the placebo analgesic response is one of the most well-understood models of placebo [8,17,34].
Despite considerable advances in understanding placebo mechanisms and effects, debate persists regarding the acceptability of therapeutic placebo use [28]. Whereas the ethics of placebo-controlled/randomized-controlled trials (RCTs) has been well established [11,22,27,44], the placebo treatment debate continues to incite disagreement among healthcare providers, bioethicist, and researchers [4]. Interventional placebo use opponents tout a variety of arguments, including that placebo use would damage the provider-patient relationship and/or cause psychological distress [10]. These arguments are primarily driven by placebos association with deceptive means and presumed negative consequences of deceiving patients [29]. Medical associations rarely adopt policies/guidelines regarding clinical placebo use, although some organizations prohibit covert use [3]. However, healthcare providers frequently use placebo interventions, often unbeknownst to patients [13,14,18,26,39].
Recent research has challenged the claims of placebo use opponents. A survey among primary care patients revealed that most were amenable to placebos despite the use of deception [5]. Furthermore, amongst both a non-clinical sample and irritable bowl syndrome (IBS) patients, placebo use had no adverse effects on mood nor the strength of subsequent placebo responding, even after the use of placebo was disclosed to participants [6]. Additionally, an explicit, open-label placebo RCT for IBS patients produced large and clinically meaningful reductions in IBS symptom severity [19].
Our research group has extended this line of inquiry by examining attitudes towards placebo use in non-clinical populations. In one study, participants responded to various scenarios of patients receiving placebo treatments to alleviate pain [21]. These scenarios systematically varied the severity of the patient’s pain, the deceptiveness of the provider’s description of the treatment, and the intervention’s effectiveness. Results illustrated that, while participants viewed placebo interventions as deceptive, their perceptions of placebo acceptability were mitigated by beneficial treatment outcomes. These findings were supported in a subsequent survey exploring placebo analgesia knowledge and acceptability [20]. Finding illustrated that lay individuals were uncertain about placebo analgesia efficacy and harbored rudimentary conceptualizations of placebos.
The present investigation seeks to further the understanding of placebo analgesia acceptability through utilization of an established patient-centered survey methodology [20,21] in a chronic musculoskeletal pain syndrome sample. The aims of this study were: 1) to examine patients’ knowledge of placebo; 2) to explore the role of medical contextual factors in appraisals placebo acceptability; 3) and to understand the role of deception and treatment effectiveness in attitudes towards a placebo analgesic use. We hypothesize that, despite having limited knowledge of placebo, placebos will be deemed acceptable by chronic pain patients under certain circumstances. Additional, we hypothesize that improved pain outcomes would mitigate the negative consequences of deceptive placebo use.
2. METHODS
2.1. Subjects
Subjects were directed recruited from two university outpatient medical clinics as well as through flyers posted in the surrounding community. Participants comprised 57 adults with chronic musculoskeletal pain (females = 40 and 17 males; M age = 45.12, SD = 19.16). Study inclusion criteria were as follows: adults age 18 years or older; the ability to read English fluently; the presence of musculoskeletal chronic pain that has lasted at least three months; and internet access to complete the web-based study. Exclusion criteria included the diagnosis of cancer or any other non-musculoskeletal chronic pain etiology (e.g., neuropathic pain).
2.2. Procedure
The present study was reviewed and approved by the University of Florida Institutional Review Board. Informed consent was obtained electronically. Prior to commencing the study, participants were informed that study participation would involve assessing their attitudes towards and knowledge of novel treatments for pain, such as placebo, and would involve completing questionnaires about their pain and their thoughts about pain treatments. The stated goals of this line of research were to help develop better ways to manage chronic pain in the future. The online questionnaire took approximately 30 minutes to complete and responses were anonymous. Participants were provided the URL/web address for the study in addition to a unique login username and password. The web-based survey was comprised to three sections: 1) Placebo Knowledge; 2) Placebo Acceptability; and 3) Treatment Scenarios.
2.3. Measures
2.3.1. Placebo Knowledge, Conceptualization, and Efficacy
All survey outcome measures were rated using Visual Analogue Scale (VAS) ratings producing numerical values between 0 and 100. Anchors for individual VAS questions can be found in Tables 2 and Table 3. VAS ratings have demonstrated considerable reliability and validity for the measurement of pain and other subjective phenomenon [23,35].
Table 2.
Placebo analgesia knowledge, effectiveness, and acceptability
| Survey Questions | VAS Anchors (0 – 100) | Abbreviation | M (SD) |
|---|---|---|---|
| Placebo Conceptualization/Knowledge | |||
| Overall, is a placebo something “inert/incapable of producing an effect” or something “active/capable of producing an effect? | Completely inert; Completely active | Placebo Conceptualization | 19.93 (24.73) |
| How much knowledge do you have about placebo for pain relief? | No knowledge of placebo for pain relief; Most knowledge of placebo for pain relief imaginable | Placebo Knowledge | 37.19 (33.17) |
| Placebo Effectiveness | |||
| How effective would placebo be for your pain? | Completely ineffective; Completely effective | Placebo Effectiveness | 18.33 (18.82) |
| Placebo Acceptability | |||
| How acceptable would it be if your physician used a placebo treatment to reduce your pain without telling you? | Completely unacceptable; Completely acceptable | Deceptive Placebo Acceptability | 18.05 (23.79) |
| How acceptable would it be if your physician told you that he/she was going to use a placebo treatment to reduce your pain? | Completely unacceptable; Completely acceptable | Explicit Placebo Acceptability | 29.46 (33.01) |
| How acceptable would it be if your physician told you that he/she was going to use a placebo treatment that may enhance the effectiveness of your usual pain treatments? | Completely unacceptable; Completely acceptable | Treatment Enhancer Placebo Acceptability | 41.93 (34.36) |
| How acceptable would it be if your physician treated your pain with placebo when other established treatments were available? | Completely unacceptable; Completely acceptable | Placebo Acceptability when other EBTs are available | 20.28 (23.76) |
| How acceptable would it be if your physician treated pain with placebo when NO other established treatments were available? | Completely unacceptable; Completely acceptable | Placebo Acceptability when NO other EBTs are available | 48.21 (39.41) |
| How acceptable would it be if your physician used a placebo to see if your pain is “real”? | Completely unacceptable; Completely acceptable | Diagnostic Placebo Acceptability | 36.26 (36.81) |
Note: M, mean; SD, standard deviation.
Table 3.
Treatment scenarios dependent variables: deception, trust, and negative mood (VAS 0 – 100)
|
M (SD)
|
||||
|---|---|---|---|---|
| Survey Treatment Scenarios | Scenario Abbreviation | How deceptive would this be? | Rate your level of trust in this physician | Rate the level of negative mood you would experience |
| Imagine that you are seeing a physician to manage your pain. The doctor provides you with a prescription for your pain, and states that what you have been given has been shown to be a powerful pain reliever in some people. Two weeks after the treatment, you report that your condition has improved. You received a placebo. | Enhanced placebo instructions used and pain improves | 61.70 (32.83) | 40.25 (32.13) | 37.53 (29.39) |
| Imagine that you are seeing a physician to manage your pain. The doctor provides you with a prescription for your pain, and states that what you have been given has been shown to be a powerful pain reliever in some people. Two weeks after the treatment, you report that there is no change in your condition. You received a placebo. | Enhanced placebo instructions used and pain is unchanged | 61.42 (36.91) | 37.42 (33.54) | 55.56 (30.99) |
| Imagine that you are seeing a physician to manage your pain. The doctor provides you with a prescription for your pain, and states that what you have been given has been shown to be a powerful pain reliever in some people. Two weeks after the treatment, you report that your condition has gotten worse. You received a placebo. | Enhanced placebo instructions used and pain worsens | 71.09 (33.31) | 23.84 (24.25) | 72.14 (28.16) |
| Imagine that you are seeing a physician to manage your pain. The doctor tells you that you will randomly receive either a standard drug treatment or a placebo treatment for your pain. Two weeks after the treatment, you report that your condition has improved. You received a placebo. | Randomly assigned and pain improved | 24.98 (31.96) | 58.44 (32.44) | 23.79 (25.70) |
| Imagine that you are seeing a physician to manage your pain. The doctor tells you that you are going to randomly receive either a standard drug treatment or a placebo treatment for your pain. Two weeks after the treatment, you report that there is no change in your condition. You received a placebo. | Randomly assigned and pain is unchanged | 24.30 (31.96) | 59.86 (30.69) | 31.25 (26.94) |
| Imagine that you are seeing a physician to manage your pain. The doctor tells you that you will randomly receive either a standard drug treatment or a placebo treatment for your pain. Two weeks after the treatment, you report that your condition has gotten worse. You received a placebo. | Randomly assigned and pain worsens | 26.19 (26.19) | 54.70 (54.70) | 43.16 (33.76) |
Note: M, Mean; SD, Standard Deviation; Deception VAS anchors = “Not at all deceptive” – “Completely deceptive”; Trust VAS anchors = “No trust” – “Most trust imaginable”; Negative mood VAS anchors = “No negative mood” – “Most negative mood imaginable.”
The Placebo Knowledge section is a modified version of a previously published web-based survey [20]. Participants were asked to VAS ratings of the following: 1) perceived knowledge of placebo analgesia, 2) conceptualization of placebo, and 3) perceived effectiveness of placebo for reducing pain, and 4) placebo analgesia treatment acceptability.
Placebo knowledge was assessed using a VAS scale from “no knowledge” to “the most knowledge imaginable”; participants were asked to conceptualize placebo along a VAS continuum from something “completely inert” to “completely active.” The perceived effectiveness of placebo treatments for pain was rated from “completely ineffective” to “completely effective.”
2.3.2. Placebo Acceptability
Placebo analgesia treatment acceptability was assessed through VAS ratings of six questions: how acceptable would it be if a physician 1) overtly or 2) covertly administered a placebo treatment for pain; 3) how acceptable would it be if a physician used a placebo as a treatment enhancer or an adjunct treatment; is it acceptable for a medical provider to treat pain with placebo for a condition for which there are 4) other established treatments or 5) for a condition in which there are no other established treatments; and 6) is it acceptable for a medical provider to use a placebo to determine if a patient’s pain complaints are “real.” VAS anchors were “completely unacceptable” and “completely acceptable.”
2.3.3. Deception, Trust, and Negative Mood
The Treatment Scenarios represented a modified version of a previously published placebo survey [21]. The survey was comprised of six different hypothetical scenarios, each portraying a clinical encounter in which a patient sees a physician for pain management and subsequently receives a placebo. Our sample of chronic pain individuals was asked to review each hypothetical scenario and to respond as if they were the individual receiving the placebo intervention. After viewing each scenario, our participants responded through VAS ratings of the following: 1) deceptiveness of the hypothetical clinical encounter/placebo intervention, 2) their level of trust in the prescribing physician, and 3) the amount of negative mood they would experience if they had received the placebo treatment for their pain.
Two factors varied per scenario: 1) the healthcare provider’s description of the placebo intervention and 2) the outcome/effectiveness of the treatment. Two distinct treatment descriptions were intended to be experimental manipulations of deceptiveness: for the “high deception/enhanced placebo” scenarios, the hypothetical patient in the scenario was informed that they will receive “a treatment that has been shown to be a powerful analgesic in some people”; for the “low deception/random assignment” instructions, the patient in the scenario was informed that they will receive either a “standard drug treatment or a placebo treatment” to manage their pain. Although the “enhanced placebo” description was once proposed to be an ethically acceptable description of a placebo treatment, more recent evidence has shown that it is perceived as highly deceptive [21]. There were three levels for treatment effectiveness/outcome: upon completion of the treatment, our sample of chronic pain individuals read that the placebo intervention either worsened, had no effect, or improved the pain of the patient depicted in the scenario.
2.4. Statistical Analyses
2.4.1. Sample Size
Power analyses were performed to determine the number of subjects needed to detect sizable effects for deception, healthcare provider trust, negative mood, and placebo acceptability as primary outcomes [16]. Due to the various analyses conducted for the original publications of these surveys, several methods were used to estimate the necessary sample size. Based upon repeated measures analyses of variance (ANOVA) partial eta squared main effect sizes for deception (.69 and .09) trust in physician (.50 and .46), and negative mood (.43 and .60), and a negative mood interaction effect size (.08) [21], it was estimated that a sample size of 42 patients would be sufficient to detect effects at alpha level 0.05 and power 0.80. Based upon repeated measures ANOVA effects sizes for placebo acceptability contexts (.22, .09, .35 and .28) and one-way ANOVA acceptability subgroups Cohen’s d effect sizes (.74, .74 and .68) [20], it was estimated that a sample size of 21 patients would be sufficient to detect effects at alpha level 0.05 and power 0.80. The final sample size is based on the most conservative value needed to optimally power the study, plus 20 percent more patients to buffer for potential participant drop out. Thus, based upon previously published placebo acceptability studies, recruitment of 50 pain patients was deemed sufficient to adequately power the study. Seven additional participants completed the study after recruitment ended and were thus included in the final sample.
2.4.2. Survey Analyses
Analyses were performed using IBM SPSS version 20 (IBM Inc, Armok New York). Means and standard deviations were calculated for all VAS outcomes and frequencies were calculated for all categorical outcomes. A one-factor repeated measures ANOVA was conducted for the six placebo acceptability treatment contexts. Three 2 × 3 (treatment instructions x treatment outcome) repeated measures ANOVAs were conducted on deception, trust, and negative mood outcomes. Significant omnibus F tests were followed by simple contrasts or post hoc comparisons for all ANOVA analyses.
3. RESULTS
3.1. Sample Demographics
Sample demographic statistics can be found in Table 1.The ethnic/racial composition of the sample was as follows: 75.4% Caucasian; 7% African American/black; 7% Asian or Pacific Islander; 3.5% Indian; 3.5% Hispanic; and 3.5% “Other”. Average annual income for participants was $31,121 (SD = $41,081), with income ranging from $0 to $220,000. Over half the sample had either “some college education” (35.1%) or a college degree (21.1%). Most participants reported their relationship status as married (42.1%) or single (45.6%).
Table 1.
Participant demographics and chronic pain characteristics (n = 57)
| Demographics/Characteristic | M(SD) | Percentage |
|---|---|---|
| Age (years) | 45.12 (19.16) | - |
| Income ($ thousands) | 31 (41) | - |
| Sex | ||
| Female | - | 70% |
| Male | - | 30% |
| Ethnicity | ||
| Caucasian | - | 75.4% |
| African American/black | - | 7% |
| Indian | - | 3.5% |
| Hispanic | - | 3.5% |
| Asian or Pacific Islander | - | 7% |
| Other | - | 3.5% |
| Education | - | |
| No high School Education | - | 1.8% |
| High School Diploma/GED | - | 12.3% |
| Some College Education | - | 35.1% |
| College Degree | - | 21.1% |
| Some Graduate Education | - | 7.0% |
| Graduate School Degree | - | 22.8% |
| Marital Status | ||
| Single | - | 45.6% |
| Living With Partner | - | 7% |
| Married | - | 42.1% |
| Divorced | - | 3.5% |
| Separated | - | 1.8% |
| Current Pain Intensity (VAS 0 = “no pain”; 100 =“worst pain imaginable”) | 41.89 (25.84) | - |
| Usual Pain Intensity (VAS 0 = “no pain”; 100 =“worst pain imaginable”) | 51.18 (23.85) | - |
| Years experiencing chronic pain | 6.34 (7.34) | - |
| Participants who uses OTC meds for pain | - | 66.7% |
| Participants who use prescription meds for pain | - | 35.1% |
| Participants who use “commentary and alternative” treatments for pain | - | 36.8% |
| Current Pain Intensity (VAS 0 = “no pain”; 100 =“worst pain imaginable”) | 41.89 (25.84) | - |
| Diagnoses endorsed by participants | ||
| Low back pain | - | 63.2% |
| Fibromyalgia | - | 28.1% |
| Osteoarthritis | - | 19.3% |
| Irritable Bowel Disease | - | 15.8% |
| Other | - | 40.4% |
Note: VAS, Visual Analogue Scale; M, mean; SD, Standard Deviation, n, sample size; %, percentage; GED, General Educational Development Certificate of High School Equivalency.
Participants reported having chronic pain for an average of 6.34 years (SD = 7.34). The mean VAS rating of current pain intensity was 41.89 (SD = 25.84). Several pain diagnoses were endorsed, with many reporting multiple diagnoses: 63.2% endorsed low back pain (LBP), 22.8% endorsed Fibromyalgia, 19.3% endorsed osteoarthritis, and 15.8% endorsed IBS. Many individuals (40.4%) also endorsed additional diagnoses, such as “scoliosis,” “herniated disk,” “Temporomandibular Joint Disorder (TMJ),” and “unknown.”
3.2. Placebo Knowledge, Conceptualization, and Efficacy
All descriptive statistics for survey outcomes are presented in Tables 2 – 4. Descriptive statistics suggest that our sample of chronic pain patients primarily conceptualized placebos as “inert” (M = 19.93), endorsed low to moderate knowledge of placebos (M = 37.19), and perceived placebos as fairly ineffective for reducing pain (M =18.33) (Table 2).
Table 4.
Placebo acceptability one-factor ANOVA
| Main effect x Contrasts | P | ES (ηp2) |
|---|---|---|
| Acceptability Contexts | <.001** | .175 |
| Deceptive Placebo - Explicit Placebo | .039* | .074 |
| Deceptive Placebo < Treatment Enhancer | <.001** | .264 |
| Deceptive Placebo – When other EBTs are available | .514 | .008 |
| Deceptive Placebo < when NO other EBTs are available | <.001** | .388 |
| Deceptive Placebo < Diagnostic Placebo | <.001** | .223 |
| Explicit Placebo < Treatment Enhancer | .001* | .174 |
| Explicit Placebo – when other EBTs are available | .026* | .085 |
| Explicit Placebo < when NO other EBTs are available | .001* | .185 |
| Explicit Placebo – Diagnostic Placebo | .273 | .021 |
| Treatment Enhancer > When other EBTs are available | <.001** | .276 |
| Treatment Enhancer – when NO other EBTs are available | .182 | .032 |
| Treatment Enhancer – Diagnostic Placebo | .332 | .017 |
| When other EBTs are available < when NO other EBTs are available | <.001** | .407 |
| When other EBTs are available - Diagnostic Placebo | .002* | .160 |
| When NO other EBTs are available – Diagnostic Placebo | .036* | .076 |
Note: P, p-value; ES (ηp2), partial eta squared effect size;
Indicates significant difference (p < .05);
Indicates significant difference (p < .001).
3.3. Placebo Acceptability
Significant differences were found between the six placebo acceptability outcomes, with these contextual factors accounting for 17.5% for the variance in acceptability (Table 4). Ordered from lowest to highest acceptability, they were: 1) deceptive placebo use; 2) use when other established treatments were available; 3) explicit placebo use (i.e., informing patient prior to use); 4) diagnostic use for pain; 5) use as a treatment enhancer; and 6) acceptability when no other treatments were available. Placebo use when no other established treatments were available was significantly more acceptable than deceptive placebo (p < .001, ηp2 = .388), explicit placebo (p < .001, ηp2 = .185), and placebo when other established treatments were available (p < .001, ηp2 = .407). Similarly, placebo as an adjunct treatment/treatment enhancer was more acceptable than deceptive placebo (p < .001, ηp2 = .264), explicit placebo (p < .001, ηp2 = .174), and placebo used when other established treatments were available (p < .001, ηp2 = .276).
3.4. Deception, Trust, and Negative Mood
Deceptive/enhanced placebo instructions were rated as highly deceptive (p < .001, ηp2 = .544) (Table 5; Figure 1), with participants endorsing the most negative mood (p < .001, ηp2 = .421) (Table 5; Figure 3) and least trust in their health care provider (p < .001, ηp2 = .378) (Table 5; Figure 2) when responding to these treatment scenarios.
Table 5.
Deception, trust, and negative mood repeated measures (2 × 3) ANOVAs
| Factors/Contrast | F | P | ES |
|---|---|---|---|
| Deception | |||
| Instructions | F(1, 56) = 66.85 | <.001** | ηp2 = .544 |
| Enhanced placebo > RA | - | <.001** | ηp2 = .544 |
| Tx Outcome | F(2, 112) = 2.69 | .072 | ηp2 = .046 |
| Instruction x Tx Outcome | F(2, 122) = 1.36 | .260 | ηp2 = .024 |
| Trust in Healthcare Provider | |||
| Instructions | F(1, 56) = 33.97 | <.001** | ηp2 = .378 |
| RA > Enhanced placebo | - | <.001** | ηp2 = .378 |
| Tx Outcome | F(2, 112) = 7.86 | .001* | ηp2 = .123 |
| Improves > Worsens | - | .001* | ηp2 = .180 |
| No Change > Worsens | - | <.001** | ηp2 = .204 |
| Improves – No Change | - | .823 | ηp2 = .001 |
| Instructions x Tx Outcome | F(1.71, 95.81) = 2.93 | .066 | ηp2 = .050 |
| Negative Mood | |||
| Instructions | F(1, 56) = 40.80 | <.001** | ηp2 = .421 |
| Enhanced placebo > RA | - | <.001** | ηp2 = .421 |
| Tx Outcome | F(2, 112) = 42.08 | <.001** | ηp2 = .429 |
| Worsens > Improves | - | <.001** | ηp2 = .546 |
| Worsens > No Change | - | <.001** | ηp2 = .324 |
| No Change > Improves | - | <.001** | ηp2 = .275 |
| Instructions x Tx Outcome | F(2, 112) = 3.27 | .042 | ηp2 = .055 |
| Enhanced Placebo | |||
| Improves < No Change | - | <.001** | d= .602 |
| Improves < Worsens | - | <.001** | d= 1.213 |
| No Change < Worsens | - | .001* | d= .564 |
| Random Assignment | |||
| Improves – No Change | - | .190 | d= .286 |
| Improves < Worsens | - | <.001** | d= .651 |
| No Change < Worsens | - | .014* | d= .393 |
| Pain Improves | |||
| Enhanced Placebo > RA | - | .006* | d= .502 |
| No Change in Pain | |||
| Enhanced Placebo > RA | - | <.001** | d= .845 |
| Pain Worsens | |||
| Enhanced Placebo > RA | - | <.001** | d= .629 |
Note: RA, Random assignment instructions; F, F statistics; P, p value; ηp2, partial eta squared effects size; d = Cohen’s d effect size;
Indicates significant deviation (p < .05);
Indicates significant deviation (p < .001).
Figure 1.
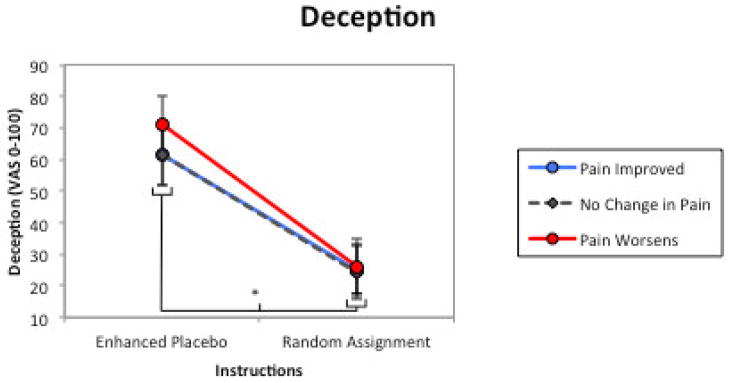
Deception. The influence of treatment descriptions/instructions and treatment outcomes on appraisal of treatment deceptiveness in six hypothetical placebo intervention scenarios. There was a significant main effect of treatment outcome but not treatment instructions. * = significant main effect (p < .05); error bars are 95% confidence intervals.
Figure 3.
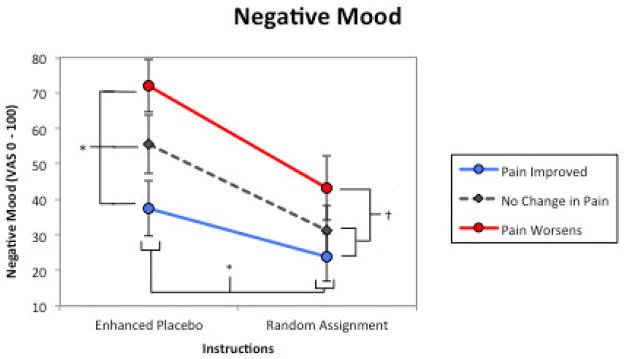
Negative Mood. The influence of treatment descriptions/instructions and treatment outcomes on participants’ negative mood in six hypothetical placebo intervention scenarios. There was significant main effect for treatment instructions and treatment outcomes, and a significant instructions x outcome interaction. * = significant main effect (p < .05); † = significant main interaction (p < .05) error bars are 95% confidence intervals.
Figure 2.
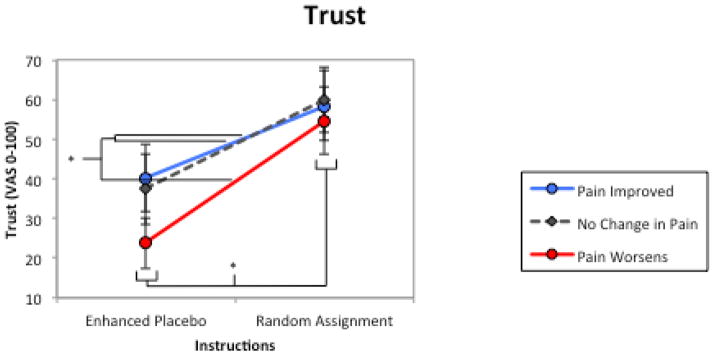
Trust. The influence of treatment descriptions/instructions and treatment outcomes on participants’ trust in their healthcare provider in six hypothetical placebo intervention scenarios. There was significant main effect for treatment instructions and treatment outcomes. * = significant main effect (p < .05); error bars are 95% confidence intervals.
While treatment effectiveness/outcome (i.e., whether pain improves, remains the same, or worsens) had no impact on perceptions of placebo deceptiveness (p = .072), treatment outcome had a significant effect on provider attributions (Table 5) as an analgesic treatment response improved trust in the provider (p < .001, ηp2 = .123) and greatly reduced negative mood (p < .001, ηp2 = .396).
There was a significant outcome x instruction interaction (Table 4; Figure 3). In scenarios depicting random assignment to placebo treatment, participants reported no difference in negative mood between pain improved and no change outcomes. However, when viewing scenarios where deceptive instructions were given, participants reported greater negative mood when pain status was unchanged than when it improved. Additionally, in scenarios where patients received a beneficial treatment response, mood ratings were the same whether they received a deceptive or non-deceptive treatment description.
4. DISCUSSION
Substantial evidence highlights the utility of placebo analgesic effects - real, potentially potent events mediated by psychological and neurobiological factors - for reducing pain [12]. Although the mechanisms underlying these effects are acknowledged among those who study placebo factors, it is unlikely that this knowledge has disseminated to the lay public [20]. To date, relatively few studies have addressed the patients’ perspectives regarding interventional placebo use. The overarching goal of this line of scientific inquiry was to use an empirical, patient-centered approach to answer essential questions about placebo treatment acceptability. The aims of the present study were to investigate chronic pain patients’ knowledge of and attitudes towards the clinical use of placebos using a validated survey methodology. Specifically, we sought to explore the role of deception and treatment efficacy on placebo treatment attributions, as well as examine the role of contextual factors in determining placebo acceptability. Our results illustrated that knowledge of placebos and their efficacy for reducing pain was relatively low among those suffering from chronic musculoskeletal pain. However, individuals with chronic pain were rather pragmatic in their appraisals of placebo acceptability, as receiving a placebo analgesic response attenuated negative mood and improved trust in the prescribing provider. Additionally, the context within which the placebo was used played an important role in influencing the acceptability of a placebo intervention for pain. These findings mirror the results of previous investigations of placebo acceptability in pain-free individuals.
4.1. Deception
It was hypothesized that placebo interventions that were non-deceptive and effective for relieving pain would be perceived as less deceptive, and individuals with chronic pain would endorse decreased negative mood and greater trust in the prescribing clinician. Comparable to findings observed in studies with non-clinical samples [21], the deceptiveness of a placebo treatment was exclusively determined by the overtness of the treatment instructions, accounting for over half the variance in treatment deceptiveness (ηp2 = .544). The enhanced placebo instructions, once proposed as an ethically acceptable description of a placebo treatment [33,41,42], were rated as highly deceptive, a finding coinciding with recent evidence [21]. Conversely, “random assignment” descriptions – where there was an explicit possibility of receiving a placebo intervention – were rated as relatively non-deceptive. Although in our previous survey research there was a marginal effect of treatment outcome on deception, in this study a beneficial treatment response had no impact on perceptions of treatment deception (p = .072, ηp2 = .046). These findings suggest that the description of the placebo intervention is important to patients, and unless it is explicitly stated, they may feel misled, even if the treatment works.
4.2. Trust and Negative Mood
Patient negative mood and trust in the prescribing clinician were both influenced by treatment deception/description and treatment outcome, supporting our hypotheses, with trust more influenced by the treatment deception/description (ηp2 = .378) than its efficacy (ηp2 = .123). Specifically, trust ratings were predominantly determined by the non-deceptive description and pain worsening status; there were no differences between no change and pain improved treatment outcomes. On the other hand, negative mood was strongly and approximately equally influenced by treatment instructions (ηp2 = .421) and outcome (ηp2 = .429), with graded increases in negative mood observed when participants received the “enhanced placebo” instructions and as intervention outcomes worsened. The interaction of these two factors suggest that patients would be much more upset if their pain remained unchanged following a deceptive placebo treatment, as opposed to when randomly assigned to treatment. Overall, theses findings suggest that patients are rather practical when making appraisals of placebo treatments – put simply, as long as it alleviates pain, then the intervention is tolerable, even if the treatment description is misleading.
4.3. Placebo Acceptability
Consistent with findings from previous studies, the acceptability of a placebo treatment for pain was highly dependent on the context in which it is delivered [20], with contextual factors accounted for nearly 18% of the variability in placebo acceptability. Deceptive placebo administration and placebo use when other established treatments were available were viewed as most unacceptable treatment scenarios. In contrast, placebos were most acceptable when there were no other well-established treatments available and when placebo was used as a treatment enhancer/adjunct. It was unexpected that placebo use to “determine if a patients’ pain is real” was considered as relatively acceptable, as diagnostic placebo use is one area in which informed researchers and clinicians can reasonably agree constitutes an unethical and unacceptable practice [38]. Another unexpected finding was that overt, yet non-deceptive use of placebo was perceived as relatively unacceptable by our sample. We believe these finding serve as corroborative evidence that, although placebo use may be relatively acceptable under certain circumstances, lay knowledge of placebo mechanisms is likely rudimentary and largely uninformed by extant empirical evidence.
4.4. Implications
More research is greatly needed to determine how to best use our understanding of placebo mechanisms to augment existing pain management practices. While the focus of the present investigation was the application of placebos in pain management, it is important to note that there are other ways to utilize the benefits of placebo mechanisms without actual placebos through enhancing provider-patient communication [9]. However, we feel that these results have important implications for our understanding of placebo acceptability, particularly in light of how frequently placebos treatments are used by healthcare providers today [15]. The findings suggest that, independent of effect sizes, individuals suffering from pain conditions bereft of well-established treatments may find placebo as a satisfactory alternative treatment. Also, placebo interventions were most acceptable when used to complement established pain treatments to improve overall therapeutic efficacy. This is a promising finding, as it implies that “dose extender” placebo use may be an acceptable application for treating pain. Although dose extending models have not been demonstrated for the treatment of pain, placebos have been used in this manner to treat both psoriasis [1] and attention deficit disorder [36]. It is also very important to consider the findings within the context that our sample’s understanding of placebo. Subjects endorsed relatively low knowledge of placebo effects and found them to have marginal efficacy for reducing pain. Research is greatly needed to determine what role education may play in placebo treatment acceptability. Specifically, we would hypothesize that an education intervention addressing the neurobiological mechanisms (e.g., activation in pain reducing areas of the brain, release of endogenous opioids and cannabinoids) and psychological mechanisms (e.g., expectancy, classical conditioning, reduced negative emotions, observational learning) underlying placebos would greatly enhance perceptions of placebo acceptably.
4.5. Limitations
Several limitations to this study should be considered when interpreting the findings. These findings are from a chronic musculoskeletal pain population and may not generalize to individuals suffering from acute pain or chronic pain from a different etiology. Additionally, individuals without knowledge of or access to the Internet (e.g. some elderly adults, those with limited financial resources) could not participate in this study, perhaps limiting the generalizability of the findings. While deception was successfully manipulated through two distinct treatment instructions, the “random assignment” instructions may not be representative of typical clinical encounter. Although random treatment assignment is common in the clinical trial setting, this is largely non-existent in typical medical encounters. Future iterations studies should substitute random assignment instructions with instructions that are equally non-deceptive and clinically applicable. More research is necessary to elucidate the magnitude and duration of placebo analgesic effects before they can be systemically applied in clinical practice. Finally, the authors acknowledge that it may be difficult to interpret the responses of participants who reported having absolutely no knowledge of placebo (i.e. zero on a 0–100 VAS). However, this only occurred for three participants, and since all participants were informed in study advertisements, during screening, and through informed consent that they would have to answer questions regarding placebo, it is assumed that they had some base knowledge. We elected to keep these participants in the final analyses since their exclusion still produced identical results.
4.6. Conclusions
The present investigation represents a meaningful addition to the placebo acceptability literature. Using an established survey methodology, we were able to assess the important role of medical treatment context in determining placebo acceptability, as well as examine the importance of treatment effectiveness and deception on mood and relationship with healthcare providers. Patients’ knowledge of placebo effects was limited, and more research is needed to investigate the role of education in placebo acceptability. We believe that this line of research will contribute to a fruitful discussion of placebo ethics, and will extend knowledge of placebo benefits to chronic pain patients and clinicians with the goal of improving existing pain management practices.
Summary.
Patients with chronic musculoskeletal pain have a limited understanding of placebo mechanisms, but may consider placebos an acceptable treatment for pain under certain medical contexts.
Acknowledgments
This manuscript was supported by Grant 5R01AT001424-06 to (M.R.) from the National Center for Complementary and Alternative Medicine (NCCAM) of the NIH. The authors thank Shankar Manamalkav for his technical assistance in creating the web-based survey.
Footnotes
The authors have no conflicts of interests.
Author contributions:
N.K. designed the study, collected, analyzed and interpreted the data, and wrote the paper. M.R. supervised every stage of the study, assisted with study design, and provided paper edits. R.H. and R.S. assisted with participant recruitment and paper edits/comments. All authors have approved the final paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, Kimball AB, Fiorentino D. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosomatic Medicine. 2010;72(2):192–197. doi: 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedetti F, Durando J, Vighetti S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. PAIN®. 2014;155(5):921–928. doi: 10.1016/j.pain.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Bostick N, Sade R, Levine M, Stewart D. Placebo use in clinical practice: report of the American Medical Association Council on Ethical and Judicial Affairs. Journal of Clinical Ethics. 2008;19(1):58–61. [PubMed] [Google Scholar]
- 4.Cahana A, Romagnioli S. Not all placebos are the same: a debate on the ethics of placebo use in clinical trials versus clinical practice. Journal of Anesthesia. 2007;21(1):102–105. doi: 10.1007/s00540-006-0440-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen G-F, Johnson M. Patients’ attitudes to the use of placebos: results from a New Zealand survey. The New Zealand Medical Journal. 2009;122(1296):11. [PubMed] [Google Scholar]
- 6.Chung SK, Price DD, Verne GN, Robinson ME. Revelation of a personal placebo response: Its effects on mood, attitudes and future placebo responding. Pain. 2007;132(3):281–288. doi: 10.1016/j.pain.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1–2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: Psychological and neurobiological mechanisms. Pain. 2013;154(4):511–514. doi: 10.1016/j.pain.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1572):1922–1930. doi: 10.1098/rstb.2010.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cupples B, Myron G. The investigator’s duty not to deceive. IRB: Ethics and Human Research. 1985;7(5):1–6. [PubMed] [Google Scholar]
- 11.Emanuel EJ, Miller FG. The ethics of placebo-controlled trials - a middle ground. N Engl J Med. 2001;345(12):915–919. doi: 10.1056/NEJM200109203451211. [DOI] [PubMed] [Google Scholar]
- 12.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 13.Fassler M, Gnadinger M, Rosemann T, Biller-Andorno N. Use of placebo interventions among Swiss primary care providers. BMC Health Services Research. 2009;9(1):144. doi: 10.1186/1472-6963-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassler M, Gnadinger M, Rosemann T, Biller-Andorno N. Placebo interventions in practice: a questionnaire survey on the attitudes of patients and physicians. Br J Gen Pract. 2011;61(583):101–107. doi: 10.3399/bjgp11X556209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassler M, Meissner K, Schneider A, Linde K. Frequency and circumstances of placebo use in clinical practice - a systematic review of empirical studies. BMC Medicine. 2010;8(1):15. doi: 10.1186/1741-7015-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 17.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. The Lancet. 2010;375(9715):686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hrobjartsson A, Norup M. The use of placebo interventions in medical practice - a national questionnaire survey of danish clinicians. Eval Health Prof. 2003;26(2):153–165. doi: 10.1177/0163278703026002002. [DOI] [PubMed] [Google Scholar]
- 19.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kisaalita NR, Robinson ME. Analgesic placebo treatment perceptions: acceptability, efficacy, and knowledge. The Journal of Pain. 2012;13(9):891–900. doi: 10.1016/j.jpain.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisaalita NR, Roditi D, Robinson ME. Factors affecting placebo acceptability: deception, outcome, and disease severity. The Journal of Pain. 2011;12(8):920–928. doi: 10.1016/j.jpain.2011.02.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovach K. Distinguishing dilemmas in the ethics of placebo-controlled trials. The American Journal of Bioethics. 2002;2(2):2. doi: 10.1162/152651602317533659. [DOI] [PubMed] [Google Scholar]
- 23.Lara-Muñoz C, de Leon SP, Feinstein AR, Puente A, Wells CK. Comparison of Three Rating Scales for Measuring Subjective Phenomena in Clinical Research: I. Use of Experimentally Controlled Auditory Stimuli Archives of Medical Research. 2004;35(1):43–48. doi: 10.1016/j.arcmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Levine JD, Gordon NC, Smith R, Fields HL. Analgesic responses to morphine and placebo in individuals with postoperative pain. Pain. 1981;10(3):379–389. doi: 10.1016/0304-3959(81)90099-3. [DOI] [PubMed] [Google Scholar]
- 25.Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B, Willich SN, Melchart D. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128(3):264–271. doi: 10.1016/j.pain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Meissner K, Höfner L, Fässler M, Linde K. Widespread use of pure and impure placebo interventions by GPs in Germany. Family Practice. 2012;29(1):79–85. doi: 10.1093/fampra/cmr045. [DOI] [PubMed] [Google Scholar]
- 27.Miller FG. The ethics of placebo-controlled trials. In: Emanuel EJ, Grady C, Crouch RA, Lie R, Miller FG, Wendler D, editors. The oxford textbook of clinical research ethics. New York: Oxford University Press; 2011. pp. 261–272. [Google Scholar]
- 28.Miller FG, Colloca L. The legitimacy of placebo treatments in clinical practice: evidence and ethics. American Journal of Bioethics. 2009;9(12):39–47. doi: 10.1080/15265160903316263. [DOI] [PubMed] [Google Scholar]
- 29.Miller FG, Wendler D, Swartzman LC. Deception in research on the placebo effect. PLoS Medicine. 2005;2(9):853–858. doi: 10.1371/journal.pmed.0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. New England Journal of Medicine. 2002;347(2):81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 31.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 32.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. PAIN. 2001;93(1):77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 33.Price DD, Craggs J, Nicholas Verne G, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127(1–2):63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thoughts. Annual Review of Psychology. 2008;59:2.1–2.26. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 35.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. PAIN. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 36.Sandler A, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? Journal of Developmental & Behavioral Pediatrics. 2010;31(5):369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological Bulletin. 2004;130(2):324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan M, Terman GW, Peck B, Correll DJ, Rich B, Clark WC, Latta K, Lebovits A, Gebhart G. APS position statement on the use of placebos in pain management. The Journal of Pain. 2005;6(4):215–217. doi: 10.1016/j.jpain.2005.01.347. [DOI] [PubMed] [Google Scholar]
- 39.Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing placebo treatments: results of national survey of US internists and rheumatologists. BMJ. 2008:337. doi: 10.1136/bmj.a1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Tulder MW, Furlan AD, Gagnier JJ. Complementary and alternative therapies for low back pain. Best Practice & Research Clinical Rheumatology. 2005;19(4):639–654. doi: 10.1016/j.berh.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: An empirical investigation. Pain. 2003;105(1–2):17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 42.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115(3):338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 43.White P, Bishop FL, Prescott P, Scott C, Little P, Lewith G. Practice, practitioner, or placebo? A multifactorial, mixed-methods randomized controlled trial of acupuncture. Pain. 2012;153(2):455–462. doi: 10.1016/j.pain.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 44.World Medical A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]


