Abstract
The projection from retina to the superior colliculus in mice is organized in a retinotopic map that develops through the formation and guidance of interstitial branches extended by retinal ganglion cell axons. Bidirectional branch guidance along the lateral-medial collicular axis is critical to mapping the dorsal-ventral retinal axis. EphB receptor tyrosine kinases expressed in an overall low to high dorsal-ventral retinal gradient have been implicated in this mapping in response to the graded low to high lateral-medial expression of a ligand, ephrin-B1, in the superior colliculus. However, the relative contributions of EphBs and ephrin-B1 are not well understood. We examined EphB1, EphB2, and EphB3 mutant mice and find that each has ectopic arborizations of retinal axon branches lateral to their appropriate termination zone, with no qualitative differences in aberrant mapping, suggesting a similar role for each EphB. However, the frequency of cases with map defects progressively rises in compound EphB mutants coincident with the number of EphB null alleles from one to five of the six total alleles indicating that EphB level is critical. We analyzed branch extension in vitro and find that dorsal branches, with low EphB levels, exhibit a negative response to ephrin-B1, whereas ventral branches, with high EphB levels, exhibit a positive response to ephrin-B1. Using EphB mutant retina, we show that both of these differential branch extension responses are dependent on EphB level. Our findings show a bifunctional action of ephrin-B1 regulated by EphB levels that can account for the bidirectional extension of interstitial branches required to establish a retinotopic map.
INTRODUCTION
The projection of retinal ganglion cells (RGCs) to the superior colliculus (SC) is the predominant system for studying mechanisms of topographic map development. The dorsal-ventral (DV) axis of the retina maps along the lateral-medial (LM) SC axis. However, the initial projection of RGC axons is diffuse and has only a coarse topographic order within the SC (Simon and O’Leary, 1992a). RGC axons extend far posterior to the location of their future termination zone (TZ), and axons originating from neighboring RGCs are dispersed across the entire LM axis of the SC, although biased for the LM position of their future TZ (Figure 1A). However, most axons are located either medial or lateral to their future TZ and connect to it through branches that form interstitially along the axon shaft (Simon and O’Leary, 1992b, 1992c). Interstitial branches are directed either medially or laterally along the LM axis to their correct TZ depending upon the initial LM position of their parent RGC axons in the SC. Thus, the DV retinotopic map is established by the bidirectional guidance of interstitial branches along the LM axis to the topographically appropriate SC region of their TZ.
Figure 1. Retinocollicular map development and the roles of EphBs.
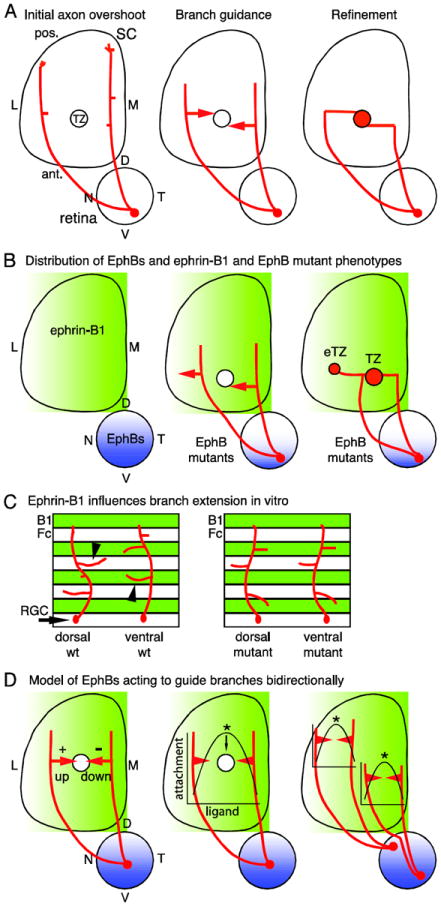
(A) Retinal ganglion cell (RGC) axons enter the superior colliculus (SC) at its anterior (ant.) border and overshoot the future termination zone (TZ) into posterior (pos.) SC (left). Axons then form branches which extend along the medial (M) - lateral (L) SC axis in a guided manner towards the TZ (middle). Branches that reach the nascent TZ arborize and contribute to a dense array of connections (right). D, dorsal; N, nasal; T, temporal; V, ventral. (B) EphB1, EphB2, EphB3, and EphB4 are distributed in RGCs in an overall high ventral - low dorsal gradient (blue) and ephrin-B1 is distributed in a high-M-to-low-L gradient (green) in the SC during retinocollicular map development. In EphB mutant mice, RGC axons from VT retina form a TZ in the appropriate location, but in addition form an ectopic TZ (eTZ) laterally (center). (C) In vitro, dorsal retinal axons prefer to extend branches on control lanes (Fc), avoiding ephrin-B1. Ventral retinal axons prefer to extend branches on ephrin-B1 lanes (B1). Both preferences are lost in EphB mutants. (D) A potential model for the roles of EphBs is informed by in vitro studies (Huynh-do et al., 1999). RGCs have a different point on the ephrin-B1 gradient at which adherence is maximal, dependent on their EphB level. Thus, distinct branch responses to the ephrin-B1 gradient are based not only on the EphB level of the parent RGC, but also the position of the branch on the gradient.
Several molecules have been implicated in DV retinotopic mapping, most prominently the EphBs and ephrin-Bs (Feldheim and O’Leary, 2010). Ephrin-B1 is expressed in a low-to-high LM gradient across the SC (Hindges et al., 2002), whereas EphB1, EphB2, and EphB3 are expressed in an overall low-to-high DV gradient by RGCs (Hindges et al. 2002; Thakar et al. 2011). Two studies (Hindges et al., 2002; Thakar et al., 2011) have reported that EphB1/EphB2 and EphB2/EphB3 double mutants, as well as each individual mutant of EphB1, EphB2, and EphB3, have DV mapping defects, demonstrating a role for EphB forward signaling for each receptor (Figure 1B). Significantly, each of these EphB mutants has lateral, ectopic TZs for central and ventral RGCs, attributed to a defect in LM branch guidance (Hindges et al., 2002).
Here we first examined EphB null allelic combinations more in depth and find that every allelic combination has similar DV mapping defects, but the frequency of cases with defects rises with the number of null EphB alleles. Thus, the overall level of EphBs is a critical factor in DV mapping rather than distinct functional contributions from each EphB type. To test the hypothesis that the differential guidance of interstitial branches is due to their bifunctional responses to ephrin-B1 mediated by EphB level, we have used the protein stripe assay to examine branch response to ephrin-B1. We show in this assay that dorsal and ventral RGCs exhibit differential branch extension and, by using retina from EphB mutants, that the differential response to ephrin-B1 by branches extended from retinal axons is dependent on EphB level. Further, these responses are EphB-dependent and, importantly, recapitulate the responses observed in vivo for RGC axons in wild type and EphB mutant mice. Our findings provide a mechanism consistent with a bifunctional action of ephrin-B1 regulated by EphB levels that can account for the bidirectional extension of interstitial branches from RGC axons required to establish a DV retinotopic map.
RESULTS
EphB1 is required for DV retinocollicular mapping
We first analyzed EphB1 mutant mice to determine the characteristics and frequency of retinocollicular map defects in mice homozygous or heterozygous for an EphB1 null allele, providing baseline data for our analysis of the contributions of EphB null alleles to DV mapping. Focal injections of the axon tracer, DiI, were made near the middle of the DV axis of peripheral temporal retina to label RGC axonal projections in the contralateral SC. Mice were injected at P7 and analyzed at P8, when in wild type mice (WT) the projection is topographically mature. Such a focal injection densely labels a single TZ in anterior SC at the appropriate LM location and never labels arborizations outside the immediate area of the TZ (Figure 2). In EphB1+/- mice, a comparable DiI injection results in a normal appearing TZ in the appropriate location in the SC, but in addition ectopic TZs (eTZs) are labeled lateral to the appropriate TZ in 39% of EphB1+/- mice (n=39 mice, p<0.03 Fisher’s exact test; Figure 2B). EphB1-/- mice have qualitatively similar defects, with all eTZs found lateral to the appropriate TZ, but at a higher frequency than EphB1+/- mice (48% with eTZs, n=23 mice, p<0.02; Figure 2C). Injections of DiI into dorsal retina in EphB1-/- mice reveal no aberrancies in retinocollicular mapping in a limited number of cases (data not shown; n=4).
Figure 2. EphB1 is required for appropriate retinocollicular mapping.
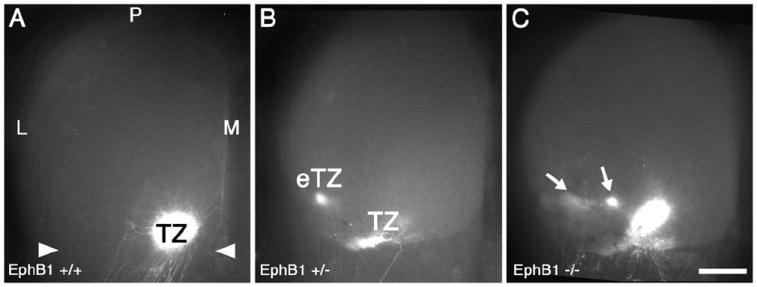
(A) Dorsal view of the superior colliculus (SC) of a WT mouse at P8, one day after injection of DiI into temporal retina. A dense, focal termination zone (TZ) in the appropriate location in anterior (arrowheads) SC is evident. (B and C) The SC of EphB1+/- and EphB1-/- mice one day after an injection of DiI similar to that in (A). (B) In 39% of EphB1+/- cases, ectopic termination zones (eTZs) of temporal RGC axons are evident lateral (L) to a normal appearing TZ. (C) Similarly, in EphB1-/- mice, 48% of cases have an eTZ positioned laterally. Scale bar=500μm. M, medial; P, posterior.
EphB1, EphB2, and EphB3 null alleles produce similar aberrancies in DV retinocollicular maps with frequency of aberrant maps correlated to number of null alleles
To investigate the relative contribution of EphB1, EphB2, and EphB3 to DV retinotopic mapping, we analyzed retinocollicular maps in mice with combinations of null alleles for each receptor. We examined 18 of the 27 possible allelic combinations for triple mutants, with at least 9 cases examined for ten of the combinations, and at least 3 cases for an additional eight combinations (182 total mice; Figure 3D). We have not been able to generate viable triple homozygous mutants, although Thakar et al. (2011) have reported them.
Figure 3. Number of EphB1, EphB2, and EphB3 null alleles corresponds to frequency, but not severity, of aberrant mapping phenotype.
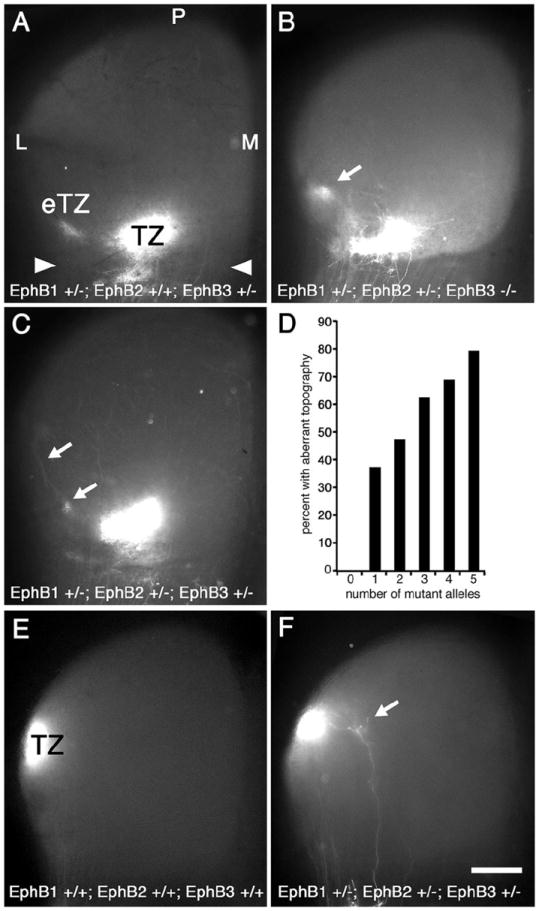
Dorsal view of the superior colliculus (SC; anterior SC marked by arrowheads) of P8 mice injected with DiI in temporal retina one day earlier. Mice deficient for one or more alleles of EphB1, EphB2, and/or EphB3 have retinotopic defects. (A) EphB1+/-; EphB2+/+; EphB3+/- mice have an appropriately positioned termination zone (TZ) as well as an ectopic TZ (eTZ, arrows) lateral (L) to the main TZ. Qualitatively similar defects are observed in (B) EphB1+/-; EphB2+/-; EphB3-/- mice and in (C) EphB1+/-; EphB2+/-; EphB3-+/- mice. (D) Graph correlating the number of combined mutant EphB1, EphB2, and EphB3 alleles to the presence of an aberrant retinotopic map. Each additional missing allele increases the frequency of an aberrant map. For 0-5 missing EphB alleles, n=10, 48, 36, 32, 32, and 24, respectively (right, 182 total cases with temporal DiI injections). Fisher’s exact test indicates statistical significance at p<0.03 for one missing EphB allele, p<0.01 for two and three missing alleles, p<0.001 for four and five missing alleles. (E) SC from a wild type mouse after injection of DiI in dorsal retina showing a dense TZ in the appropriate position in lateral SC (n=6). (E) DiI injection in dorsal retina of an EphB1+/-; EphB2+/-; EphB3-+/- mouse reveals, in addition to a normal TZ, a small eTZ positioned medially (arrow) in some cases (n=7). Scale bar=500μm. M, medial; P, posterior.
In mice with one or more mutant alleles of EphB1, EphB2, or EphB3, and in every combination of EphB1, EphB2, and EphB3 null alleles examined in sufficient numbers, including null mutants for each EphB, we find an aberrant topographic map in a subset of cases with one or more eTZs present lateral to a normal appearing TZ at the topographically appropriate site (Figure 3). Axon branches are found to extend along the LM axis from the appropriate TZ to the eTZs, often ending with clearly identified arborizations forming an eTZ (Figure 3C). We find no substantial qualitative difference in phenotype and the overall severity of the phenotype and the number of eTZs does not correlate with the number of null alleles nor with a null allele for any specific EphB receptor. For example, EphB1+/-; EphB2+/+; EphB3+/- mice have defects similar to EphB1+/-; EphB2+/-; EphB3-/- mice (Figure 3B) and to EphB1+/-; EphB2+/-; EphB3-+/- mice (Figure 3C). However, the total number of null alleles for EphB1, EphB2, and EphB3 does correlate strongly with the frequency of cases with eTZs in the SC (Figure 3D). All 18 allelic combinations examined have mapping aberrancies, ranging from 33% (3 of 9) aberrant cases for EphB1+/+; EphB2+/+; EphB3+/- mice with one null of six possible null alleles to a high of 86% (12 of 14) aberrant cases for EphB1-/-; EphB2+/-; EphB3-/- mice, with 5 null alleles. These findings indicate that each of the EphB receptors expressed by RGCs has similar functions in DV retinocollicular mapping and that the overall level of EphB expression determines the probability of developing a normal DV map.
The analyses described above focuses on RGC projections from the middle of the DV retinal axis within temporal retina. To investigate the potential for mapping errors in more dorsal retinal locations we performed a set of injections of DiI into peripheral dorsal retina near the NT midpoint in wild type, and EphB mutants. In wild type mice a dense TZ is present in mid-SC on the lateral periphery (Figure 3E; n=6). However, in one of seven cases with three mutant alleles (14%), in addition to the normal TZ in the appropriate position in lateral SC, we find a small arbor medial to the TZ (Figure 3F).
EphB-dependent responses of dorsal and ventral retinal axon branches to ephrin-B1 in vitro
We used the protein stripe assay to test the influence of ephrin-B1 on retinal axon branch extension and to determine whether dorsal and ventral retina exhibit distinct responses due to their differences in EphB levels (Figure 4). Cells dissociated from dorsal and ventral retina from WT and EphB compound mutant mice with 3 null alleles, either EphB1+/-; EphB2-/- or EphB1+/-; EphB3-/-, were plated at low density on alternating lanes of protein substrates containing ephrin-B1-Fc or as a control, human-Fc (i.e. control-Fc).
Figure 4. EphB-dependent bifunctional response to ephrin-B1 in vitro.
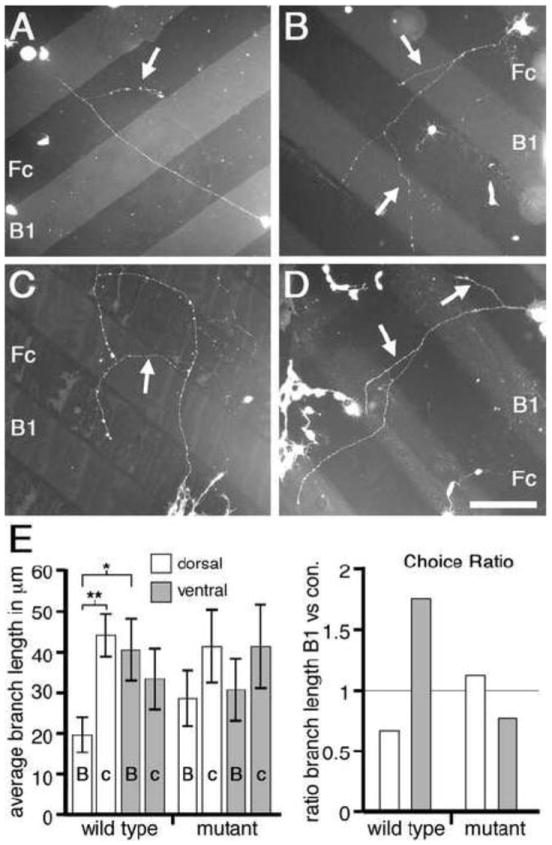
(A to D) Dissociated mouse retinal cells plated on alternating stripes of ephrin-B1-Fc (B1) and control human-Fc (Fc). Branches (arrows) form along axon shafts and extend. Cells from WT dorsal retina (A) form branches which preferentially extend upon Fc lanes; cells from WT ventral retina (B) form branches which preferentially extend upon B1 lanes. In contrast, cells from either dorsal (C) or ventral (D) EphB mutant retina extend branches which do not strongly prefer either lane. (E, left) Graph quantifies branch extension in absolute terms. Statistics details presented in Results. (E, right) Choice Ratio (C.R.) of lane preferences are normalized for initial axon and branch point position and show that WT lane choices are not present in EphB mutant retina. A C.R. less than one is a choice for control-Fc lanes, a C.R. greater than one is a choice for ephrin-B1 lanes. * p<0.03; ** p<0.001; Scale bar=150μm.
General growth parameters of retinal axons and branches in protein stripe assay
We first analyzed the general growth and branching characteristics of retinal cell axons in this assay and found them to be indistinguishable based on either genotype (WT or EphB mutant) or retinal origin (dorsal or ventral). Overall, retinal cells from each of these four categories have primary axon length averages ranging from 440μm to 510μm with the average number of branches in each category ranging from 3.2 to 4 per axon. The average branch length in each category is approximately 70μm. A small, statistically insignificant bias for the control-Fc lane was observed in primary axon growth and branch formation (see below). However, the similarity in the overall growth characteristics of both dorsal and ventral retina from EphB mutants compared to WT mice indicate that the growth of primary axons and their formation and extension of interstitial branches is not significantly affected in EphB-deficient retina nor by DV retinal position, and therefore the level of endogenous EphB.
WT dorsal and ventral retinal axons exhibit differential responses to ephrin-B1
Although the basic growth characteristics of retinal axons from dorsal and ventral WT or EphB compound mutant mice do not differ in this assay, we find that the extension of interstitial branches is significantly influenced by ephrin-B1 in an EphB-dependent manner. Within WT cultures, branches of dorsal retinal axons preferentially extend on control-Fc lanes (average branch length of 44+/-5μm), compared to lanes containing ephrin-B1-Fc (average branch length of 19+/-5μm; p<0.001, Student’s t-test; Figures 4A and 4E). In contrast, branches from WT ventral retinal axons show no preference for control-FC lanes, and in fact show a small bias for preferential extension on ephrin-B1-Fc lanes compared to control-Fc lanes (averages of 40+/-8μm and 33+/-8μm, respectively; p=0.37; Figures 4B and 4E). Thus, the behavior of ventral retinal axon branches is significantly different from the behavior of dorsal retinal axon branches on ephrin-B1-Fc lanes (p=0.023 for branch extension on ephrin-B1 lanes for dorsal WT retina versus ventral WT retina). These in vitro preferences reflect in vivo DV retinotopic mapping in which dorsal retina with low levels of EphB maps to the low end of the ephrin-B1 gradient in lateral SC and ventral retina with high levels of EphB maps to the high end of the ephrin-B1 gradient in medial SC.
Responses of EphB mutant dorsal and ventral retinal axons to ephrin-B1 in vitro
To test whether these differences between WT dorsal and ventral retinal axons in extension of interstitial branches is mediated by EphBs, we repeated the protein stripe assay but used dissociated cells from dorsal and ventral retina of EphB compound mutant mice. We find that dorsal axon branches from EphB mutant retina extend to a similar average length (41+/-10μm) on control-Fc lanes as those from WT retina (p=0.80), but extend 49% further (28+/-7μm) than WT dorsal axon branches on ephrin-B1-Fc lanes, eliminating the statistical significance in growth preference of WT dorsal retina for the control lane (p>0.1; Figures 4C and 4E). Ventral axon branches from EphB mutant retina also show a diminished preference for branch extension on ephrin-B1-Fc lanes compared to WT with the average branch extension on ephrin-B1-Fc lanes declining by 24% to 30+/-8μm (p=0.45), whereas the extension on control lanes increases by 24% to an average of 41+/-11μm (p=0.57; Figures 4D and 4E). The significant distinction in growth preference exhibited by retinal axon branches of dorsal versus ventral WT retinal cells is abolished in EphB mutant retina, as ventral and dorsal EphB mutant retina respond similarly to ephrin-B1-Fc and control-Fc lanes (p>0.8 for ephrin-B1 lanes; p>0.95 for control-Fc lanes). These findings demonstrate that the differential low-to-high DV expression of EphBs in RGCs generates the preference of interstitial branches from ventral retinal axons to extend on ephrin-B1 versus a control substrate compared to dorsal retinal axon branches.
‘Choice Ratio’ confirms retinal branch responses to ephrin-B1 in vitro reflects DV mapping in vivo
To focus specifically on the issue of branch guidance and facilitate comparison across categories, we calculated a single metric, termed the ‘Choice Ratio’ (C.R.), which normalizes the data for a bias in the growth of primary retinal axons and the position of branch points along them on the control-Fc lanes (Figure 4). Overall, ~60% of primary axon length and 70% of all branch points are on the control-Fc lanes, with the percentages varying across categories; although the variation is not statistically significant it does predispose the calculations presented above towards avoidance of ephrin-B1. We define the C.R. as the ratio of branch length per 100 units of primary axon length on ephrin-B1-Fc lanes to control-Fc lanes. Thus, a C.R. below one indicates a choice for branch extension on control-Fc lanes and a C.R. above one indicates a choice for branch extension on ephrin-B1-Fc lanes (Figure 4E).
WT dorsal retina has a C.R. well below one (0.66) whereas WT ventral retina has a C.R. well above one (1.76). Taken together, these data show a clear distinction between dorsal and ventral retinal axon branches in their growth behavior on ephrin-B1 substrates, mimicking in vivo DV mapping. Further, dorsal retina from EphB mutants has a C.R. of 1.12, indicating only a slight growth preference of dorsal axon branches for ephrin-B1-Fc lanes, consistent with EphB mutant dorsal retina showing a strongly diminished repulsion to ephrin-B1 compared to WT dorsal retina. Ventral retina from EphB mutants has a C.R. of 0.76, indicating that the preference for WT ventral retinal axon branches to extend preferentially on ephrin-B1 is lost (Figure 4E). The C.R. for EphB mutant ventral retina is most similar to the C.R. for WT dorsal retina. This switch in response indicates that extension preferences of retinal axon branches is EphB-dependent and convertible within the DV population from a positive to a negative response to the same concentration of ephrin-B1.
DISCUSSION
EphB forward signaling controls DV retinotopic mapping in mice (Hindges et al., 2002, Thakar et al., 2011) and frogs (Mann et al., 2002). However, the distinction between EphB receptors in the process remains unknown. We have analyzed EphBs implicated in mapping and find that each individual null allele, as well as every allelic combination examined, has a percentage of cases with qualitatively similar aberrancies, strongly indicating that the individual role of each EphB is similar. Though the qualitative appearance of the aberrant retinocollicular maps in EphB mutants is similar, we find an increase in the percentage of cases with aberrancies to rise with the number of EphB null alleles and conclude that the overall level of EphBs is more important than EphB identity. Further, we find distinct in vitro responses from dorsal and ventral retina to ephrin-B1 that are EphB dependent and consistent with a bifunctional role in branch guidance in vivo.
We demonstrate in vitro that EphB level is a critical component in determining whether RGC axon branch response to ephrin-B1 is positive versus negative, a fundamental feature of DV mapping. In the protein stripe assay retinal axon branches with high levels of EphB respond positively to a substrate of ephrin-B1 compared to branches with low EphB levels which respond negatively to that same concentration of ephrin-B1. Critically, we demonstrate that both responses are diminished in retina from EphB mutants. Thus, dorsal retinal axon branches, which in WT prefer not to extend on ephrin-B1 substrates, in EphB mutants demonstrate no significant response and extend at least equally well on ephrin-B1 and control lanes. In contrast, ventral retinal axon branches, which in WT show a small bias for ephrin-B1 lanes, in EphB mutants demonstrate a reversal in response and show a small bias for control-Fc lanes. These bifunctional responses match the in vivo requirements for branch guidance in that each axon is capable of directed branch extension medially up the ephrin-B1 gradient and laterally down the ephrin-B1 gradient in the SC (Figure 1D). In contrast, we find a mild negative influence on primary axon guidance in vitro that does not change with EphB level, suggesting that branch guidance and primary axon guidance are distinct processes. This finding is also consistent with in vivo observations of RGC axon mapping in which there is no apparent response of the RGC primary axon growth cone to the gradient of endogenous ephrin-B1 (Yates, et al., 2001; Hindges, et al., 2002) nor to ectopic domains of high expression of ephrin-B1 created by electroporation (McLaughlin et al., 2003a). These data support a model in which RGC axon branches respond to ephrin-B1 as an attractant and as a repellent and that these responses are due to the total EphB level relative to the level of ephrin-B1 (Figure 1D). This bifunctional response is similar to cell attachment assays in which increased attachment is favored at a peak concentration of ephrin-B1, set by the level of EphB expressed by the cell (Huynh-do et al., 1999).
Our data are consistent with a bifunctional action of ephrin-B1, acting through EphB1, EphB2, and EphB3, to guide RGC axon branches laterally or medially along the LM gradient of ephrin-B1 in the SC. This bidirectional RGC axon branch guidance is the critical mechanism of DV retinotopic mapping (Simon and O’Leary 1992b, 1992c; Hindges et al, 2002). Interestingly, though, EphB mutants have a TZ in the appropriate location, in addition to an eTZ, a phenomenon observed in the vast majority of mutants for axon guidance molecules with retinocollicular mapping defects (McLaughlin and O’Leary, 2005). The retinal expression of EphB4, though weak, may explain the presence of an appropriately positioned TZ in every case (Hindges et al., 2002). Furthermore, other guidance molecules implicated in DV retinal axon guidance (e.g. L1, semaphorins, wnt/ryk; Demyanenko and Maness, 2003; Liu et al., 2004; Schmitt et al., 2006) may partially compensate for the lack of EphB1, EphB2, and EphB3 and simply provide a small bias along the ML axis of the SC sufficient to influence branching enough for other guidance activities to act normally to form a correctly positioned TZ from a subset of RGCs (e.g. spontaneous waves of electrical activity in retina; McLaughlin et al., 2003b). Interestingly, L1 interacts with the EphB system, suggesting additional complexity for the roles of guidance molecules in mapping (Dai et al., 2012).
The frequency of defects in EphB1 mutant mice is comparable to that reported for EphB2, EphB3 double mutant mice (Hindges et al., 2002) and other mutants with retinocollicular defects (e.g. ephrin-A5-/-; Frisén et al., 1998). We cannot discount a higher percentage of animals with retinocollicular defects than reported here, as our criteria includes only those animals with clear defects visible in a dorsal view of a wholemount SC. Additionally, Henkemeyer and colleagues report a similar percentage of ectopic TZs in EphB knockout animals with one or two EphB alleles lacking (Thakar et al., 2011). However, when using kinase inactive EphB alleles (putative dominant negatives) and with more than two mutant alleles they report a somewhat higher percentage of mapping defects present than we report here. Though our results generally agree, Thakar et al. report a small percentage of WT cases with aberrant maps. Additionally, Thakar and colleagues describe multiple small eTZs in some mutant cases, a phenotype we rarely see. Both current reports indicate a higher percentage of mutants than initially reported suggesting either an enhanced ability to detect retinocollicular aberrancies with DiI injections and SC wholemounts or perhaps reflecting a larger role for EphB1 as it is expressed more prominently than EphB3 (Hindges et al., 2002).
Reverse signaling via ephrin-Bs, which has been reported to influence RGC axon mapping in Xenopus tectum (Mann et al., 2002) and mouse SC (Thakar et al., 2011; but see Hindges et al., 2002), might contribute, but it would also be greatly attenuated in EphB mutants. Further, though we find evidence of eTZs from dorsal RGC axon projections, the defects are less frequent and less prominent that those from more ventral retinal locations. Thakar and colleagues do not find aberrant retinocollicular projections from dorsal retina in ephrin-B1 mutants, though ephrin-B1 is highly expressed in dorsal retina during mapping (Hindges et al., 2002; Thakar et al., 2011) and reverse signaling through ephrin-B1 in axon guidance decisions is possible (Bush and Soriano, 2009). However, mice mutant for ephrin-B2 or EphBs, do have defects consistent with reverse signaling, though even in these mutants the effect is most pronounced for ventral retina and the defects for dorsal RGCs are found at a low rate (Thakar, et al., 2011). Furthermore, the retinocollicular mapping defects from dorsal RGCs in ephrin-B2 mutants appear distinct from EphB mutants in that the eTZs are closely associated with the primary TZ and seem to lack clear separation from it (Thakar et al., 2011). As reported above we find a low frequency of minor defects in EphB mutants for dorsal RGC axons which would, in theory, reveal a role for reverse signaling due to the loss of EphBs from the SC in the mutants examined (Hindges et al., 2002). We cannot rule out that EphB4 is primarily involved in reverse signaling and is maintained in the EphB mutants analyzed here. It is possible the mild phenotype we observe in dorsal retinal mapping in EphB mutants is due to forward signaling, which would be consistent with our in vitro results indicating dorsal retina from EphB mutants is slightly biased for extension on ephrin-B1 lanes.
It has become apparent that the multiple guidance systems employed by RGCs in the SC provide information that, in total, leads to a dynamic mapping system that cannot be completely disorganized by the loss of one particular mechanism (Feldheim and O’Leary, 2010). Within those systems the multiple EphBs expressed by retina contribute by responding to their ligand, ephrin-B1, in a context-dependent bifunctional manner that is consistent across receptor type.
EXPERIMENTAL METHODS
Animals
The generation of EphB mutant mice has been described (Henkemeyer et al., 1996; Orioli et al., 1996; Williams et al., 2003). Genotyping was done by PCR (Hindges et al., 2002; Williams, et al., 2003). All procedures were IACUC approved.
Axon Labeling
Anterograde labeling was done as described (Hindges et al., 2002). DiI-dimethylformamide solution (Molecular Probes) was injected into the retina of anesthetized P7 mice; one day later, contralateral SC whole mounts were analyzed blind to genotype. Retinas were dissected, flat-mounted, and examined under fluorescence to confirm that all labeled RGC axons originated solely from a single focal injection site.
Stripe Assay
Striped substrates were prepared as described (Lim et al., 2008) using 10μg/ml ephrin-B1-Fc and human-Fc (R&D Systems). Dorsal and ventral retinal thirds were dissected from P1 pups, dissociated, and cells were plated at low density (approximately 20 cells/mm2), incubated at 37C for two days, and stained with vital dye (5 (and-6) carboxyfluoroscein diacetate, succinimidyl ester, Molecular Probes). Isolated axons were analyzed blind to genotype, lane composition, and lane position. Branch points (n=107 WT; n=55 mutant), axons, and branches were measured in MetaMorph. We control for a slight bias in RGC primary axon position for human-Fc lanes by dividing the length of branches on each lane by the total axon length on that same lane for each condition (‘Choice Ratio’).
Acknowledgments
This work is funded by NIH R01 EY007025 and the Vincent J. Coates Chair in Molecular Neurobiology (D.D.M.O). We thank Berta Higgins and Haydee Gutierrez for expert technical assistance, Kyucheol Cho for comments on the manuscript, and Mark Henkemeyer for providing EphB mutant mice.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bush JO, Soriano P. Ephrin-B1 regulates axon guidance by reverse signaling through a PDZ-dependent mechanism. Genes & Development. 2009;23:1586–1599. doi: 10.1101/gad.1807209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Dalal JS, Thakar S, Henkemeyer M, Lemmon VP, Harunaga JS, Schlatter MC, Buhusi M, Maness PF. EphB regulates L1 phosphorylation during retinocollicular mapping. Mol Cell Neurosci. 2012;50:201–210. doi: 10.1016/j.mcn.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Maness PF. The L1 cell adhesion molecule is essential for topographic mapping of retinal axons. J Neurosci. 2003;23:530–538. doi: 10.1523/JNEUROSCI.23-02-00530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, O’Leary DDM. Visual Map Development: Bidirectional Signaling, Bifunctional Guidance Molecules, and Competition. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisén J, Yates PA, McLaughlin T, Friedman GC, O’Leary DDM, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DDM. EphB forward signaling controls directional branch extension and arborization required for dorsal ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y-S, McLaughlin T, Sung T-C, Santiago A, Lee K-F, O’Leary DDM. p75NTR mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Berndt J, Su F, Tawarayama H, Shoji W, Kuwada JY, Halloran MC. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci. 2004;24:310–318. doi: 10.1523/JNEUROSCI.4287-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris WA, Holt CE. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461–473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, Yates PA, O’Leary DDM. Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal ventral retinotopic mapping. Development. 2003a;130:2407–2418. doi: 10.1242/dev.00467. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003b;40:1147–1146. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DDM. Molecular gradients and development of retinotopic maps. Annual Review of Neuroscience. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996;15:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu C-C, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DDM. Responses of retinal axons in vivo and in vitro to molecules encoding position in the embryonic superior colliculus. Neuron. 1992a;9:977–989. doi: 10.1016/0896-6273(92)90249-d. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DDM. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992b;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DK, O’Leary DDM. Influence of position along the medial-lateral axis of the superior colliculus on the topographic targeting and survival of retinal axons. Brain Res Dev Brain Res. 1992c;69:167–172. doi: 10.1016/0165-3806(92)90155-p. [DOI] [PubMed] [Google Scholar]
- Thakar S, Chenaux G, Henkemeyer M. Critical roles for EphB and ephrin-B bidirectional signalling in retinocollicular mapping. Nature Communications. 2011;2:431. doi: 10.1038/ncomms1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AR, McLaughlin T, O’Leary DDM. Topographic specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. Journal of Neuroscience. 2001;21:8548–8463. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


