Abstract
CNS immune signaling contributes to deleterious opioid effects including hyperalgesia, tolerance, reward, and dependence/withdrawal. Such effects are mediated by opioid signaling at TLR4, presumptively of glial origin. Whether CNS endothelial cells express TLR4 is controversial. If so, they would be well positioned for activation by blood-borne opioids, contributing to opioid-induced pro-inflammatory responses. These studies examined adult primary rat CNS endothelial cell responses to (-)-morphine or its mu-opioid receptor (MOR) inactive metabolite morphine-3-glucuronide (M3G), both known TLR4 agonists. We demonstrate that adult rat CNS endothelial cells express functional TLR4. M3G activated NFκB, increased tumor necrosis factor-α (TNFα) and cyclooxygenase-2 (COX2) mRNAs, and released prostaglandin E2 from these cells. (-)-Morphine-induced upregulation of TNFα mRNA and prostaglandin E2 release were unmasked by pre-treatment with nalmefene, a MOR antagonist without TLR4 activity (unlike CTAP, shown to have both MOR- and TLR4-activity), suggestive of an interplay between MOR and TLR4 co-activation by (-)-morphine. In support, MOR-dependent Protein Kinase A (PKA) opposed TLR4 signaling, as PKA inhibition (H-89) also unmasked (-)-morphine-induced TNFα and COX2 mRNA upregulation. Intrathecal injection of CNS endothelial cells, stimulated in vitro with M3G, produced TLR4-dependent tactile allodynia. Further, cortical suffusion with M3G in vivo induced TLR4-dependent vasodilation. Finally, endothelial cell TLR4 activation by lipopolysaccharide and/or M3G was blocked by the glial inhibitors AV1013 and propentofylline, demonstrating endothelial cells as a new target of such drugs. These data indicate that (-)-morphine and M3G can activate CNS endothelial cells via TLR4, inducing proinflammatory, biochemical, morphological, and behavioral sequalae. CNS endothelial cells may have previously unanticipated roles in opioid-induced effects, in phenomena blocked by presumptive glial inhibitors, as well as TLR4-mediated phenomena more broadly.
Keywords: endothelial cell, CTAP, nalmefene, M3G, morphine, TLR4
1. Introduction
Studies of opioid-induced CNS immune signaling have focused exclusively on astrocytes and microglia. The mu opioid receptor (MOR) agonist (-)-morphine upregulates the expression of their activation markers (Song and Zhao, 2001, Raghavendra et al., 2004), triggers TLR4 signaling (Wang et al., 2012) and NFκB activation (Kawai and Akira, 2006, Hutchinson et al., 2011) by these cells, as well as proinflammatory cytokine production in the CNS (Johnston et al., 2004, Raghavendra et al., 2004, Hutchinson et al., 2008a). Such opioid-induced effects have generated considerable interest, as blocking proinflammatory cytokines and/or treatment with putative glial inhibitors enhances (-)-morphine analgesia and suppresses opioid adverse effects including reward, tolerance, dependence/withdrawal, and paradoxical pain (Hutchinson et al., 2011, Hutchinson et al., 2012). Such data led to the conclusion that glia can powerfully modulate opioid actions.
However, another cell type beyond glia bears consideration. CNS endothelial cells are bathed by blood-borne substances, including opioids. These cells can be activated by select blood-borne substances, such as inflammatory mediators, causing release of proinflammatory mediators (Matsumura et al., 1998, Singh and Jiang, 2004, Quan, 2008). Whether this holds true for opioids is unknown. While opioid-induced signaling has been argued to occur via glial TLR4 activation (Hutchinson et al., 2011), it is controversial whether CNS endothelial cells also express TLR4 (Singh and Jiang, 2004, Verma et al., 2006, Nagyoszi et al., 2010, Shih and Yang, 2010). If so, endothelial cells might contribute to proinflammation from (-)-morphine and/or its long-lived, predominant MOR inactive metabolite, M3G, which both signal through TLR4 (Lewis et al., 2010). Notably, M3G is largely peripherally restricted given its low blood-brain barrier penetration (De Gregori et al., 2012). Thus, if CNS endothelial cells express TLR4, they may have a unique and as yet uncharacterized role in opioid-induced signaling, and hence diverse opioid actions, via detection of this major blood-borne metabolite.
While controversy surrounds TLR4 expression by CNS endothelial cells, it is well accepted that these cells express MORs (Stefano et al., 1995, Wilbert-Lampen et al., 2007). MOR/TLR4 interactions have been suggested for some classical immune cells, with MOR and TLR4 signaling having opposing actions (Roy et al., 1998, Welters et al., 2000). Whether such occurs for CNS endothelial cells is entirely unknown. But should it occur, it would make TLR4 signaling by the long-lived, peripherally restricted, MOR inactive morphine metabolite M3G all the more intriguing, as the result of TLR4 signaling would be predicted to differ in the presence versus absence of MOR ligands.
The present study characterizes TLR4 expression and opioid-induced function in adult rat CNS endothelial cells. The relative contributions of TLR4 versus classical opioid receptor signaling were also examined. To test whether TLR4-activated CNS endothelial cells are sufficient to alter in vivo responses, in vitro activated CNS endothelial cells were injected into the lumbar intrathecal space to test for increases in nociceptive hypersensitivity, and cortical vasodilation was assessed as a classical inflammatory response. Lastly, the putatively glia-targeting inhibitors, propentofylline (phosphodiesterase inhibitor (Sweitzer and De Leo, 2011)), and AV1013 (like ibudilast, a macrophage migration inhibitory factor (MIF) inhibitor (Cho et al., 2010)) were tested to define whether they also block CNS endothelial cell activation, as such a result would have broad ramifications for the use of such agents to conclude glial involvement in diverse phenomena.
2. Materials and Methods
2.1 Subjects
Pathogen-free adult male outbred Sprague Dawley rats (300-400 g; Harlan Laboratories) were used for Experiments 1-5, 7 and 8. Pathogen-free adult male inbred Lewis rats (275–300 g; Harlan Laboratories) were used for Experiment 6 For all experiments, rats were housed two or four per cage in a temperature-controlled environment (23±2°C) with a 12 hr light/dark cycle (lights on at 0700 hr), with standard rat chow and water available ad libitum. All procedures occurred in the light phase. Rats were allowed 1 week of acclimation to the colony room before experimentation. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
2.2 Drugs
(-)-Morphine sulfate was a gift from Mallinckrodt, Inc. (St. Louis, MO). (-)-Nalmefene hydrochloride and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) were purchased from Tocris Bioscience (Ellisville, MO). Lipopolysaccharide (LPS; Escherichia Coli, serotype 0111:B4), M3G and H-89 were purchased from Sigma (St. Louis, MO). LPS-RS (a TLR4 competitive receptor antagonist naturally produced by Rhodobacter sphaeroides) was purchased from Invivogen (San Diego, CA). AV1013 (Cho et al., 2010) was a gift from MediciNova (San Diego, CA). Propentofylline was a gift from Solace Pharmaceuticals (Cambridge, MA). (+)-Naloxone and (+)-morphine were a gift from Dr. Kenner Rice. (-)-Morphine, (+)-morphine and M3G were dissolved in sterile 0.9% saline (Abbott Laboratories, North Chicago, IL) and stored at 4°C as stock 10 mM solutions. For in vitro experiments, (-)-morphine, (+)-morphine and M3G were further diluted in culture medium. LPS, LPS-RS, nalmefene hydrochloride, CTAP, propentofylline, and AV1013 were freshly dissolved in culture medium for use. H-89 was freshly dissolved in 1.5 % DMSO.
2.3 Endothelial cell isolation and culture
Endothelial cells were isolated from adult rat brain and spinal cord tissue and established as primary cultures, as described previously (Perriere et al., 2005, Verma et al., 2006). This method yields cultures that are >98% pure, which was confirmed with positive immunostaining for von Willebrand factor, negative immunostaining for markers of fibroblasts (prolyl 4-hydroxylase) and astrocytes (glial fibrillary acidic protein) (data not shown), and visual inspection of the cells, which had the typical spindle-shaped morphology of CNS endothelial cells and formed confluent monolayers that were longitudinally aligned and non-overlapping, as described previously (Perriere et al., 2005). Briefly, rats were anesthetized with isoflurane then decapitated. The brain was dissected out of the skull and the spinal cord was removed by hydraulic extrusion with ice-cold physiological saline. The tissue was processed using sterile technique as follows: tissue was incubated in an enzymatic digestion solution, containing collagenase type II (Invitrogen, Carlsbad, CA) and DNase I (Sigma), triturated with a 25 ml pipette, then incubated at 37°C for 40 min. Following the incubation, the mixture was again triturated then centrifuged, and the pellet was centrifuged in a solution of 20% bovine serum albumin in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen) to remove neurons and glia, leaving behind a pellet of microvessels. The microvessels were then placed in an enzymatic digestion solution, containing collagenase/dispase (Roche, Indianapolis, IN) and DNase I, and incubated at 37°C for 30 min. The mixture was centrifuged, and the pellet was centrifuged through a 33% Percoll gradient (Amersham Biosciences, Piscataway, NJ) to purify the microvessel fragments. The microvessel fragments were washed with DMEM then suspended in basal medium (DMEM/F-12 (Invitrogen) containing 100 U/ml penicillin-streptomycin (Sigma), 50 μg/ml gentamicin (Sigma), 2 mM GlutaMAX-I (Invitrogen), 20% fetal bovine serum (Invitrogen), and 1 ng/ml basic fibroblast growth factor (Sigma)), supplemented with 4 μg/ml puromycin (a translation inhibitor; Sigma) which selectively kills microvessel cell types other than endothelial cells, due to high expression of the efflux pump P-glycoprotein selectively on endothelial cells (Cordon-Cardo et al., 1989, Beaulieu et al., 1997). This method does not alter endothelial cell viability (Perriere et al., 2005).
Microvessel fragments from the CNS tissues of 4 rats were pooled and seeded onto eight 10 cm petri dishes (coated with a mixture of extracellular matrix proteins including fibronectin (Sigma), collagen type IV (Sigma), and collagen type I (Sigma), then maintained (37°C, 5% CO2) for 48 hr in DMEM/F-12 with puromycin, followed by DMEM/F-12 without puromycin, until the endothelial cells reached confluence (1 week post-isolation). The cells were then passaged onto 24 well microplates (again coated with the mixture of extracellular matrix proteins as above) and used for experiments once they again reached confluence. Such cultures of confluent monolayers of CNS endothelial cells polarize because they are grown on a substrate of extracellular matrix proteins, with luminal (blood) and abluminal (brain) properties that model in vivo conditions (Joo, 1993, Deli et al., 2005). For each in vitro experiment an n of 6 wells within a 24 well microplate was used for each concentration/time point.
2.4 mRNA extraction, cDNA synthesis, and real-time PCR
To analyze gene expression in cultured CNS endothelial cells, supernatants were removed from cells growing in 24 well microplates, 800 μl of TRIzol reagent (Invitrogen) was added to each well, and the plates were frozen at -80°C until later analysis. Each sample was later transferred to an eppendorf tube. Total RNA was isolated from the cells based on the method of Chomczynski and Sacchi (1987). Chloroform (200 μl) was added to each sample, which was then vortexed and centrifuged (12,000 × g) for 15 min at 4°C to achieve phase separation of nucleic acid. Isopropyl alcohol (500 μl) was added to the aqueous phase to precipitate nucleic acid. Samples were vortexed (1 min) and incubated at room temperature for 10 min followed by centrifugation (12,000 × g) for 10 min at 4°C. Nucleic acid precipitates were washed twice in 75% ethanol (1 ml) and centrifuged (7500 × g) for 5 min at 4°C. Samples were not DNase-treated because the primers used were designed to exclude genomic DNA (see below). Total RNA was reverse transcribed into cDNA using the SuperScript II First Strand Synthesis System for RT-PCR (Invitrogen). RNA (2 μg) was incubated for 5 min at 65°C in a total reaction volume of 12 μl containing random hexamer primers (5 ng/μl) and dNTPs (1 mM). Samples were chilled on ice for at least 1 min. A cDNA synthesis buffer (7 μl) as described by the manufacturer was added to the reaction and incubated at 42°C for 2 min. Reverse transcriptase (1 μl; 200 units SuperScript II) was added to the reaction and incubated at 25°C for 10 min followed by 42°C for 50 min. The reaction was terminated by heating to 70°C for 15 min. cDNA was diluted 2-fold in nuclease-free water and stored at −20°C.
PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). cDNA (1 μl) was added to a reaction master mix (25 μl) containing 2.5 mM MgCl2, HotStar Taq DNA polymerase, SYBR Green I, dNTPs, fluorescein (10 nM) and gene specific primers (500 nM each of forward and reverse primer). For each sample, triplicate reactions were conducted in 96-well plates (BioRad, Hercules, CA). Pipetting was performed blind as to experimental condition. PCR cycling conditions consisted of a hot-start activation of HotStarTaq DNA polymerase (94°C, 15 min) and 40 cycles of denaturation (95°C, 15 s), annealing (57–59°C, 30 s), and extension (72°C, 30 s). Formation of PCR product was monitored in real-time using the MyiQ Single-Color Real-Time PCR Detection System (BioRad). Fluorescence of SYBR Green I was captured at 72°C. Threshold for detection of PCR product above background was set at 10× the standard deviation of mean background fluorescence for all reactions. Background fluorescence was determined from cycles 1–5 prior to exponential amplification of product and subtracted from raw fluorescence of each reaction/cycle. Threshold for detection of PCR product fell within the log-linear phase of amplification for each reaction. Threshold cycle (CT; number of cycles to reach threshold of detection) was determined for each reaction.
Primers
Primers were designed to span exon - intron boundaries. The following sequences were used:
TLR4
| TCCCTGCATAGAGGTACTTC | Forward |
| CACACCTGGATAAATCCAGC | Reverse |
TNFα
| CAAGGAGGAGAAGTTCCCA | Forward |
| TTGGTGGTTTGCTACGACG | Reverse |
COX-2
| CAGGAGAGAAAGAAATGGCTGC | Forward |
| TGGTCTCCCCAAAGATAGCATC | Reverse |
Relative gene expression was determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001, Pfaffl, 2001). The mean cycle threshold (CT) of triplicate measures was computed for each sample. Sample mean CT of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin (as housekeeping genes) was subtracted from the sample mean CT of the respective gene of interest (ΔCT). The sample with the absolute highest mean ΔCT was selected as a calibrator and subtracted from the mean ΔCT of each experimental sample (ΔΔCT). 2−ΔΔCT yields fold-change in gene expression of the gene of interest normalized to the housekeeping gene expression and relative to the calibrator sample.
2.5 In-cell Western blots
Cells were grown in 24 well microplates, and stimulated as per Experiment 3 (see below). In-cell Western blots were performed blind as to experimental condition. For each well, the medium was removed, then 1 ml of fresh 4% paraformaldehyde was added and incubated for 20 min at room temperature. The cells were permeabilized by washing five times in phosphate-buffered saline (PBS) + 0.1% Triton X-100 for 5 min per wash on a shaker at room temperature, then incubated in Odyssey blocking buffer (750 μl per well; LI-COR, Lincoln, NE) for 1 hr on a shaker at room temperature. The blocking buffer was removed and replaced with a solution containing primary antibody diluted in Odyssey blocking buffer. On each plate, 1 well was incubated with Odyssey blocking buffer that did not contain primary antibody, to serve as a negative control for the plate. NF-κB (p65 subunit; Millipore, Billerica, MA; 1:500) which recognizes an epitope overlapping the nuclear location signal of the p65 subunit of the NF-κB heterodimer and thus selectively binds to the activated form of NF-κB (Zabel et al., 1993) was used. The plates were incubated overnight at 4°C on a shaker, then washed the next morning 4 times with PBS + 0.1% Tween-20 for 5 min per wash at room temperature on a shaker. The cells were then incubated with a secondary antibody solution in Odyssey blocking buffer, containing secondary antibody, 0.2% Tween-20, and the cell stains DRAQ5 (Biostatus Limited, Leicestershire, United Kingdom) and Sapphire700 (LI-COR), both of which emit fluorescence at 700 nm. The control well secondary antibody solution contained only 0.2% Tween-20 and secondary antibody in Odyssey blocking buffer. Secondary antibody was IRDye 800CW conjugate of goat-anti-mouse-IgG (LI-COR), which emits fluorescence at 800 nm, and was diluted at 1:800. The plates were incubated for 1 hr at room temperature on a shaker, protected from light, then washed 4 times with PBS + 0.1% Tween-20 for 5 min per wash at room temperature on a shaker while still protected from light. The wash buffer was then removed and the plate was tapped firmly against paper towels to remove as much buffer as possible. The plates were scanned and analyzed using an Odyssey IR scanner with Odyssey imaging software (LI-COR). The antibody signals were analyzed for each well as the average 800 nm-channel integrated intensity normalized to the 700 nm-channel integrated intensity to correct for well-to-well variations in cell number. Results are expressed as percent relative responses compared to vehicle-treated control wells.
2.6 In vitro HEK293-hTLR4 antagonist assay
As previously described (Hutchinson et al., 2008a) a human embryonic kidney-293 (HEK293) cell line stably transfected to express human TLR4 was used to assess TLR4 activity (Invivogen; 293-htlr4a-md2cd14). This HEK293 cell line expresses high levels of TLR4, the required TLR4 co-signaling molecules (MD-2 and CD14) and an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by several transcription factors such as NF-κB and AP-1. TLR4 activity in the cells is assessed by measuring the expression of secreted alkaline phosphatase (SEAP) protein that is produced as a consequence of TLR4 activation. All assessments were conducted under modified conditions as outlined previously (Hutchinson et al., 2008a), using artificial cerebrospinal fluid (aCSF) as the culture media.
2.7 In silico TLR4/MD-2 docking simulations
In order to prioritize the docking calculations and to provide a possible mechanistic framework for the in silico docking simulations it was a priori hypothesized that the TLR4 and MD2 could exist in a range of possible conformational states ranging from a pre-activation state of individual membrane bound TLR4 and soluble extracellular MD2 through to a complete signaling heterodimer of TLR4 and MD2.
To examine the in silico docking of ligands to the TLR4/MD2 complex, the crystal structure of the human TLR4-human MD2-E.coli LPS Ra complex program database (pdb) file was obtained from RCSB Protein Data Bank (PDBID: 3FXI) as published by Park et al. (2009). All ligands, water, and cofactors were removed from the file via Molegro Molecular Viewer, thus eliminating exogenous water molecules and artifacts from crystallization from future docking simulations. The modified .pdb files were further prepared using MGLTools 1.5.6.RC2 (http://mgltools.scripps.edu/) with polar hydrogens added. Ligands for docking were gathered using PubChem isomeric SMILES then converted to .pdb using a structure file generator (http://cactus.nci.nih.gov/services/translate/) and validated by visual inspection.
The 4 macromolecules in the MD-2-TLR4 heterodimer .pdb file were separated into four separate .pdb files, resulting in TLR4-A, TLR4-B, MD-2-C and MD-2-D facilitating the creation of the range of possible conformational states using Molegro Molecular Viewer. Docking simulations were conducted for all ligands (agonists and antagonists) to each of these conformational states. Docking was conducted for all ligands ((-)-morphine, M3G, (-)-nalmefene, and CTAP) using Vina (version 1.1.2) (Trott and Olson) within PyRx (version 0.8) (Wolf, 2009). An exhaustiveness factor of 8 was used for all simulations, with the Vina search space dimensions and center defined for each macromolecule using the auto-maximize function.
2.8 Biophysical characterization of M3G binding to human MD-2
MD-2 protein was prepared as described (Wang et al., 2012, Wang et al., 2013). Fluorescence measurements were performed on a Fluorolog-3 spectrofluorimeter (Horiba Jobin Yvon, Edison, NJ). All measurements were carried out under room temperature in a 2×10 mm quartz cell (Starna Cells, Atascadero, CA,). 280 nm was chosen as the excitation wavelength of MD-2 intrinsic Tyr and Trp fluorescence and emission at 300- 450 nm was measured. Appropriate controls were subtracted from spectra obtained on the samples.
0.5 μM MD-2 was titrated with different concentrations of M3G, and the fluorescence intensity at 337 nm was plotted against M3G concentration. The raw data was fitted by non-linear least square method using the equation: F=0.5 × (2 × F0 - FPL × (KD + [LT] + [PT]-((KD + [LT]+ [PT])2 – 4 × [LT] × [PT])0.5)), where [F], the observed fluorescence; F0, initial fluorescence of protein in the absence of ligand; FRL, adjustable parameter for protein–ligand complex molar fluorescence; KD, dissociation constant; [LT], total concentration of the ligand; [PT], total protein concentration. Roxithromycin, which has been reported to show no apparent binding to MD-2 (Resman et al., 2008), served as a negative control compound. Protein A was used as a negative control protein to eliminate the possibility of binding to Protein A tag.
2.9 Enzyme immunoassay (EIA) for prostaglandin E2
Following stimulation, cell supernatants were removed and frozen at -80°C until assay. Prostaglandin E2 protein in cell supernatants were measured with an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI; and R&D Systems, Minneapolis, MN), per the manufacturer's instructions. Samples were pipetted blind as to experimental condition.
2.10 Acute intrathecal injection of CNS endothelial cells
To prepare endothelial cells for injection, the culture medium was removed following drug stimulation (see Experiment 6 design, below) and the cells were rinsed twice with PBS. The cells were then incubated with 0.05% trypsin containing EDTA (Invitrogen) for 2 min at 37°C. DMEM/F-12 containing 10% FBS was added to stop the enzyme, and the cells were centrifuged for 5 min at 180 × g. The supernatant was removed and cells were resuspended in PBS at a density of 1,000 cells per 10 μl.
Rats were lightly anesthetized with isoflurane. The lumbar region of the back was shaved and cleaned with 70% ethanol. An 18 gauge guide needle, with the hub removed, was inserted into the L5/6 intervertebral space. A PE-10 catheter was threaded through the guide needle, pre-marked such that the distal end of the PE-10 tubing rested over the L4–L6 lumbar spinal cord. Cells were injected over 15 s (10 μl of PBS containing 1,000 cells followed by 6 μl of sterile PBS flush) with a 30 s delay before removing the catheter and guide needle. Each animal was anesthetized for a maximum of 5 min, and none exhibited neurological damage from the procedure.
2.11 von Frey test for tactile allodynia
Testing was conducted blind with respect to group assignment. Rats received at least three 60 min habituations to the test environment prior to behavioral testing. The von Frey test (Chaplan et al., 1994) was performed at the distal region of the heel in the hindpaws, within the region of sciatic innervation as previously described in detail (Chacur et al., 2001, Milligan et al., 2001). Assessments were made prior to (baseline) and on days 1, 2, 3, 4 and 7 post injection. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to the left vs. right hindpaws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer designated 3.61 (0.407 g) to 5.18 (15.136 g) filaments. The behavioral responses were used to calculate absolute threshold (the 50 % probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method (Harvey, 1986, Treutwein and Strasburger, 1999), as described previously (Milligan et al., 2000, Milligan et al., 2001). This fitting method allows parametric analyses that otherwise would not be statistically appropriate (Milligan et al., 2000, Milligan et al., 2001). Absolute thresholds for the left paw are presented for each group on a logarithmic scale.
2.12 Craniotomy surgery
Rats were anesthetized to a surgical plane using an intraperitoneal injection of ketamine-xylazine-acepromazine (45-9-1.5 mg/kg body weight), placed on a regulated heating pad, and maintained with sub-sequent injections throughout the experiment so that the corneal reflex could barely be elicited. A bilateral craniotomy was performed over both hemispheres extending 2 mm rostral to bregma to 1 mm caudal of lambda and from the mid-sagittal suture to 2 mm below the zygomatic arch, exposing a maximal area of the surgically accessible cortex. The dura was reflected and the exposed cortex regularly irrigated with aCSF containing: 135 mM NaCl; 3 mM KCl; 2 mM MgCl; and 2 mM CaCl– pH 7.4 at 37° C.
2.13 Statistical analyses
For Experiment 1, data were analyzed by one-way ANOVA or two-way ANOVA with Dunnett's, Tukey or Holm-Sidak posthoc tests where appropriate. For Experiment 3, data were analyzed by two-way ANOVA with repeated measures and a Holm-Sidak posthoc test. For Experiments 4 (SEAP data), 5 and 8, data were analyzed by two-way ANOVA with a Holm-Sidak posthoc test. For Experiment 6, baseline values were compared between groups using a one-way ANOVA, with differences between treatment groups determined by two-way ANOVA with repeated measures and a Holm-Sidak posthoc test. For Experiment 7, data were analyzed by paired t-test of all the naive groups compared to all the aCSF or aCSF+NAL. Data are presented as mean ± standard error of the mean. Significance was set at P < 0.05.
2.14 Experiment 1: Do cultured adult rat CNS endothelial cells express functional TLR4 receptors?
Cultured adult rat primary CNS endothelial cells were first assayed for TLR4 mRNA expression under basal conditions. Upon observation of robust TLR4 expression in these adult rat cells, this experiment then tested the effect of LPS on TLR4 and TNFα gene expression (0, 1, or 10 ng/ml LPS for 4 hr); and release of PGE2 (0, 10 ng/ml, 100 ng/ml, or 1000 ng/ml LPS for 24 hr). To define whether LPS-induced effects are blocked by a TLR4 antagonist, LPS-RS (10 ng/ml) was added to separate cultures 1 hr prior to a 4 hr or 24 hr exposure to LPS, and the endothelial cells then assayed for TNFα mRNA (after a total incubation of 5 hr) or release of PGE2 (after a total incubation of 25 hr). For each study, n = 6 wells per condition were used.
2.15 Experiment 2: Does M3G bind to MD-2?
An in silico docking analysis was conducted to model the binding of M3G and (-)-morphine to TLR4, MD-2, the TLR4/MD-2 dimer, and the complete signaling heterodimer of TLR4 and MD-2. An M3G/MD-2 binding biophysical binding assay was also performed to validate in silico docking (n=3).
2.16 Experiment 3: Does (-)-morphine or M3G cause activation of NFκB in cultured adult rat CNS endothelial cells?
Cultures of CNS endothelial cells were grown to confluence in 24 well microplates. The cells were then stimulated with (-)-morphine (0, 0.01μM, 0.1μM, 1 μM, 10 μM or 100 μM) or M3G (0, 0.01 μM, 0.1 μM, or 1 μM) for 0.5 hr, 2 hr, or 4 hr, and then processed for In-Cell Western blots to measure levels of activated NFκB. For each study, n = 6 wells per condition were used.
2.17 Experiment 4: Do the known opioid receptor antagonists CTAP and nalmefene antagonize TLR4 in cultured adult rat endothelial cells?
Using the HEK293-hTLR4 assay, CTAP and nalmefene were tested for their ability to block LPS-induced SEAP expression by incubating a range of concentrations for each (0, 0.1 nM, 1 nM, 10 nM, 100 nM, 1 μM, or 10 μM) with 0.1 ng/ml or 1 ng/ml LPS for 24 hr in aCSF, then measuring SEAP levels. Additionally, an in silico docking analysis was conducted to model the binding of CTAP and nalmefene to TLR4, MD-2, the TLR4/MD-2 dimer, and the complete signaling heterodimer of TLR4 and MD-2. For each in vitro study, n = 6 wells per condition were used.
2.18 Experiment 5: Does (-)-morphine, (+)-morphine and/or M3G upregulate proinflammatory gene expression in cultured adult rat CNS endothelial cells?
This experiment measured changes in TNFα and COX-2 mRNA expression in cultured CNS endothelial cells in response to a 4 hr stimulation with a range of concentrations of (-)-morphine and (+)-morphine (0, 0.01 μM, 0.1 μM, 1 μM, 10 μM, or 100 μM) or M3G (0, 0.001 μM, 0.01 μM, 0.1 μM, or 1 μM). In separate studies, cultured CNS endothelial cells were treated with 0, 10 nM, or 100 nM nalmefene for 1 hr prior to 4 hr treatment with 0 or 10 μM (-)-morphine; or the cells were treated with 0 or 10 ng/ml LPS-RS for 1 hr prior to 4 hr treatment with M3G (0, 0.001 μM, 0.01 μM, 0.1 μM, or 1 μM). In separate studies, cultured CNS endothelial cells were treated with 0 or 30 μM of H-89 (a PKA inhibitor that acts by competitive inhibition of the ATP binding site (Engh et al., 1996)) for 1 hr prior to 4 hr treatment with a range of concentrations of (-)-morphine (0, 0.01 μM, 0.1 μM, 1 μM, 10 μM, or 100 μM). For each study, n = 6 wells per condition were used.
2.19 Experiment 6: Do adult rat CNS endothelial cells stimulated in vitro with (-)-morphine or M3G cause allodynia when adoptively transferred to rats via lumbar intrathecal injection?
CNS endothelial cells cultures were prepared from donor Lewis rats (an inbred strain, used to ensure syngeneic recipients of adoptively transferred cells). Recipient Lewis rats were habituated to the von Frey testing environment and baseline hindpaw sensitivity was measured. Confluent cultures of CNS endothelial cells (from Lewis rats) in 10 cm dishes were stimulated with saline, 0.1 μM M3G, 0.1 μM M3G and 10 ng/mL LPS-RS, or 10 μM (-)-morphine for 18 hr, a time-point chosen to be within the 24 hr time window used for release studies in Experiment 5. The next morning, the rats received pre-injection baseline testing while the cells were prepared for injection. The cells were washed in PBS then injected (1,000 cells in 10 μL of PBS, as described above) and the rats were re-tested at 1, 2, 3 and 24 hr post-injection for PBS and (-)-morphine treated cells, and 1, 2, 3, 4, 6, 24 and 48 hr post-injection for saline, M3G and M3G/LPS-RS treated cells.
2.20 Experiment 7: Does epicortical suffusion of M3G cause vasodilation?
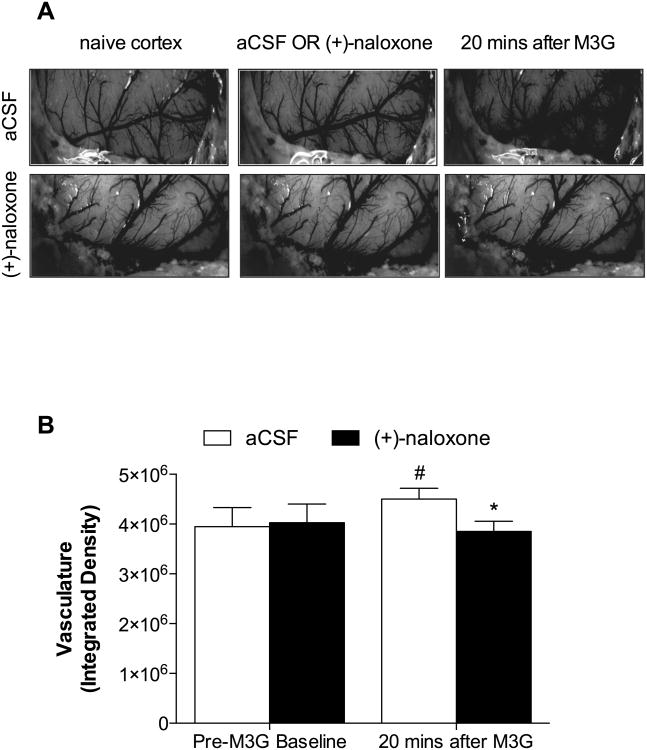
Images of naïve cortex were taken 10 min post-surgery, 10 min following epicortical suffusion of either aCSF or a TLR4 antagonist (+)-naloxone (4 μg/ml, 100 μl dose) (Hutchinson et al., 2008c, Hutchinson et al., 2012, Lewis et al., 2012), and 20 min following epicortical suffusion of M3G (100 μg/ml, 100 μl dose), alone (left hemisphere) or after pretreatment with (+)-naloxone (right hemisphere).
Images were obtained with a Nikon E5000 (Nikon Inc., Tokyo, Japan), using a microscope MDC-A relay adapter lens (UR-E6), light levels were held constant at 7,200 Ix, and exposures were taken at ISO 100, f4, 1/16. Images were opened with ImageJ and the number of pixels and the average pixel mean gray values were measured and multiplied, giving an integrated densitometric measure. Two measurements were taken of the macrovasculature and microvasculature (middle cerebral artery and the inferior cerebral veins); the measurements were then averaged to obtain a single integrated density value per rat.
2.21 Experiment 8: Do the putative glial inhibitors propentofylline or AV1013 inhibit adult rat CNS endothelial cell activation in response to LPS or M3G?
CNS endothelial cell cultures were pre-treated for 1 hr with propentofylline (100 μM), AV1013 (100 μM), or vehicle control (culture medium). These concentrations were chosen based on previously published in vitro experiments (Ohkubo et al., 1991, Cho et al., 2010, Matsui et al., 2010). LPS (10 ng/ml), M3G (0.1 μM), or vehicle control (culture medium) were then added for an additional 4 hr, and the cells were processed for analysis of TNFα gene expression. n = 6 wells per condition were used.
3. Results
3.1 Experiment 1: Primary adult rat CNS endothelial cells in vitro express functional TLR4 receptors
There are no prior reports in the literature of functional TLR4 expression in rat CNS endothelial cells, at any age. Transcription of TNFα mRNA is a known output of TLR4 signaling, while PGE2 release from brain endothelial cells is a major component of the CNS response to systemic gram-negative bacterial infection (Beutler et al., 2006, Quan, 2008). These two end-points were therefore used to assess whether the cultured CNS endothelial cells used in the present experiments exhibit functional TLR4 signaling and output.
Analysis of cultured adult rat CNS endothelial cells by real-time PCR revealed basal expression of TLR4 mRNA (Figure 1A). As the primers were designed to span exon - intron boundaries, this reflects true expression of TLR4 mRNA and excludes the possible confound of genomic DNA contamination. Expression of TLR4 mRNA was not changed by acute exposure (4 hr) to the classic TLR4 agonist LPS (Figure 1A; F2,8 = 2.8, P = 0.09). To test whether these cells exhibit functional responsivity to TLR4 signaling, cultured CNS endothelial cells were treated with LPS and assayed for changes in TNFα mRNA expression and release of PGE2, after 4 and 24 hr, respectively. LPS elicited a concentration-dependent increase in TNFα mRNA expression (Figure 1B; LPS concentration × LPS-RS: F2,18 = 5.3, P < 0.05; LPS concentration: F2,18 = 19.2, P < 0.001; LPS-RS: F1,18 = 18.7, P < 0.001). Pre-incubation with the TLR4 antagonist LPS-RS significantly attenuated LPS-induced TNFα mRNA expression at 1 (P < 0.05) and 10 ng/ml (P < 0.001). LPS also elicited a concentration-dependent increase in PGE2 release (Figure 1C; F3,20 = 35.0, P < 0.001). Pre-treatment with 10 ng/ml LPS-RS significantly attenuated PGE2 release induced by 100 ng/ml LPS (a concentration that elicited robust but not maximal PGE2 release) (Figure 1D; F2,14 = 118.9, P < 0.001). These data therefore provide evidence that functional TLR4 signaling is present on adult rat CNS endothelial cells.
Figure 1.
Primary adult rat CNS endothelial cells in vitro express functional TLR4 receptors. (A) Primary adult rat CNS endothelial cells express TLR4 mRNA, which is not altered by a 4 hr incubation with 1 ng/ml or 10 ng/ml LPS. (B) A 4 hr incubation with 1 ng/ml or 10 ng/ml LPS produces significant upregulation of TNFα mRNA, which is blocked by 1 hr pre-treatment with 10 ng/ml LPS-RS. (C) LPS concentration-dependently upregulates PGE2 release during a 24 hr incubation. (D) A 1 hr pre-treatment with 10 ng/ml LPS-RS inhibited the upregulated PGE2 release in response to 100 ng/ml LPS. Data are presented as mean ± SEM; n = 6 wells per condition. *P < 0.05, ** P < 0.01, ***P < 0.001, LPS-treated vs. treatment-matched controls; ##P < 0.01, LPS + LPS-RS-treated vs. LPS-treated cells.
3.2 Experiment 2: M3G binds MD-2
To determine whether M3G, the predominant opioid receptor inactive metabolite of morphine, could induce TLR4 signaling, in silico docking simulations and a binding assay were performed.
3.2.1 In silico docking simulations: M3G and (-)-morphine
Putative TLR4 agonists M3G and (-)-morphine displayed similar docking energies to TLR4 and MD-2 alone, with both ligands docking to the LPS binding domain of MD-2. Interestingly, M3G demonstrated profoundly reduced docking energy requirements (i.e. more readily docks) for state 3 (TLR4/MD-2 dimer) and state 4 (TLR4/MD-2 heterodimer) (Figure 2A) (Park et al., 2009). The same progression of lowering docking energies was not observed for (-)-morphine, indicating that morphine does not show a preference for the different conformations of TLR4 and will interact equally with them. Upon further examination of the docking results sequence from state 2 (MD-2 alone) to state 3 (MD-2/TLR4 dimer) to state 4 (TLR4/MD-2 heterodimer) for M3G, it appears that M3G may be guided down a docking energy gradient to its final state 4 docking location nestled between the two TLR4 proteins that for the heterodimer with MD2 (Figure 2B). While (-)-morphine does not have the same docking energy gradient, suggesting decreased affinity, compared to M3G, its state 4 docking conformation is also located in adjacent locations between the two TLR4 proteins in the functional heterodimer (data not shown).
Figure 2.
M3G binds to MD-2. (A) (-)-Morphine and M3G displayed similar docking energies to TLR4 and MD-2 alone. M3G, but not (-)-morphine, demonstrated profoundly reduced docking energies for the TLR4/MD-2 dimer and TLR4/MD-2 heterodimer. (B) Visualization of the docking conformations of M3G in panel C from state 2 to state 4 chaperoning the M3G into its final preferred docking conformation. (C) On a binding assay, M3G binds to MD-2 and cause the quenching of MD-2 intrinsic fluorescence at a dissociation constant (KD) of 1.5 ± 0.3 μM. By contrast, the negative control roxithromycin shows no MD-2 binding activity. (D) M3G shows negligible binding to the negative control Protein A protein.
3.2.2 Biophysical characterization of M3G binding to MD-2
To validate the in silico docking, an M3G/MD-2 binding assay was performed. M3G binds to MD-2 and causes the quenching of MD-2 intrinsic fluorescence (Figure 2C). By fitting the curve to a one-site binding model, a dissociation constant (KD) of 1.5 ± 0.3 μM was obtained, which is stronger than the affinity of morphine binding to MD-2 (4.3 ± 3.3 μM) (Wang et al., 2012). As a comparison, roxithromycin, a compound used as a negative control in previous report (Resman et al., 2008), shows no MD-2 binding activity. Furthermore, M3G shows negligible binding to the negative control Protein A (Figure 2D). Taken together, these data suggest M3G specifically binds to MD-2, indicating that M3G may be a more potent TLR4 signaling modulator than morphine.
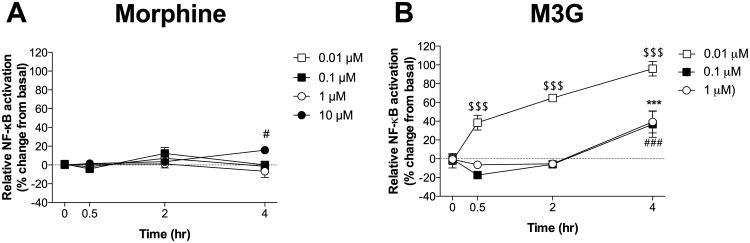
3.3 Experiment 3: M3G induces greater NF κB activation than (-)-morphine in adult rat CNS endothelial cells in vitro
NFκB has been implicated in the transcriptional regulation of a wide variety of downstream targets, including cytokines, growth factors, and adhesion proteins, and is a downstream target of TLR4 signaling (Muller-Ladner et al., 2002, Kraus et al., 2003, Law et al., 2004, Kawai and Akira, 2006, Hutchinson et al., 2011). Hence, NFκB activation was assessed as a marker of TLR4 activation by (-)-morphine and M3G.
3.3.1 (-)-Morphine-induced activation of NF κB
In general, (-)-morphine produced at most small increases in NFκB activation (Figure 3A). There was a significant interaction between time and (-)-morphine concentration (F9,60 = 2.2, P < 0.05), but no main effects of time (F3,60 = 2.6, P = 0.06) or (-)-morphine concentration (F3,20 = 2.1, P = 0.2). Only 10 μM (-)-morphine caused NFκB activation, which became reliable at 4 hr (P < 0.05).
Figure 3.
(-)-morphine and M3G cause activation of the transcription factor NF-κB in adult rat CNS endothelial cells in vitro. (A) Significant NFκB activation is observed in CNS endothelial cells after 4 hr exposure to 10 μM (-)-morphine. (B) 0.01 μM M3G produces significant NFκB activation observable after 0.5 hr, which continues to increase over time through at least 4 hr. In contrast, 0.1 μM M3G and 1 μM M3G produce a slightly delayed effect, with NFκB activation observed after 4 hr. Data are presented as mean ± SEM; n = 6 wells per condition. $$$P < 0.001 vs. baseline (time = 0 min) (0.01 μM); ***P < 0.001 vs. baseline (time = 0 min) (0.1 μM); #P < 0.05, ###P < 0.001 vs. baseline (time = 0 min) (10 μM).
3.3.2 M3G-induced activation of NFκB
M3G induced greater NFκB activation than did (-)-morphine at equimolar concentrations (Figure 3B). There was a significant interaction between time and M3G concentration (F6,45 = 7.7, P < 0.001) as well as main effects of time (F3,45 = 47.1, P < 0.001) and M3G concentration (F2,15 = 36.3, P < 0.001). The observed effects did not exhibit classic concentration-dependency. The most effective concentration of M3G, 0.01 μM, elevated NF κB activation over 0.5, 2, and 4 hr (P < 0.001). The 2 higher concentrations of M3G tested here, 0.1 μM and 1 μM, elicited no significant change at 0.5 or 2 hr, but did significantly increase NFκB activation at 4 hr (P < 0.001). Therefore, NFκB is more potently induced by M3G than (-)-morphine.
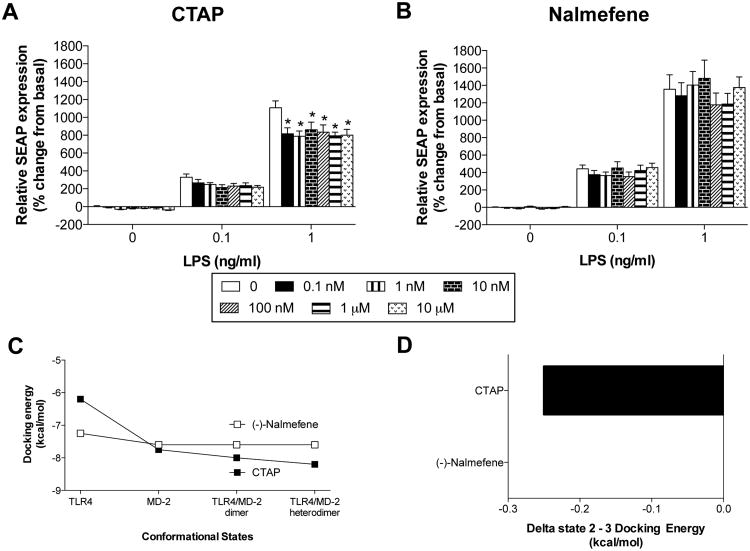
3.4 Experiment 4: The MOR antagonist CTAP, but not nalmefene, inhibits LPS-induced TLR4 activation in TLR4 over-expressing HEK293 cells
As both M3G and (-)-morphine signal through TLR4, but only (-)-morphine signals through MOR, it is possible that the divergent effect on NFκB activation observed in Experiment 3 is due to MOR suppression of TLR4 signaling, as suggested in several previous studies in neutrophils, monocytes and macrophages (Roy et al., 1998, Welters et al., 2000). To selectively assess the role of classical MOR signaling in response to (-)-morphine in endothelial cells, an opioid receptor antagonist was needed that does not also modulate TLR4 signaling. Hence, the opioid receptor antagonists CTAP and (-)-nalmefene were selected (MOR selective and universal, respectively (Michel et al., 1985, Kramer et al., 1989)), and tested for TLR4 antagonism. CTAP was chosen for study here as prior work showed it to block (-)-morphine-induced microglial migration and this blockade by CTAP was used to conclude mediation by MOR (Horvath and DeLeo, 2009). (-)-Nalmefene was chosen because its stereoisomer, (+)-nalmefene, has been shown to be inactive at TLR4 using both an in silico docking analysis and the same HEK293-hTLR4 assay as used here (Hutchinson et al., 2010b). As ongoing studies in our laboratory have documented that (+)- and (-)-isomers do not always exert the same effects on TLR4 signaling (Wang et al., unpublished observations), it could not be assumed that (-)-nalmefene would mirror the TLR4 inhibition we previously reported for (+)-nalmefene (Hutchinson et al., 2010b).
3.4.1. In vitro HEK293-hTLR4 antagonist assay
In this assay, there was no interaction between CTAP concentration and LPS concentration (F12,105 = 1.7, P = 0.09), but significant main effects of LPS concentration (F2,105 = 731.3, P < 0.001) and CTAP concentration (F6,105 = 4.2, P < 0.001). CTAP tended to inhibit SEAP expression induced by 0.1 ng/ml LPS, though this did not reach statistical significance. At an LPS concentration of 1 ng/ml, CTAP did significantly inhibit LPS-induced SEAP expression at all concentrations tested (P < 0.001; Figure 4A). In contrast, there was no interaction between nalmefene concentration and LPS concentration (F12,167 = 0.4, P = 0.9), while there was a main effect of LPS concentration (F2,167 = 369.2, P < 0.001), but not nalmefene concentration (F6,165 = 0.8, P = 0.5). Nalmefene did not significantly inhibit LPS-induced SEAP expression at any concentration of LPS or nalmefene tested (Figure 4B). These data therefore suggest that, unlike CTAP, (-)-nalmefene is not a TLR4 antagonist and therefore appropriate for use to block MOR but not TLR4.
Figure 4.
CTAP blocks LPS-induced TLR4 activation in TLR4 over-expressing HEK293 cells, but nalmefene does not. (A) When co-incubated with 1 ng/ml LPS, a range of CTAP concentrations (0.1 nM – 10 μM) inhibited LPS-induced SEAP expression. (B) When co-incubated with 1 ng/ml LPS, a range of nalmefene concentrations (0.1 nM – 10 μM, in multiples of ten) had no effect on LPS-induced SEAP expression. (C) Nalmefene and CTAP displayed different docking energies when docked to TLR4 alone but not MD-2 alone. However, upon examination of the protein dimers, CTAP, but not nalmefene, demonstrated profoundly reduced docking energies for the TLR4/MD-2 dimer and TLR4/MD-2 heterodimer. (D) Both nalmefene and CTAP docking occupied the LPS binding domain of MD-2 in spatially overlapping conformations as the agonists M3G and (-)-morphine occupied in state 2. Therefore, the only differentiation between these two ligands through this method was the difference between these two was the state 2 to state 3 binding energy difference presented here. Data are presented as mean ± SEM; n = 6 wells per condition. P < 0.001 vs. treatment-matched control.
3.4.2. In silico docking simulations: CTAP and nalmefene
The TLR4/MD-2 docking capacity of CTAP and nalmefene were also examined. Both ligands docked with some preference for MD-2 (state 2) rather than native TLR4 (state 1; Figure 4C), although this was much more profound for CTAP than (-)-nalmefeme. In both cases the ligands occupied the LPS binding domain of MD-2 in spatially overlapping conformations as the agonists M3G and (-)-morphine occupied in state 2 (MD-2 alone). CTAP displayed the greatest difference between in vitro active and inactive cases was the state 2 (MD-2 alone) to state 3 (MD-2/TLR4 dimer) binding energy difference with the active ligand CTAP having decreased binding energy resulting from the transition, while nalmefene had no change in energy requirements (Figure 4D). These data suggest that similar to M3G, CTAP requires less energy to dock to the complex heterodimer and thus the active form of the complex.
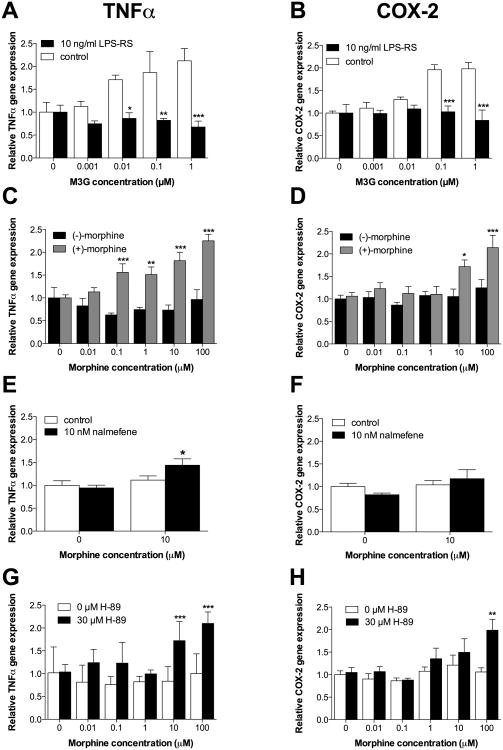
3.5 Experiment 5: MOR signaling masks TLR4-dependent proinflammatory gene expression in a PKA dependent fashion in adult rat CNS endothelial cells in vitro. Implications for morphine versus M3G results
In Experiment 3, M3G induced greater NFκB activation, compared to equimolar (-)-morphine. One explanation for the differential effects is that (-)-morphine, signaling through TLR4 and MOR, elicits MOR-mediated modulation of TLR4 activation, as described above. Based on such a premise, one would predict that a) sole TLR4 agonists (no MOR activity), should induce a greater proinflammatory response in CNS endothelial cells, than dual TLR4 and MOR agonism; b) antagonism of MOR, but not TLR4 (e.g., using (-)-nalmefene), should unmask (-)-morphine-induced proinflammatory signaling in CNS endothelial cells, but not effect the M3G-induced proinflammatory responses, and c) inhibition of (-)-morphine-induced activation of PKA (Borner et al., 2009) which in turn suppresses NFκB activity (Minguet et al., 2005) should unmask (-)-morphine-induced proinflammatory signaling in CNS endothelial cells. As in Experiment 1, TNFα mRNA transcription was assayed as an output of TLR4 signaling. COX-2 mRNA expression was also measured given the significance of PGE2 release from brain endothelial cells in the context of infection and the importance of COX-2 mRNA upregulation for generating an inflammatory PGE2 response (Quan, 2008). M3G and (+)-morphine have previously been shown to initiate TLR4 signaling, but not that of MOR, (Hutchinson et al., 2010a, Hutchinson et al., 2010b, Lewis et al., 2010). Hence, M3G and (+)-morphine were used to test the hypothesis that TLR4 signaling would induce greater upregulation of TNFα and COX-2 mRNA expression than concurrent MOR and TLR4 signaling in response to (-)-morphine. CNS endothelial cells were also pre-treated with LPS-RS in a separate experiment to confirm prior reports that M3G-induced upregulation of TNFα and COX-2 mRNA expression occurs via TLR4 signaling. The universal opioid receptor antagonist, (-)-nalmefene, was shown to not antagonize TLR4 in Experiment 4. Hence, CNS endothelial cells were pre-treated with (-)-nalmefene to test the hypothesis that blocking MOR signaling would unmask upregulation of TNFα and COX-2 mRNA expression in response to (-)-morphine. Finally, CNS endothelial cells were pre-treated with H-89, a PKA inhibitor that acts by competitive inhibition of the ATP binding site, to test the hypothesis that blocking PKA signaling would unmask upregulation of TNFα and COX-2 mRNA expression in response to (-)-morphine (Engh et al., 1996).
3.5.1 Comparison of (-)-morphine-, (+)-morphine and M3G-induced changes in gene expression
The response profile of M3G was first defined, followed by a separate study that compared responses induced by (-)-morphine vs. (+)-morphine. Separate studies were required given limitations of cell availability. (+)-morphine was also included here as a comparison to M3G given that (+)-morphine is also a MOR inactive, TLR4 agonist (Hutchinson et al., 2010a).
Cultured adult rat CNS endothelial cells were incubated for 4 hr with M3G concentrations ranging from 0.001 – 1 μM, preceded by 1 hr pre-incubation with 0 (vehicle) or 10 ng/ml LPS-RS. There was a concentration dependent reliable increase in both TNFα mRNA (Figure 5A; M3G concentration × LPS-RS: F4,32 = 3.6, P < 0.05; M3G concentration: F4,32 = 2.0, P = 0.12; LPS-RS: F1,8 = 53.9, P < 0.001) and COX2 mRNA (Figure 5B; M3G concentration × LPS-RS: F4,32 = 6.8, P < 0.001; M3G concentration: F4,32 = 4.8, P < 0.01; LPS-RS: F1,8 = 71.28, P < 0.001). Pre-incubation with LPS-RS induced a significant suppression of M3G-induced TNFα expression at 0.01 (P < 0.05), 0.1 (P < 0.01), and 1 μM (P < 0.001), and COX2 expression at 0.1 (P < 0.001), and 1 μM (P < 0.001), compared to control. TNFα gene expression did not mirror the dose-response profile of NFκB found in Figure 3B, which may be explained by induction of additional transcription factors (Means et al., 2000), not assayed herein. These data support that M3G induces pro-inflammatory mediator expression in endothelial cells in a TLR4 dependent fashion.
Figure 5.
(-)-morphine signaling through classical opioid receptors masked upregulation of proinflammatory gene expression, while M3G signaling via TLR4 caused upregulation of proinflammatory gene expression, in adult rat CNS endothelial cells in vitro. (A) A 4 hr exposure to a range of M3G concentrations elevated TNFα gene expression; these effects are blocked by 1 hr pre-treatment with 10 ng/ml LPS-RS. (B) A 4 hr exposure to a range of M3G concentrations elevated COX2 gene expression; these effects are blocked by 1 hr pre-treatment with 10 ng/ml LPS-RS. (C) A 4 hr incubation with a range of (+)-morphine concentrations elevated TNF-α gene expression, in contrast to (-)-morphine. (D) A 4 hr incubation with a range of (+)-morphine concentrations elevated COX2 gene expression, in contrast to (-)-morphine. (E) A 1 hr pre-treatment with 10 nM nalmefene unmasked a significant upregulation of TNFα gene expression induced by 4 hr treatment with 10 μM (-)-morphine. (F) A 1 hr pre-treatment with 10 nM nalmefene did not unmask a significant upregulation of COX2 gene expression induced by 4 hr treatment with 10 μM (-)-morphine. (G) A 1 hr pre-treatment with H-89 unmasked a significant upregulation of TNFα gene expression induced by 4 hr treatment with a (-)-morphine concentration range. (H) A 1 hr pre-treatment with H-89 unmasked a significant upregulation of COX2 gene expression induced by 4 hr treatment with a (-)-morphine concentration range. Data are presented as mean ± SEM; n = 6 wells per condition. *P < 0.05, **P < 0.05, ***P < 0.001 vs. treatment-matched control.
In the second study, primary adult rat CNS endothelial cells were incubated for 4 hr with (-)-morphine. Here, the response was compared to that induced by (+)-morphine. This was done to provide to define whether a second, structurally distinct non-opioid TLR4 agonist would produce a similar effect as observed for M3G, above. For both morphine isomers, concentrations used ranged from 0.01 - 100 μM. There was a significant effect for TNFα (Figure 5C; Concentration × Drug: F5,45 = 5.4, P < 0.001; Concentration: F5,45 = 5.0, P < 0.001; Drug: F1,9 = 58.6, P < 0.001) and COX2 (Figure 5D; Concentration × Drug: F5,40 = 3.1, P < 0.05; Concentration: F5,40 = 7.3, P < 0.001; Drug: F1,8 = 9.7, P < 0.05). (+)-Morphine induced significant elevations in TNFα mRNA at 0.1 (P < 0.001), 1 (P < 0.01), 10 and 100 μM (P < 0.001), compared to (-)-morphine. (-)-Morphine, in contrast failed to increase TNFα gene expression at any concentration tested. (+)-Morphine also induced significant elevation in COX2 gene expression at 10 (P < 0.05) and 100 μM (P < 0.001). This contrasts (-)-morphine, which again had little to no effect on this measure. The failure of (-)-morphine, along with the efficacy of both (+)-morphine and M3G (above), suggests that the proinflammatory effects observed occur when TLR4, not MOR, is activated.
3.5.2 (-)-Morphine-induced changes in gene expression after nalmefene pretreatment
Based on the results of Experiment 4, which defined (-)-nalmefene as an opioid antagonist that did not inhibit TLR4, (-)-nalmefene was employed to explore whether (-)-morphine induced gene expression in the face of MOR inhibition. As (-)-morphine activates TLR4 in addition to MOR, this strategy was followed in order to uncover its TLR4-mediated effects. Cultures of primary adult rat CNS endothelial cells were incubated with 10 nM nalmefene for 1 hr prior to a 4 hr stimulation with 10 μM (-)-morphine. There was a significant elevation in TNFα mRNA (Figure 5E; (-)-Morphine × Nalmefene: F1,10 = 6.3, P < 0.05; (-)-Morphine: F1,10 = 16.0, P < 0.05; Nalmefene: F1,10 = 1.1, P = 0.3), but not COX2 mRNA (Figure 5F; (-)-Morphine × Nalmefene: F1,10 = 6.3, P < 0.05; (-)-Morphine: F1,10 = 16.0, P < 0.05; Nalmefene: F1,10 = 1.1, P = 0.30). The effect on TNFα mRNA was accounted for by (-)-nalmefene significantly elevating (-)-morphine-induced TNFα gene expression, compared to control (P < 0.05). This result suggests that there is an interaction between intracellular MOR and TLR4 signaling cascades in adult rat CNS endothelial cells.
3.5.3 (-)-Morphine-induced changes in gene expression after H-89 pretreatment
As an additional means of exploring the effect of MOR signaling in adult rat CNS endothelial cells, H-89 was employed as a PKA inhibitor. This was done as MOR agonists (but not TLR4 agonists) activate PKA, so a change in the response to (-)-morphine in the presence of H-89 would reflect blockade of MOR signaling. Cultures of primary CNS endothelial cells were incubated with 30 μM H-89 for 1 hr prior to a 4 hr stimulation with (-)-morphine concentrations ranging from 0.01 - 100 μM. There was a significant elevation of both TNFα (Figure 5G; (-)-Morphine × H-89: F5,48 = 3.8, P < 0.001; (-)-Morphine: F5,48 = 5.1, P < 0.001; H-89: F1,48 = 34.8, P < 0.001), and COX2 (Figure 5H; (-)-Morphine × H-89: F5,40 = 2.6, P < 0.05; (-)-Morphine: F5,40 = 5.6, P < 0.001; H-89: F1,8 = 4.6, P = 0.06). This effect was accounted for, not by the actions of (-)-morphine alone, but rather by (-)-morphine in the presence of H-89. The PKA inhibitor H-89 unmasked (-)-morphine-induced TNFα gene expression at 10 and 100 μM (P < 0.001), and COX2 gene expression at 100 μM (P < 0.01), compared to vehicle control. These results indicate that PKA activation, mostly likely as a result of (-)-morphine signaling through MOR, suppresses pro-inflammatory mediator induction.
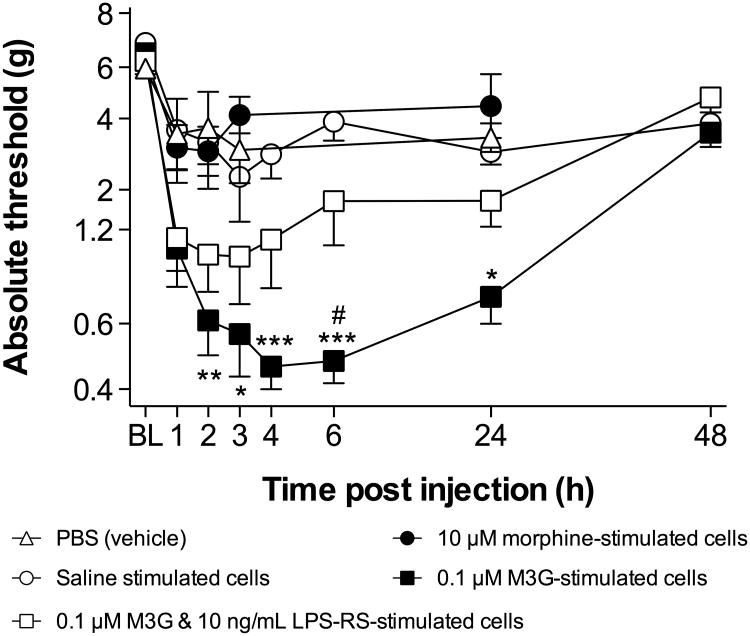
3.6 Experiment 6: M3G- but not LPS-RS-costimulated- or (-)-morphine-stimulated adult rat CNS endothelial cells produce hindpaw tactile allodynia when injected into the intrathecal space surrounding the lumbar enlargement
In addition to measuring CNS endothelial cell responsivity to opioids in vitro, we sought to address whether opioid-stimulated CNS endothelial cells could have an impact on behavior in vivo. To assess this, alterations in sensory processing were assessed to parallel similar adoptive transfer experiments previously performed using microglia, astrocytes and peripheral immune cells (Tsuda et al., 2003, Coull et al., 2005, Narita et al., 2006, Grace et al., 2011a, Zhang et al.). A simple yet powerful way to test this is to stimulate cultured cells, inject them into the lumbar intrathecal space, and measure how this affects hindpaw sensitivity to tactile stimuli. Based on the in vitro data presented above, we hypothesized that CNS endothelial cells treated with (-)-morphine would not alter hindpaw tactile sensitivity, whereas CNS endothelial cells treated with M3G would, and that the effects of M3G would be blocked by co-treatment with LPS-RS.
Primary CNS endothelial cells were stimulated for 18 hr with saline, 10 μM (-)-morphine, 0.1 μM M3G, or 0.1 μM M3G and 10 ng/mL LPS-RS. The (-)-morphine and M3G concentrations were chosen to be consistent with Experiment 5. After the stimulation in vitro, cells were washed in PBS, and 1,000 cells suspended in 10 μl of PBS were injected into the intrathecal space surrounding the lumbar enlargement. Use of fresh PBS as the vehicle for injection ensured that any effects on hindpaw tactile sensitivity were not due to signaling molecules released during the stimulation in vitro, but rather to local effects of the injected cells occurring after injection.
There were no differences between treatment groups at baseline. There was a significant interaction between time and treatment (F24,174 = 4.1, P < 0.001), and significant main effects of time (F6,174 = 11.0, P < 0.001) and treatment (F4,29 = 8.3, P < 0.001; Figure 6). Injection of PBS alone produced no significant effect, although a mild and transient decrease in absolute paw withdrawal threshold was noted at 1 hr post-injection. Injection of saline- or (-)-morphine-stimulated CNS endothelial cells also produced no significant effect on withdrawal thresholds. In contrast, injection of M3G-stimulated CNS endothelial cells produced a significant increase in tactile allodynia from 2 to 24 hrs post injection, compared to those animals receiving PBS alone and saline- or (-)-morphine-stimulated cells (P < 0.05). Injection of endothelial cells co-treated with M3G and LPS-RS caused an increase in tactile allodynia that was not statistically distinguishable from saline-stimulated cells, but was significantly attenuated, compared to M3G-stimulated cells at 6 hr post injection (P < 0.05).
Figure 6.
M3G- but not (-)-morphine-stimulated CNS endothelial cells produce hindpaw tactile allodynia when adoptively transferred into the intrathecal space surrounding the lumbar enlargement. Allodynia is evident within 1 hr post-injection. Data are presented as mean ± SEM; n = 8 rats per group. *P< 0.05, **P< 0.01, ***P < 0.001 M3G stimulated cells vs. saline stimulated cells; #P < 0.05 M3G stimulated cells vs. M3G + LPS-RS stimulated cells.
3.7 Experiment 7: Epicortical suffusion of M3G causes vasodilation
Experiment 6 demonstrated that intrathecal adoptive transfer of M3G- but not (-)-morphine-stimulated endothelial cells induced a behavioral response indicative of proinflammation, given the pronociceptive roles of other non-neuronal cells (Grace et al., 2011b, Grace et al., 2014). An additional experiment was performed to determine whether CNS endothelial cells stimulated with M3G in vivo could impact endothelial cell function. Vasodilation was selected as a classical inflammatory in vivo response of endothelial cells. Therefore, a bilateral craniotomy was performed and the exposed cortex was suffused unilaterally with vehicle (artificial CSF; aCSF) or (+)-naloxone, followed by bilateral M3G. The TLR4 antagonist (+)-naloxone (Hutchinson et al., 2008c, Hutchinson et al., 2012, Lewis et al., 2012) was selected for this experiment, as LPS-RS has poor diffusion in vivo.
There were no significant differences between baseline values and vehicle hemispheres (Fig 7A; P = 0.881), so the data were pooled for comparison following M3G application. The results revealed that vasodilation (Fig 7B) was significantly increased 20 minutes following M3G application in the vehicle hemisphere, compared to the hemisphere pretreated with (+)-naloxone (P < 0.05). At 20 minutes post-M3G, the (+)-naloxone pretreated hemisphere did not statistically differ from the pooled baseline (P = 0.625), compared to the vehicle hemisphere (P < 0.05).
Figure 7.
Epicortical suffusion of M3G induces vasodilation. Representative images following M3G application in (A) pretreated hemisphere with aCSF vehicle (top panel) or (+)-naloxone (bottom panel, showing markedly increased vasodilation and enlarged micro/macro vasculature in the vehicle hemisphere following M3G treatment. (B) Integrated density of the vasculature following M3G application, significant increases were found in the M3G-treated hemisphere compared to baseline and (+)-naloxone treated hemispheres. Data are presented as mean ± SEM; n = 8 rats per group. #P < 0.05 vs. combined baseline; *P < 0.05 vs. aCSF 20 mins after M3G.
3.8 Experiment 8: Adult rat CNS endothelial cell activation is suppressed by drugs (propentofylline, AV1013) commonly thought of as glial inhibitors
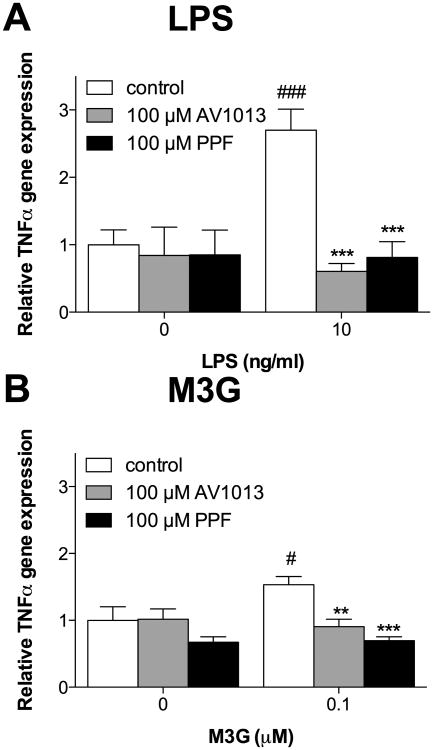
One question that naturally arises is whether adult rat CNS endothelial cells may potentially be contributing to phenomena commonly attributed to microglia and/or astrocytes alone. This is an important question given the breadth of studies utilizing drugs widely referred to as glial inhibitors or immunomodulators. These include phosphodiesterase (PDE) inhibitors (e.g., propentofylline) and macrophage MIF inhibitors (e.g., AV1013, a non-PDE-inhibitor derivative of ibudilast) (Cho et al., 2010, Sweitzer and De Leo, 2011). Both inhibit reactive gliosis (Cho et al., 2010). Here, the two compounds were tested for their ability to block activation of adult rat CNS endothelial cells in response to TLR4 agonists, LPS and/or M3G. (-)-Morphine was not tested due to its negligible induction of immune signaling in CNS endothelial cells above. AV1013 was used instead of the parent compound ibudilast (AV411) because the two compounds are mechanistically similar, but AV1013 is both soluble in aqueous buffer and, importantly, is devoid of PDE inhibition. Thus, unlike ibudilast (AV411), AV1013 avoids this overlap in mechanism of action with propentofylline so to provide a broader view of how glia-targeting compounds may affect adult rat CNS endothelial cells.
In adult rat CNS endothelial cells, significant effects for TNFα mRNA expression were observed in the presence of LPS (10 ng/ml) (Figure 8A; LPS × Drug: F3,29 = 6.1, P < 0.01; LPS: F1,29 = 6.3, P < 0.05; Drug: F3,29 = 8.6, P < 0.001). LPS significantly elevated TNFα mRNA expression (P < 0.001). No significant effects of immunomodulators on basal TNFα expression were observed, but LPS-induced TNFα expression was significantly attenuated by pre-treatment with 100 μM AV1013 or 100 μM propentofylline (P < 0.001). Significant effects for TNFα mRNA expression were also observed in the presence of M3G (0.1 μM) (Figure 8B; M3G × Drug: F2,23 = 3.6, P < 0.05; M3G: F1,22 = 2.1, P = 0.16; Drug: F2,23 = 10.7, P < 0.001). Like LPS, M3G significantly elevated TNFα mRNA expression (P < 0.05). No significant effects of immunomodulators on basal TNFα expression were observed. However, M3G-induced upregulation of TNFα mRNA expression was abolished by pre-treatment with 100 μM AV1013 (P < 0.01), 100 μM propentofylline (P < 0.001). Hence, the action of these drugs is extended to endothelial cells. This suggests that a re-evaluation of the literature is needed where use such compounds leads to the conclusion that glia, rather than other cell types, underlie their effects.
Figure 8.
Propentofylline and AV1013 inhibited upregulation of TNFα mRNA in response to LPS and M3G in adult rat CNS endothelial cells in vitro. (A) Cells treated with vehicle for 1 hr, followed by 10 ng/ml LPS for 4 hr, exhibit significant upregulation of TNF-α mRNA. This upregulation is completely blocked by a 1 hr pre-treatment with AV1013 or propentofylline. (B) Cells treated with vehicle for 1 hr, followed by 0.1 μM M3G for 4 hr, exhibit significant upregulation of TNF-α mRNA. This upregulation is completely blocked by a 1 hr pre-treatment with AV1013 or propentofylline. Data are presented as mean ± SEM; n = 6 wells per condition. #P< 0.05, ###P < 0.001 vs. 0 concentration of LPS or M3G; **P < 0.01, *P < 0.05, ***P < 0.001 vs. vehicle-pre-treatment with LPS or M3G treatment.
4. Discussion
The experiments presented here document how adult rat CNS endothelial cells respond to (-)-morphine and M3G in vitro and in vivo, and the receptors that may be involved. The data from Experiment 1 show that primary CNS endothelial cells, obtained via the isolation method of Perriere et al. (2005), express functional TLR4 receptors. Using in silico docking and the biophysical characterizations, we demonstrate that M3G binds to the accessory protein MD-2. These data were confirmed in vitro where M3G caused significant NFκB activation, which did not follow a classical concentration response. In contrast, (-)-morphine elicited little NFκB activation, which may reflect an interaction between signaling downstream of TLR4 and MOR, as previously suggested from studies of peripheral immune cells (Roy et al., 1998, Welters et al., 2000). Evidence of an interaction between MOR and TLR4 signaling was also suggested when studying changes in mRNA expression of TNFα and COX2. For these end-points, the MOR/TLR4 agonist (-)-morphine caused no effect at a wide range of concentrations tested, in contrast to the non-MOR, TLR4 agonist (+)-morphine. Pre-treating the cells with (-)-nalmefene, a classical opioid receptor antagonist (which we demonstrate to be inactive at TLR4) did unmask (-)-morphine-induced increases in TNFα mRNA expression. Furthermore, inhibition of PKA, which can be activated by MOR signaling in immune cells (Ai et al., 1999, Zhang and Pan, 2010), also unmasked induction of TNFα and COX2 mRNA expression by (-)-morphine. Therefore, the dual activation of TLR4 and MOR by (-)-morphine has opposing actions on the expression of pro-inflammatory cytokines, possibly by preventing degradation of IκBα, as previously shown (Minguet et al., 2005). Whether that occurs here remains to be defined. M3G induced increases in TNFα and COX-2 mRNA, which were antagonized by LPS-RS, indicating that M3G signals through TLR4, in agreement with our prior work (Lewis et al., 2010). We additionally demonstrated that the “MOR-selective” antagonist CTAP was also a TLR4 antagonist. The lack of concentration-dependency observed herein suggests that the concentrations tested may be at the top of the concentration-response curve, as we show that CTAP potently binds TLR4 in silico. To extend our in vitro findings, primary CNS endothelial cells were stimulated in vitro with (-)-morphine or M3G, then adoptively transferred via intrathecal injection into naïve donor animals. Only rats receiving M3G-stimulated CNS endothelial cells exhibited robust tactile allodynia, which was significant within 2 hours of injection. M3G also initiated vasodilation, in a (+)-naloxone sensitive fashion, when suffused over the cortex in vivo. Finally, we demonstrated that LPS- and/or M3G-induced activation of the cells was blocked by propentofylline, and the AV411/ibudilast derivative AV1013, indicating that the effects of these drugs extend beyond glial cells. This may be true for many of the “glial inhibitors”, as minocycline has previously been shown to attenuate pro-inflammatory cytokine release from endothelial cells (Xing et al., 2012).
There are conflicting reports as to whether CNS endothelial cells express TLR4. Nagyoszi et al. (2010) reported that TLR4 mRNA was not detectable in primary brain endothelial cells. However, the cells were isolated from the brains of two week old rats, and receptor expression in adults may not be predicted by expression in pups. Singh and Jiang (2004) reported that TLR4 mRNA was not detected in primary endothelial cells isolated from adult rat brains, but their protocol used a 24 hr isolation procedure and the cells were analyzed 24 hr after plating, which may not be sufficient time for the cells to recover normal expression following the isolation procedure. In contrast, Shih and Yang (2010) and Verma et al. (2006) provided evidence for TLR4 expression and function in mouse brain endothelial cells by measuring TLR4 mRNA expression and cytokine release in response to the classic TLR4 agonist, LPS, respectively, using adult mice-derived cells that were cultured for 4 days to 1 week between isolation and use. Further, Gosselin and Rivest (2008) used knockout mice to demonstrate that TLR4 expression in brain endothelial cells is necessary for elevated plasma corticosterone levels evoked by systemic LPS treatment. Hence, this is the first report of functional TLR4 expression in rat CNS endothelial cells, at any age.
In vivo experiments have clearly documented that opioids induce reactive gliosis and central immune signaling, characterized by increased expression and/or release of proinflammatory cytokines (Raghavendra et al., 2002, Johnston et al., 2004, Hutchinson et al., 2008a). Acute (-)-morphine analgesia is enhanced by blockade of proinflammatory cytokine signaling and by drugs including minocycline and ibudilast (Shavit et al., 2005, Cui et al., 2008, Hutchinson et al., 2008a, Hutchinson et al., 2008b, Hutchinson et al., 2009). It has recently been demonstrated that (-)-morphine binds to the accessory protein, MD2, and initiates TLR4 signaling (Wang et al., 2012). We and other have also shown that TLR4 blockade in vivo potentiates acute intrathecal (-)-morphine analgesia, and attenuates the development of analgesic tolerance, hyperalgesia, withdrawal and reinforcement behaviors (Hutchinson et al., 2010b, Due et al., 2012, Hutchinson et al., 2012, Eidson and Murphy, 2013, Theberge et al., 2013). Some of these findings have recently been disputed, but caution should be exercised when drawing conclusions. For example, in vitro work in the HEK293-hTLR4 reporter cell line found only minor activation following opioid agonist stimulation (Stevens et al., 2013). However, the sole use of NFκB activation as a surrogate for TLR4 signaling has been recently critiqued (Watkins et al., 2014). In another recent study, morphine-induced hyperalgesia, but not tolerance, was attenuated following in vivo blockade of TLR4 (Ferrini et al., 2013). However, Ferrini et al. (2013) dosed (+)-naloxone acutely, which may not account for longer-acting neuroinflammatory metabolites (e.g. M3G) that were likely blocked by our repeated (+)-naloxone dosing regimen (Hutchinson et al., 2010b). Nonetheless, morphine does induce expression of mediators, such as BDNF, that may be independent of TLR4 (Ferrini et al., 2013). Other studies have explored opioid effects in mouse strains with mutations in the Tlr4 gene (Ferrini et al., 2013, Fukagawa et al., 2013, Mattioli et al., 2014). Not only are some TLR4 agonists are documented to signal around point mutations (Goodridge et al., 2007), but developmental, compensatory signaling pathways may also be activated in the absence of TLR4 (Grace et al., 2014), leaving conclusions based solely on mutant mouse models in need of further investigation. Nevertheless, the data presented here strongly suggest a role for CNS endothelial cells in analgesic tolerance, hyperalgesia, withdrawal and reinforcement behaviors, as our data show that CNS endothelial cells respond to M3G with proinflammatory signaling that is TLR4-dependent, sufficient to alter neuronal functioning, and sensitive to blockade with the so-called glial inhibitors propentofylline and AV1013.
At equimolar concentrations, we have shown that M3G more potently induces TLR4 signaling than does (-)-morphine. Herein, we propose two explanations. The first is that our demonstration that M3G binds to MD-2 with greater affinity than does (-)-morphine in a biophysical assay. The second is that dual activation of TLR4 and MOR by (-)-morphine may have opposing actions on NFκB activation, and hence the expression of pro-inflammatory cytokines. The presence of such an interaction is supported by the fact that MOR-inactive, but TLR4 active isomer (+)-morphine induced greater pro-inflammatory cytokine gene expression than did (-)-morphine, and that (-)-morphine pro-inflammatory signaling was unmasked by the non-TLR4, MOR antagonist (-)-nalmefene. Our data suggest that MOR may inhibit pro-inflammatory signaling via PKA, which can be activated by MOR (Ai et al., 1999, Zhang and Pan, 2010), as inhibition of PKA also unmasked pro-inflammatory signaling by (-)-morphine. This may potentially be due to suppression of NFκB activity, by preventing degradation of the NFκB inhibitor, IκBα, shown previously in B cells (Minguet et al., 2005).
Translation of our in vitro data requires consideration of several factors. First, cells stimulated in vitro are, as was the case here, typically in a static environment, such that factors released by the cells in response to a stimulus could provide autocrine feedback and confound the primary response to the stimulus. Secretions of CNS endothelial cells exposed to opioids in vivo would diffuse away from the cells in either the blood or CNS parenchyma, potentially altering the way the cells respond. Second, CNS endothelial cells in vivo exist within a framework defined by many other cell types that were absent from the in vitro experiments performed here, including neurons, glia, pericytes, and peripheral immune cells. Future in vitro studies will address how CNS endothelial cells stimulated by (-)-morphine or M3G interact with co-cultured glia and/or peripheral immune cells, as well as in vivo responses to TLR4 agonists and antagonists. Finally, (-)-morphine pharmacokinetics in vivo are such that following a single bolus administration of (-)-morphine, (-)-morphine is rapidly metabolized and cleared, and subsequently, M3G concentration in the blood increases and persists (Mazoit et al., 2007). This in combination with the oppositional MOR and TLR4 signaling in CNS endothelial cells presented here suggests that in vivo, a proinflammatory response to (-)-morphine and M3G might build over time and persist from CNS endothelial cells. Additional arguments for this are the observations that MOR is downregulated under conditions of (-)-morphine tolerance and by heterologous desensitization caused by chemokines (Koch and Hollt, 2008, Heinisch et al., 2011), which could again limit anti-inflammatory signaling downstream of MOR while allowing persistent proinflammatory signaling downstream of TLR4.
The relevance of CNS endothelial cell activation to CNS dysfunction is a relatively new field, and studies tend to focus on breakdown of the blood-brain barrier rather than on proinflammatory signaling of the endothelial cells per se (Grammas et al., 2011). Our finding that M3G-activated CNS endothelial cells produce tactile allodynia when delivered via intrathecal injection underscores the potential significance of activated CNS endothelial cells in vivo. In comparison with studies documenting increased pain sensitivity in rodents in response to intrathecal injection of activated microglia or astrocytes, our results suggest that activated CNS endothelial cells are at least as potent as these two glial cell types in altering spinal nociception, as we observed allodynia in response to M3G-stimulated cells within two hours of injection while studies using astrocytes or microglia injected at least as many cells as we did but did not document associated changes in pain sensitivity until 2-3 hr post-injection (Tsuda et al., 2003, Coull et al., 2005, Narita et al., 2006, Zhang et al., 2011). Although non-significant from controls, LPS-RS costimulation did not completely abolish the allodynia induced by adoptive transfer of M3G-stimulated endothelial cells. This trend may be due to incomplete TLR4 blockade by LPS-RS, resulting in partial activation. Beyond the spinal cord, our data indicate that M3G activates endothelial cells in the brain, as supported by induction of the classical vasodilatory response to M3G. (-)-Morphine- and M3G-induced activation of endothelial cells lining the blood-brain barrier in drug reward areas of the brain could have important modulatory effects on neuronal function in those areas, potentially contributing to opioid-associated reward and addiction.
Given the clear significance of TLR4 signaling in various CNS disorders including ischemic damage, neuropathic pain, neuroinflammation caused by alcohol or stress, and experimental multiple sclerosis and Alzheimer's disease (Walter et al., 2007, Caso et al., 2008, Marta et al., 2008, Watkins et al., 2009, Yang et al., 2010, Pascual et al., 2011, Wu et al., 2012, Grace et al., 2014), and the observations that CNS endothelial cells are TLR4-expressing immunocompetent cells, these cells may well prove to have a significant TLR4-dependent role in various CNS disorders. In order to better understand how endothelial cells contribute to any given CNS disorder, it will be critical for the field to devise methods that clearly separate endothelial cell functionality from that of other cells.
Highlights.
Adult rat CNS endothelial cells express functional TLR4
The opioid receptor inactive morphine metabolite M3G activates TLR4-dependent inflammatory signaling in endothelial cells
Nalmefene is a mu opioid receptor antagonist without TLR4 activity, whereas CTAP is an antagonist at both receptors
Stimulation of endothelial cells by M3G induces in vivo responses of decreased mechanical thresholds and vasodilation
The putative glial inhibitors AV1013 and propentofylline also inhibit the inflammatory response of endothelial cells.
Acknowledgments
This work was supported by the American Pain Society Future Leaders in Pain Research Small Grants Program (KMR 2009-2011); F32NS066665 from NINDS (KMR 2010-2013); NIH grants DA024044, DE017782, and DA023132 (LRW); American Australian Association Merck Company Foundation (MRH) and Sir Keith Murdoch (PMG) Fellowships; a National Health and Medical Research Council CJ Martin Fellowship (ID 465423, MRH 2007-2010; ID 1054091, PMG 2013-); and an Australian Research Council Research Fellowship (DP110100297, MRH 2011-). The work of the Drug Design and Synthesis Section, CBRB, NIDA, and NIAAA was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
Conflicts of interest: Kirk Johnson served as Chief Scientific Officer of MediciNova, which is developing ibudilast for CNS indications. No other authors have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai W, Gong J, Yu L. MAP kinase activation by mu opioid receptor involves phosphatidylinositol 3-kinase but not the cAMP/PKA pathway. FEBS letters. 1999;456:196–200. doi: 10.1016/s0014-5793(99)00949-7. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Borner C, Warnick B, Smida M, Hartig R, Lindquist JA, Schraven B, Hollt V, Kraus J. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. Journal of immunology. 2009;183:882–889. doi: 10.4049/jimmunol.0802763. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M. Morphine metabolism, transport and brain disposition. Metabolic brain disease. 2012;27:1–5. doi: 10.1007/s11011-011-9274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. Journal of neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Murphy AZ. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33:15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. The Journal of biological chemistry. 1996;271:26157–26164. doi: 10.1074/jbc.271.42.26157. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nature neuroscience. 2013;16:183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa H, Koyama T, Kakuyama M, Fukuda K. Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. Journal of anesthesia. 2013;27:93–97. doi: 10.1007/s00540-012-1469-4. [DOI] [PubMed] [Google Scholar]
- Goodridge HS, McGuiness S, Houston KM, Egan CA, Al-Riyami L, Alcocer MJ, Harnett MM, Harnett W. Phosphorylcholine mimics the effects of ES-62 on macrophages and dendritic cells. Parasite immunology. 2007;29:127–137. doi: 10.1111/j.1365-3024.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Bishop A, Somogyi AA, Mayrhofer G, Rolan PE. Adoptive transfer of peripheral immune cells potentiates allodynia in a graded chronic constriction injury model of neuropathic pain. Brain Behav Immun. 2011a;25:503–513. doi: 10.1016/j.bbi.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nature reviews Immunology. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Rolan PE, Hutchinson MR. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun. 2011b;25:1322–1332. doi: 10.1016/j.bbi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Harvey LO., Jr Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- Heinisch S, Palma J, Kirby LG. Interactions between chemokine and mu-opioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain Behav Immun. 2011;25:360–372. doi: 10.1016/j.bbi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008a;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010a;167:880–893. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008b;22:1248–1256. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the Neuroimmuno-pharmacology of Opioids: an Integrative Review of Mechanisms of Central Immune Signaling and Their Implications for Opioid Analgesia. Pharmacol Rev. 2011 doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008c;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010b;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo F. The blood-brain barrier in vitro: the second decade. Neurochem Int. 1993;23:499–521. doi: 10.1016/0197-0186(93)90098-p. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Koch T, Hollt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther. 2008;117:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kramer TH, Shook JE, Kazmierski W, Ayres EA, Wire WS, Hruby VJ, Burks TF. Novel peptidic mu opioid antagonists: pharmacologic characterization in vitro and in vivo. J Pharmacol Exp Ther. 1989;249:544–551. [PubMed] [Google Scholar]
- Kraus J, Borner C, Giannini E, Hollt V. The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol. 2003;64:876–884. doi: 10.1124/mol.64.4.876. [DOI] [PubMed] [Google Scholar]
- Law PY, Loh HH, Wei LN. Insights into the receptor transcription and signaling: implications in opioid tolerance and dependence. Neuropharmacology. 2004;47(Suppl 1):300–311. doi: 10.1016/j.neuropharm.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, Huang Y, Rice KC, Watkins LR. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain. 2012;13:498–506. doi: 10.1016/j.jpain.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marta M, Andersson A, Isaksson M, Kampe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. European journal of immunology. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- Matsui T, Svensson CI, Hirata Y, Mizobata K, Hua XY, Yaksh TL. Release of prostaglandin E(2) and nitric oxide from spinal microglia is dependent on activation of p38 mitogen-activated protein kinase. Anesth Analg. 2010;111:554–560. doi: 10.1213/ANE.0b013e3181e3a2a2. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, Nicol CJ, Milne B, Trang T, Cahill CM. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PloS one. 2014;9:e97361. doi: 10.1371/journal.pone.0097361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoit JX, Butscher K, Samii K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth Analg. 2007;105:70–78. doi: 10.1213/01.ane.0000265557.73688.32. [DOI] [PubMed] [Google Scholar]
- Means TK, Pavlovich RP, Roca D, Vermeulen MW, Fenton MJ. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. Journal of leukocyte biology. 2000;67:885–893. doi: 10.1002/jlb.67.6.885. [DOI] [PubMed] [Google Scholar]
- Michel ME, Bolger G, Weissman BA. Binding of a new opiate antagonist, nalmefene, to rat brain membranes. Methods Find Exp Clin Pharmacol. 1985;7:175–177. [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. European journal of immunology. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Gay RE, Gay S. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep. 2002;4:201–207. doi: 10.1007/s11926-002-0066-1. [DOI] [PubMed] [Google Scholar]
- Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA. Expression and Regulation of Toll-Like Receptors in Cerebral Endothelial Cells. Neurochem Int. 2010 doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshida T, Nakajima M, Miyatake M, Takagi T, Yajima Y, Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Mitsumoto Y, Mohri T. Characterization of the uptake of adenosine by cultured rat hippo-campal cells and inhibition of the uptake by xanthine derivatives. Neurosci Lett. 1991;133:275–278. doi: 10.1016/0304-3940(91)90587-j. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Perriere N, Demeuse P, Garcia E, Regina A, Debray M, Andreux JP, Couvreur P, Scherrmann JM, Temsamani J, Couraud PO, Deli MA, Roux F. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Mol Neurobiol. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyper-algesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Resman N, Gradisar H, Vasl J, Keber MM, Pristovsek P, Jerala R. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS letters. 2008;582:3929–3934. doi: 10.1016/j.febslet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Shih RH, Yang CM. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. Journal of neuroinflammation. 2010;7:86. doi: 10.1186/1742-2094-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Liu Y, Casares F, Goligorsky MS. Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. The Journal of biological chemistry. 1995;270:30290–30293. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Aravind S, Das S, Davis RL. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br J Pharmacol. 2013;168:1421–1429. doi: 10.1111/bph.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011:235–250. doi: 10.1007/978-3-642-13443-2_8. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biological psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang X, Grace PM, Pham MN, Cheng K, Strand KA, Smith C, Li J, Watkins LR, Yin H. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2713–2722. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends in pharmacological sciences. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wang X, Mustafa S, Hutchinson MR. In vivo veritas: (+)-Naltrexone's actions define translational importance: A letter in response to Skolnick et al. ‘Translational potential of naloxone and naltrexone as TLR4 antagonists’. Trends in pharmacological sciences. 2014;35:432–433. doi: 10.1016/j.tips.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Welters ID, Menzebach A, Goumon Y, Cadet P, Menges T, Hughes TK, Hempelmann G, Stefano GB. Morphine inhibits NF-kappaB nuclear binding in human neutrophils and monocytes by a nitric oxide-dependent mechanism. Anesthesiology. 2000;92:1677–1684. doi: 10.1097/00000542-200006000-00027. [DOI] [PubMed] [Google Scholar]
- Wilbert-Lampen U, Trapp A, Barth S, Plasse A, Leistner D. Effects of beta-endorphin on endothelial/monocytic endothelin-1 and nitric oxide release mediated by mu1-opioid receptors: a potential link between stress and endothelial dysfunction? Endothelium : journal of endothelial cell research. 2007;14:65–71. doi: 10.1080/10623320701346585. [DOI] [PubMed] [Google Scholar]
- Wolf LK. New software and websites for the chemical enterprise. Chemical and Engineering News. 2009;87:32. [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, Watkins LR, Somogyi AA, Hutchinson MR. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Levchenko T, Guo S, Stins M, Torchilin VP, Lo EH. Delivering minocycline into brain endothelial cells with liposome-based technology. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:983–988. doi: 10.1038/jcbfm.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Front Biosci (Schol Ed) 2010;2:1081–1091. doi: 10.2741/s119. [DOI] [PubMed] [Google Scholar]
- Zabel U, Henkel T, Silva MS, Baeuerle PA. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993;12:201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang J, Zhou Q, Xu Y, Pu S, Wu J, Xue Y, Tian Y, Lu J, Jiang W, Du D. Brain-derived neurotrophic factor-activated astrocytes produce mechanical allodynia in neuropathic pain. Neuroscience. 2011;199:452–460. doi: 10.1016/j.neuroscience.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between delta- and mu-opioid receptors. J Neurosci. 2010;30:4735–4745. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]