Abstract
The role of iron in experimental infection of mice with Trypanosoma cruzi was investigated. B6 mice had a transient parasitemia and a transient anemia, both of maximal intensity 28 d after the inoculation of T. cruzi. There was a biphasic hypoferremic host response to infection with T. cruzi with the peak hypoferremia also occurring 28 d after inoculation of the parasite. The mortality rate from infection was increased from 23% in phosphate-buffered saline-treated B6 mice to 50% in a group of B6 mice receiving iron-dextran (P less than or equal to 0.025), whereas depletion of iron stores with the iron chelator desferrioxamine B and an iron-deficient diet provided complete protection of B6 mice (P less than or equal to 0.05). The mortality rate in the highly susceptible C3H strain was reduced from 100% in the control group to 45% (P less than or equal to 0.025) in the iron-depleted group. The tissue iron stores were altered in mice receiving either iron-dextran or desferrioxamine B and an iron-deficient diet. In vitro, T. cruzi was shown to require both a heme and a nonheme iron source for an optimal growth rate. The effects of iron excess or depletion on the outcome of infection with T. cruzi correlated both with the growth requirements of the parasite for iron and with the availability of intracellular iron. Thus, it was suggested that the hypoferremic response, by sequestering iron within intracellular stores, potentially enhanced the pathogenicity of the intracellular parasites. Furthermore, the in vivo effects of iron excess and depletion correlated with an effect of iron on the growth rate and pathogenicity of the parasite.
Full text
PDF
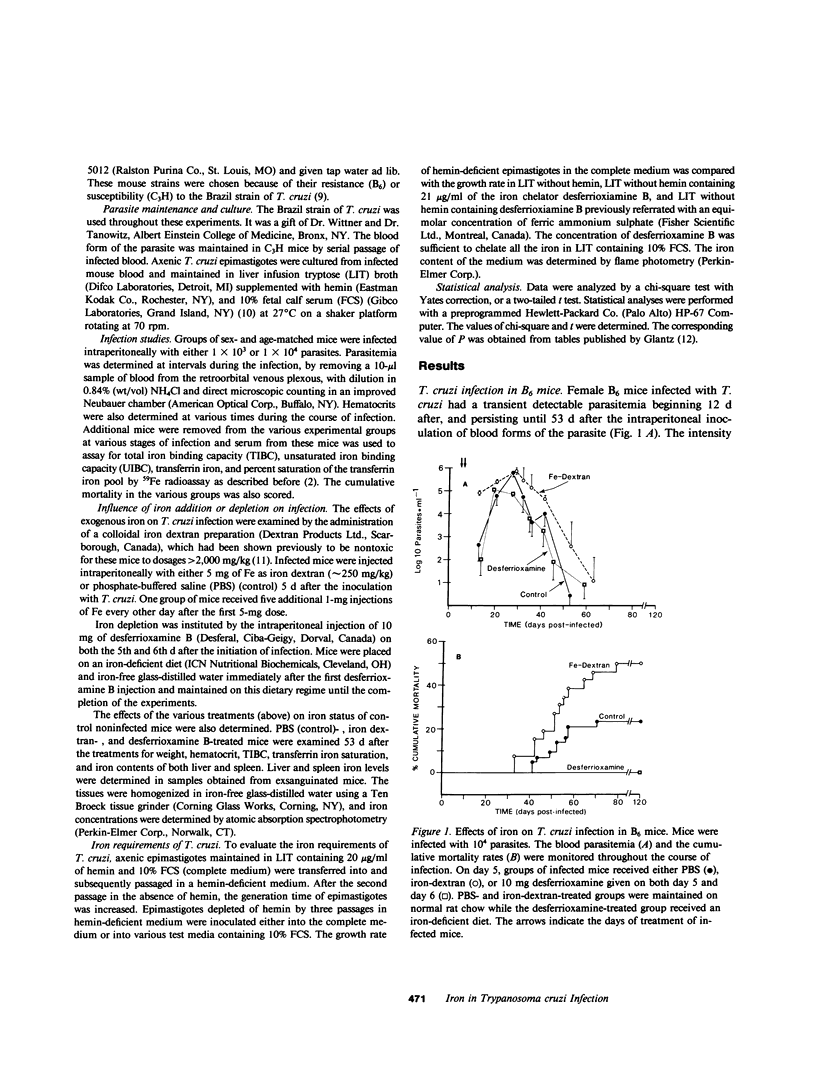
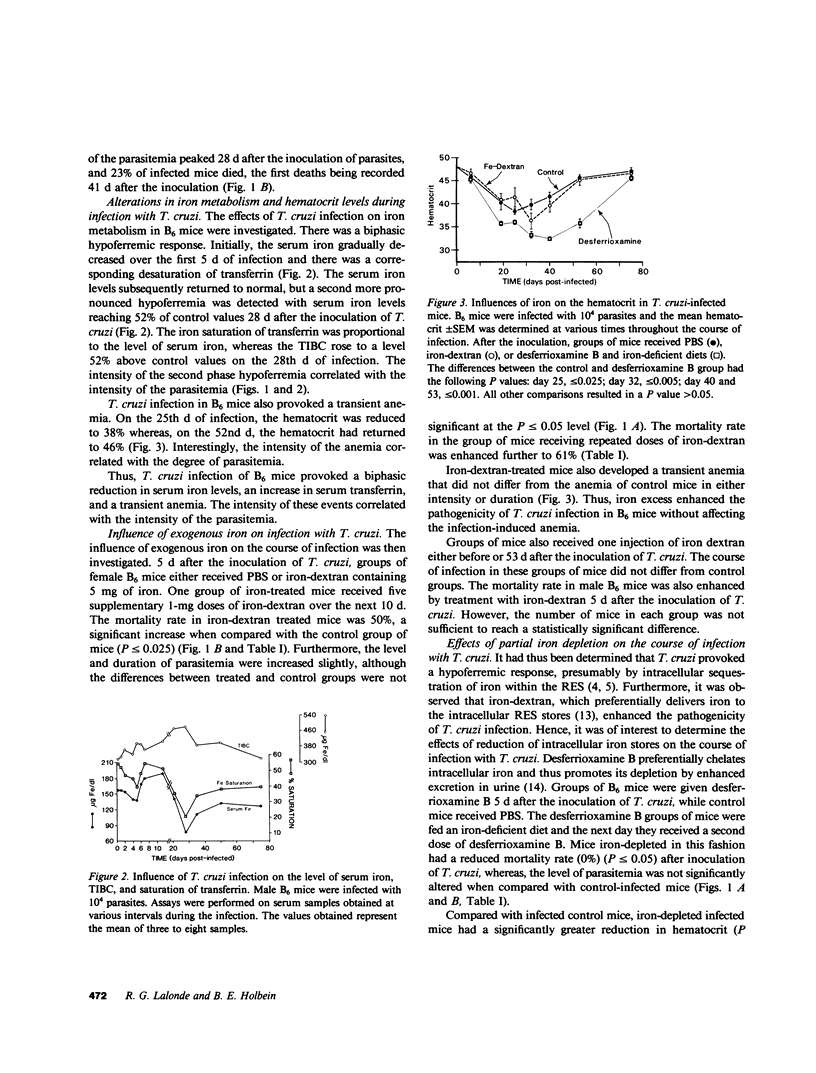
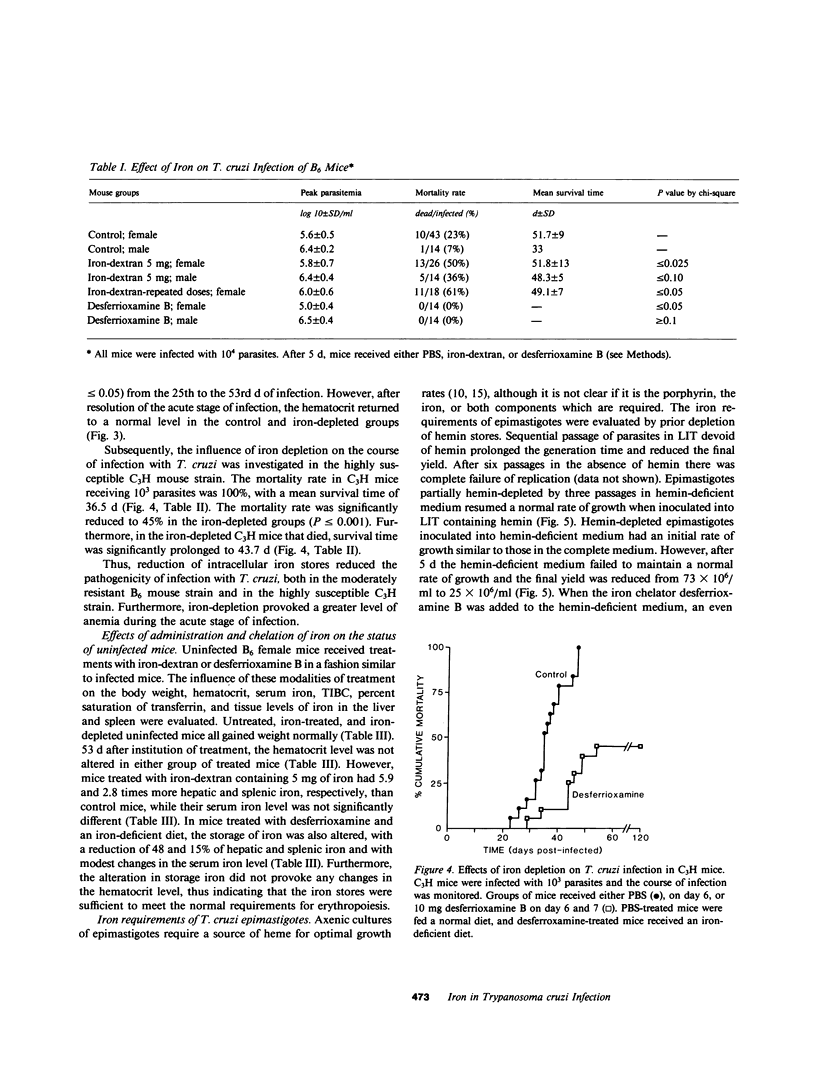



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Bretaña A., Casanova M. A., Avila A., Rodríguez F. Trypanosoma cruzi: defined medium for continuous cultivation of virulent parasites. Exp Parasitol. 1979 Aug;48(1):27–35. doi: 10.1016/0014-4894(79)90051-1. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Cover B., Gutteridge W. E. Comparison of drug sensitivities of three strains of Trypanosoma cruzi in inbred A/Jax mice. Trans R Soc Trop Med Hyg. 1981;75(2):274–281. doi: 10.1016/0035-9203(81)90334-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E. Trypanosoma cruzi: recent biochemical advances. Trans R Soc Trop Med Hyg. 1981;75(4):484–492. doi: 10.1016/0035-9203(81)90183-8. [DOI] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Jericho K. W., Likes G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect Immun. 1979 May;24(2):545–551. doi: 10.1128/iai.24.2.545-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre E. D., Holbein B. E. Turnover in the transferrin iron pool during the hypoferremic phase of experimental Neisseria meningitidis infection in mice. Infect Immun. 1983 Jan;39(1):50–59. doi: 10.1128/iai.39.1.50-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R. C., Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978 Jun;64(3):475–482. [PubMed] [Google Scholar]
- Murray M. J., Murray A. B., Murray M. B., Murray C. J. The adverse effect of iron repletion on the course of certain infections. Br Med J. 1978 Oct 21;2(6145):1113–1115. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschmann M., Ganzoni A. M. Increased resistance of iron-deficient mice to salmonella infection. Infect Immun. 1977 Sep;17(3):663–664. doi: 10.1128/iai.17.3.663-664.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. W. The cellular transformation of injected colloidal iron complexes into ferritin and hemosiderin in experimental animals; a study with the aid of electron microscopy. J Exp Med. 1959 Feb 1;109(2):197–216. doi: 10.1084/jem.109.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischmann T., Tanowitz H., Wittner M., Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978 Aug;45(2):160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


