Abstract
Ischemia is a complex phenomenon modulated by the concerted action of several cell types. We have identified that sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2 (SERCA 2) cysteine 674 (C674) S-glutathiolation is essential for ischemic angiogenesis, vascular endothelial growth factor (VEGF)-mediated endothelial cell (EC) migration and network formation. A heterozygote SERCA 2 C674S knockin (SKI) mouse shows impaired ischemic blood flow recovery after femoral artery ligation, and its EC show depleted endoplasmic reticulum (ER) Ca2+ stores and impaired angiogenic behavior. Here we studied the role of SERCA 2 C674 in the interaction between ECs and macrophages in the context of ischemia and discovered the involvement of the ER stress response protein, ER oxidoreductin-1α (ERO1). In wild type (WT) mice, expression of ERO1 was increased in the ischemic hind limb in vivo, as well as in ECs and macrophages exposed to hypoxia in vitro. The increase in ERO1 to ischemia/hypoxia was less in SKI mice. In WT ECs, both vascular cell adhesion molecule 1 (VCAM1) expression and bone marrow-derived macrophage adhesion to ECs were increased by hypoxia, and both were attenuated in SKI ECs. In WT ECs, ERO1 siRNA blocked hypoxia-induced VCAM1 expression and macrophage adhesion. In WT macrophages, hypoxia also stimulated both ERO1 and VEGF expression, and both were less in SKI macrophages. Compared with conditioned media of hypoxic SKI macrophages, conditioned media from WT macrophages had a greater effect on EC angiogenic behavior, and was blocked by VEGF neutralizing antibody. Taken together, under hypoxic conditions, SERCA 2 C674 and ERO1 enable increased VCAM1 expression and macrophage adhesion to ECs, as well as macrophage VEGF production that, in turn, promote angiogenesis. This study highlights the hitherto unrecognized interaction of two ER proteins, SERCA 2 C674 and ERO1, which mediate the EC and macrophage angiogenic response to ischemia/hypoxia.
Keywords: ischemia/hypoxia, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, endoplasmic reticulum oxidoreductin-1, macrophage, vascular endothelial growth factor
Introduction
Tissue ischemia and its multiple complications (involving brain, heart, kidney and limb) are ranked as the number one cause of morbidity and mortality in humans, and efforts continue to predict patients at risk as well as to prevent and reverse its effects. Elevated levels of reactive oxygen and nitrogen species (RONS) are important regulators of tissue responses to ischemia by inducing post-translational protein modifications, but the precise protein targets of RONS that dictate the outcome of ischemia are not well understood. Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2 (SERCA 2) is a critical Ca2+ pump that maintains endoplasmic reticulum (ER) Ca2+ stores, and regulates Ca2+ flux and normal cellular responses to ischemia. We have identified the SERCA 2 cysteine 674 (C674) thiol as a major reduction/oxidation (redox)-sensitive site which is modified by RONS to form S-glutathione (GSH) adducts, a reversible thiol adduct that causes an increase in SERCA 2 activity and Ca2+ uptake [1]. Importantly, we found that vascular endothelial growth factor (VEGF) induced endothelial cell (EC) migration was mediated by SERCA 2 GSH adducts that were required for Ca2+ influx [2]. Indeed, VEGF failed to increase SERCA 2 GSH adducts, EC Ca2+ influx, angiogenic migration or network formation in human EC expressing a SERCA 2 C674S mutant [3]. Our recent study indicates that hypoxia also increases SERCA 2 C674 glutathione adducts, and a heterozygote SERCA 2 C674S knockin (SKI) mouse has decreased adducts and impaired angiogenesis after hind limb ischemia in vivo. SKI ECs also have impaired angiogenic behavior in response to VEGF in vitro [4].
The ER is an important organelle of eukaryotic cells that functions in a variety of biological processes, including essential roles in lipid and protein biosynthesis [5, 6]. In addition to serving as the site of protein synthesis, folding and post-translational modification, ER is the major intracellular storage location for Ca2+, and maintains Ca2+ homeostasis through multiple integrated systems [7]. When ER homeostasis is disrupted, ER stress ensues, and an adaptive process called the unfolded protein response is initiated [8]. ER stress can be triggered by hypoxia, perturbation of redox status, aberrant Ca2+ regulation, and increased protein synthesis and/or accumulation of unfolded or misfolded proteins in the ER [9–12]. Hypoxia or ischemia induces ER stress, which plays dual roles in cell functions. On one side, ER stress initiates protective signals to support cell survival [13, 14]. However, an uncontrolled, aberrantly regulated or unresolved ER stress response participates in disease development. SERCA 2 plays a critical role in maintaining ER Ca2+ homeostasis and normal ER function. Decreased SERCA expression or inhibition of SERCA with thapsigargin is associated with depletion of ER Ca2+ stores and ER stress-associated apoptosis [15]. Conversely, SERCA over-expression alleviates ER stress [16]. ER oxidoreductin-1α (ERO1) plays an essential role in maintaining ER redox homeostasis and proper ER folding activity [17]. We found that ECs from SKI mice have depleted Ca2+ stores, but the role of SERCA 2 C674 on ER homeostasis under ischemia is currently unknown.
The major source of VEGF during ischemia is from macrophages, underlying their importance in ischemia-induced revascularization [18, 19]. VEGF mediates EC tip cell formation, whereas macrophages promote tip cell fusion and vascular anastomosis to add new elements to the existing vessel network [20]. Vascular cell adhesion molecule 1 (VCAM1) expressed in ECs regulates leukocyte adhesion and initiates the inflammatory response after injury. The proper coordination of ECs and macrophages is therefore critical in promoting angiogenesis after tissue ischemia. Here we studied the role of SERCA 2 C674 in the response of ECs and macrophages to ischemia/hypoxia, and found that SERCA 2 C674 interacted with ERO1 as key regulators of the inflammatory response.
Methods
Generation of the SKI heterozygote mouse [4]
The SERCA 2 C674S heterozygote knock-in mouse (SKI) was generated on a C57BL/6J background by Ingenious Targeting Laboratory, Inc. (Ronkonkoma, NY). A mutated 14.4 kb SERCA 2 exon 14 containing the C674 to S674 codon (TGT->TCC) was inserted into the endogenous SERCA2 gene locus. In this construct, a neomycin cassette was introduced into the intron between exon 14 and exon 15 for embryonic stem cells selection. The neomycin cassettes were removed in vivo by breeding in flp recombinase, and the recombinase was bred out by another round of breeding, all while maintaining the C57BL6 genetic background of the original embryonic stem cells used. This mouse was validated for absence of extra DNA inserts. Expression of the mutant allele is governed by the unaltered upstream native SERCA 2 promoter. The presence of the C674S mutation was confirmed by sequencing of both genomic DNA and cDNA from cardiac endothelial cells. Homozygous knock-in fetuses died in utero just prior to the initial period of vascular development (8–10.5 days), so only heterozygous adult animals expressing 50% of SERCA 2 C674S allele were studied. All animal usage was approved by Boston University’s Institutional Animal Care and Use Committee in accordance with the provisions of the Animal Welfare Act, Public Health Service Animal Welfare Policy, and the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Boston University Medical Campus. Animals were maintained in an AALAC approved Laboratory Animal Science Center staffed with licensed veterinarians.
Hind limb ischemia (HLI) surgical procedure [4]
8–12 week old male littermate wild type (WT) and heterozygous SKI mice were anesthetized by intraperitoneal injection of xylazine (40 mg/kg) and ketamine (100 mg/kg). The left femoral artery, vein and nerve proximal to the popliteal bifurcation site and distal to the inguinal ligament were ligated with 6-0 silk suture and excised. Gastrocnemius muscle from ischemic and non-ischemic limbs was harvested at the indicated post-operative times and snap frozen in liquid nitrogen for RNA or protein analysis or embedded in Optimal Cutting Temperature Compound (Sakura Finetek USA, Inc., Torrance, CA) for immunostaining.
Isolation and culture of murine cardiac endothelial cells [4]
We have attempted to isolate hind limb muscle endothelial cells. However, yields were unacceptably low to be feasible. As an alternative, we have established methods for isolating primary cardiac microvascular endothelial cells from mice at greater than 90% purity using a Miltenyi AutoMACS Pro magnetic cell separator (Miltenyi Biotec, Auburn, CA). These cells have typical cobblestone appearance and express the endothelial cell antigens CD31 and eNOS, and at passage 4 are 87% CD31 positive. Briefly, mice were euthanized via inhaled anesthetic overdose (isoflurane) followed by cervical dislocation. Three hearts per genotype were pooled from mice aged 4–12 weeks. Hearts were rinsed in Hank’s Balanced Salt Solution and minced with 3,000 units collagenase type II and 300 units DNAse I. Samples were incubated at 37°C with gentle agitation for 30 minutes, minced again then filtered through a 70 µM strainer. Samples were washed with Miltenyi AutoMACs running buffer and red blood cells were lysed. Cells were incubated with rat anti-mouse CD31 antibody followed by goat anti-rat IgG micro-beads, and then labeled cells were separated according to the manufacturer’s protocol. The resulting cells were resuspended in EBM-2 supplemented with 10% FBS, 50 µg/mL endothelial mitogen and 1% antibiotic-antimycotic, then seeded on 0.5% gelatin-coated plates at a density of 20,000 cells per cm2. Cells were cultured and early passages used in in vitro experiments.
Capillary-like network formation assay [4]
In vitro endothelial cell network formation was assayed using BD Matrigel™. The Matrigel assay takes advantage of the phenomenon that cultured endothelial cells will spontaneously form network structures when seeded on this complex solubilized extracellular matrix rich in laminin, collagen IV and heparan sulfate proteoglycans. Briefly, 96-well plates were coated with BD Matrigel™ for 30–60 minutes according to the manufacturer’s instructions prior to seeding endothelial cells at a density of 1 × 104 cells per well, the outgrowth of tube networks was evaluated by measurement of tube length using NIH image J software.
Endothelial cell proliferation assay
EC proliferation was evaluated by water soluble tetrazolium salt assay. Briefly, ECs were seeded in 96 well plates at a density of 5,000 cells/well and treated for 72 h, cells were then washed with PBS and incubated with WST (Roche, Indianapolis, IN) for 1 h, fluorescence was determined by microplate reader (Tecan Infinite M1000, Switzerland).
Macrophage culture and adhesion assay
Bone marrow was isolated as previously described [21] and was cultured in DMEM supplemented with 10% fetal bovine serum. 20% L929-conditioned medium was added to stimulate bone marrow differentiation into macrophages. For macrophage adhesion assay, ECs plated in 6-well plates at a density of 500,000 cells per well were exposed to hypoxia. An in vitro hypoxic environment (0.5% O2, 5% CO2, 70% humidity, 37°C) was generated using a LiveCell™ chamber system (Pathology Devices, Inc., Westminster, MD). ECs cultured under normoxic condition were used as control. After ECs were exposed to hypoxia or normoxia for 24 h, 500,000 macrophages were added and cultured, non-adherent cells were decanted after 1 h, pictures of adherent cells were taken, and cells were counted using Image J software.
Western blotting
Western blotting was performed as described previously [22]. Cells after treatment or gastrocnemius muscle from animals performed with femoral artery ligation were lysed using RIPA buffer. Equal amounts of proteins (20µg) were loaded and separated by 4–20% SDS-PAGE (Biorad, Hercules, CA). Proteins were transferred to PVDF membranes. Membranes were incubated overnight at 4°C with the primary antibodies at 1:1000 specific for SERCA2 (Bethyl laboratory, Montgomery, TX), ERO1, VCAM1 (Santa Cruz, Dallas, Texas), VEGF (Novus, Littleton, CO), β-actin (Sigma, St. Louis, MO), tubulin and heat shock protein 90 (cell signaling, Danvers, MA), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (cell signaling, Danvers, MA) at 1:2000. Blots were visualized by an ECL system (GE Healthcare, Buckinghamshire, UK). Densitometric analyses of immunoblots were performed by Image J.
Small interference RNA (si-RNA) transfection
Human aortic endothelial cells (HAECs) were plated at a density of 400,000/ well in 6-well plates and transfected with control siRNA or ERO1 siRNA (Santa Cruz, Dallas, Texas) as described by the protocol. After transfection, cells were cultured in complete medium for 72 h, and then exposed to hypoxia for indicated time. Cells were harvested for protein analysis or used for macrophage adhesion assay.
Enzyme linked immuno-sorbent assay (ELISA)
WT and SKI macrophages were seeded into 6-well plates and exposed to hypoxia for the indicated time. The culture media at baseline (0 h) and 16 h were collected, centrifuged to remove cell debris, and stored at −80°C. ELISA was performed with mouse VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Total VEGF protein concentration was adjusted to reflect VEGF level in the cell free supernatant.
Macrophage hypoxia media on EC network formation
WT and SKI macrophages were seeded into 6-well plates and exposed to hypoxia for 16 h. The culture media was centrifuged, and the supernatant was saved for ELISA or stimulation of tube formation. For EC network formation, HAECs were seeded in Matrigel coated 96-well plates at a density of 10,000 cells/well, cultured at 5% CO2, 37°C for 24 h, and then the images were captured by Nikon TS100. The outgrowth of networks was evaluated by measurement of tube length using NIH image J.
Quantitative real-time PCR
Macrophages and ECs isolated from WT and SKI mice were exposed to hypoxia for the indicated time. Total RNA was extracted with Aurum™ Total RNA Mini Kit (Bio-Rad, Hercules, CA), and cDNA was synthesized using high capacity RNA-to-cDNA™ Kit (Life Technologies, Grand Island, NY) according to manufacturer's instructions. Quantitative real-time PCR was performed using gene-specific FAM-NFQ-conjugated TaqMan primers for mouse SERCA2, VCAM1, ERO1, VEGF. A VIC-NFQ-conjugated human 18S primer was used to normalize mRNA expression levels. Expression was analyzed using the comparative CT (ΔΔCT) with StepOne™ Real Time PCR Software (Life technology, Carlsbad, CA).
Immunofluorescence
After femoral artery ligation, gastrocnemius muscle was harvested from WT and SKI mice at 3 d, tissue cryosections were incubated with antibodies specific to ERO1 and F4/80 (Abcam, Cambridge, MA) or CD31 (BD Bioscience, San Jose, CA), followed by incubation with Alexafluor antibodies (Life Technologies, Grand Island, NY) and DAPI mounting media (Vector lab, Burlingame, CA). Images were visualized and captured by Nikon ECLIPSE 80i. Infiltrated macrophages were counted with Image J and were expressed as the ratio to the total number of nuclei in the same field.
Statistic analysis
All data obtained in vitro represent three independent experiments. Results are expressed as mean ± standard error. For animal studies, 5 mice were included in each group. Statistical analysis was performed using GraphPad Prism, and comparisons were made with Student’s t-test, or one- or two-way ANOVA. A P value <0.05 was considered significant.
Results
SERCA 2 C674 is required for ERO1 induction under ischemic conditions in vivo and hypoxic conditions in vitro
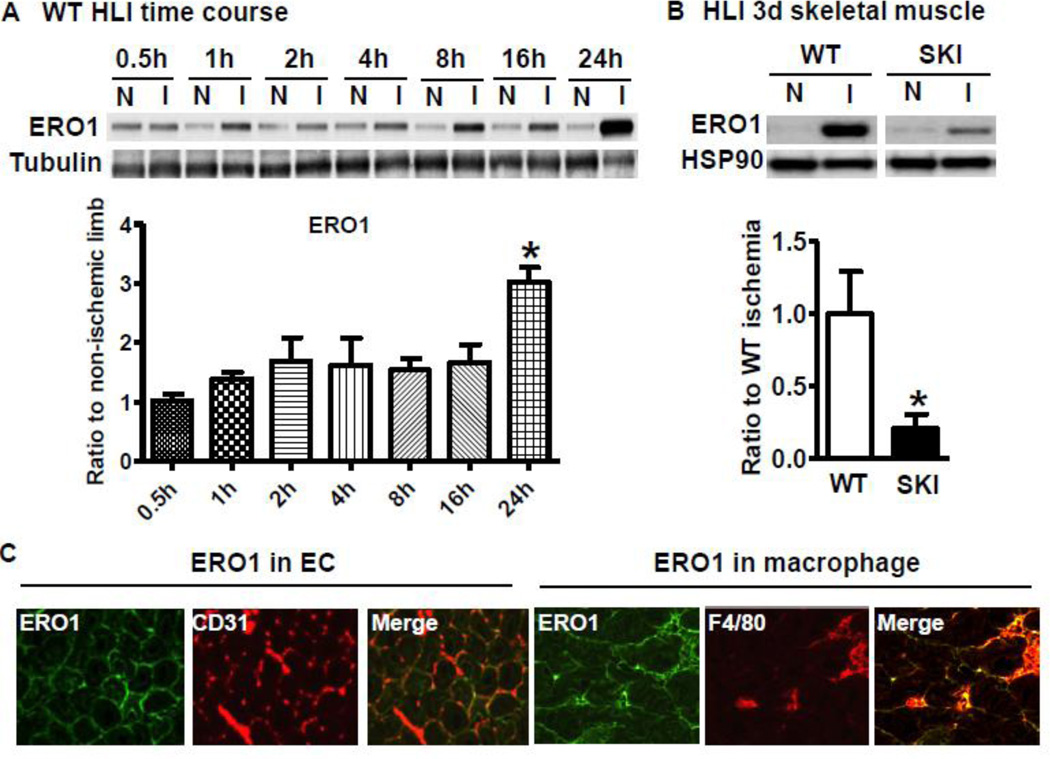
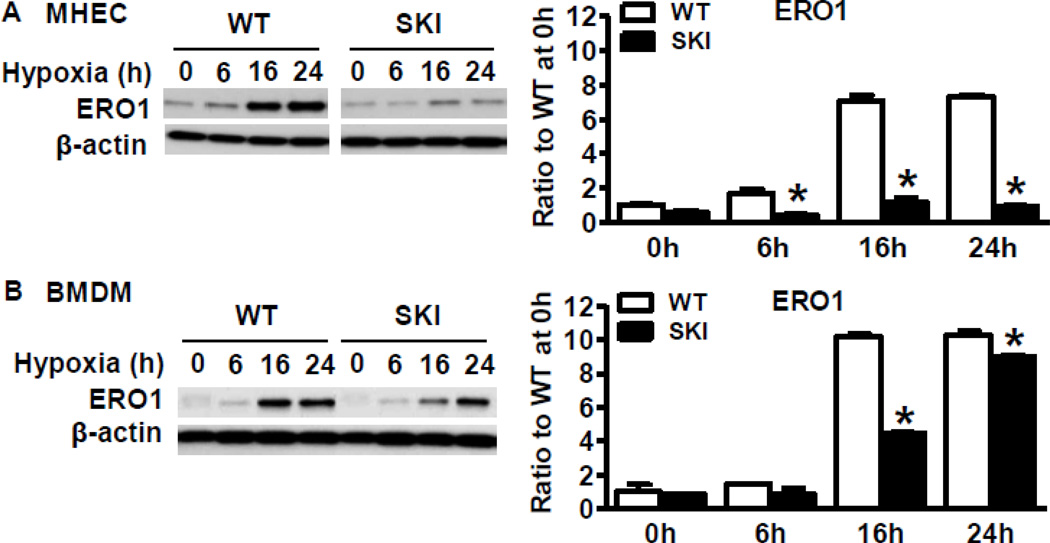
As shown in Figure 1A, ERO1 protein expression was induced in WT ischemic gastrocnemius muscle as early as 1 h after ischemia and was increased time-dependently over 24 h. Compared with robust induction of ERO1 in the WT ischemic limb 3 d after HLI, SKI ischemic limb muscle had significantly decreased induction of ERO1 (Figure 1B). To determine the potential sources of ERO1 in ischemic muscles, double immune-staining was applied to the ischemic muscle sections. As shown in Figure 1C, ERO1 was co-localized with CD31 (EC marker) and F4/80 (macrophage marker) at 3 d postsurgery. To test the expression levels in individual cells, we cultured ECs from heart and macrophages from bone marrow from both WT and SKI mice. As shown in Figure 2A and 2B, hypoxia in vitro induced robust expression of ERO1 in WT ECs (Figure 2A) and macrophages (Figure 2B), but there was significantly less ERO1 induced in SKI.
Figure 1. SERCA 2 C674 regulates ERO1 expression under ischemic conditions in vivo.
A. ERO1 expression at the indicated times in the non-ischemic muscle (N) and ischemic muscle (I) of WT mice after femoral artery ligation. Tubulin acts as a loading control. *P<0.05, vs. 0.5 h, n=5. B. Comparison of ERO1 protein expression in the hind limb muscles between WT and SKI mice at 3 d HLI. For the densitometric analysis, ERO1 protein was corrected by loading control heat shock protein 90 (HSP90). *P<0.05, vs. WT, n=5. C. Co-localization of ERO1 with CD31 (EC marker) and F4/80 (macrophage marker) in WT ischemic muscle at 3 d after HLI. Green fluorescence represents ERO1, and red fluorescence represents CD31 or F4/80.
Figure 2. SERCA 2 C674 regulates ERO1 expression under hypoxic conditions in vitro.
A. ERO1 protein expression in WT or SKI ECs exposed to hypoxia for indicated times. For the densitometric analysis, ERO1 protein was corrected by β-actin. *P<0.05, vs. WT, n=3. B. ERO1 protein expression in WT or SKI macrophages exposed to hypoxia for indicated times. For the densitometric analysis, ERO1 protein was corrected by β-actin. *P<0.05, vs. WT, n=3.
SERCA 2 C674 and ERO1 mediate endothelial cell VCAM1 expression and macrophage adhesion to ECs under hypoxia
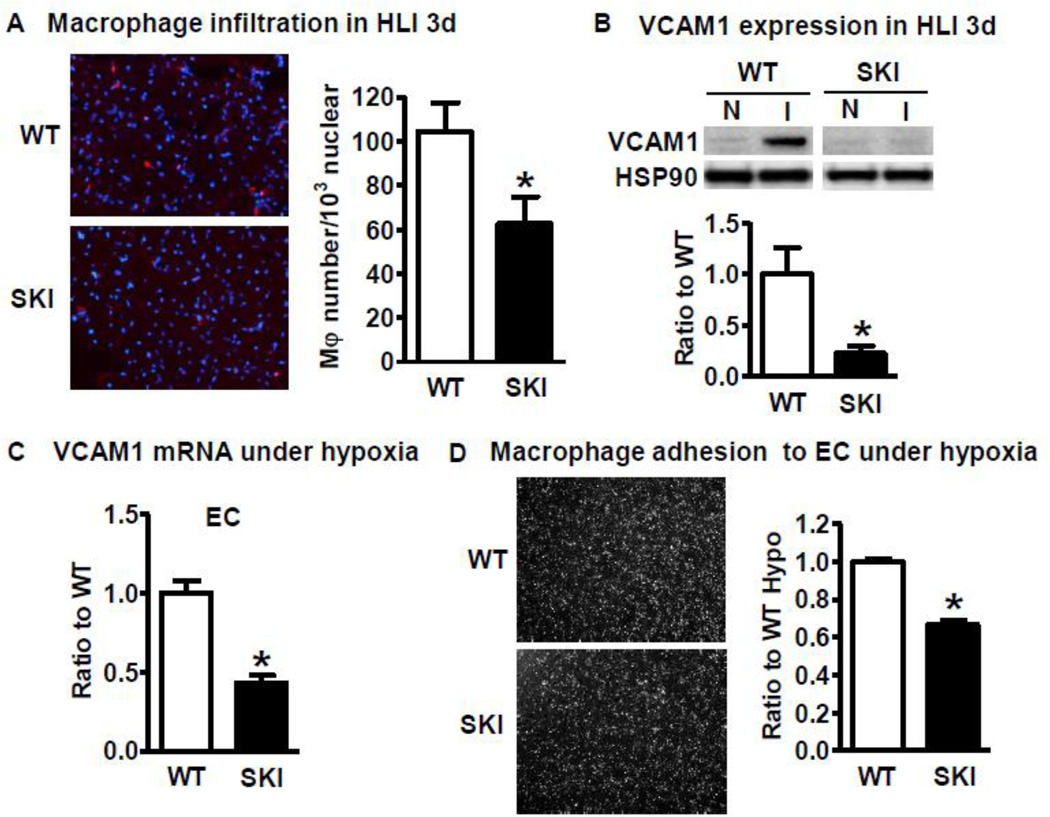
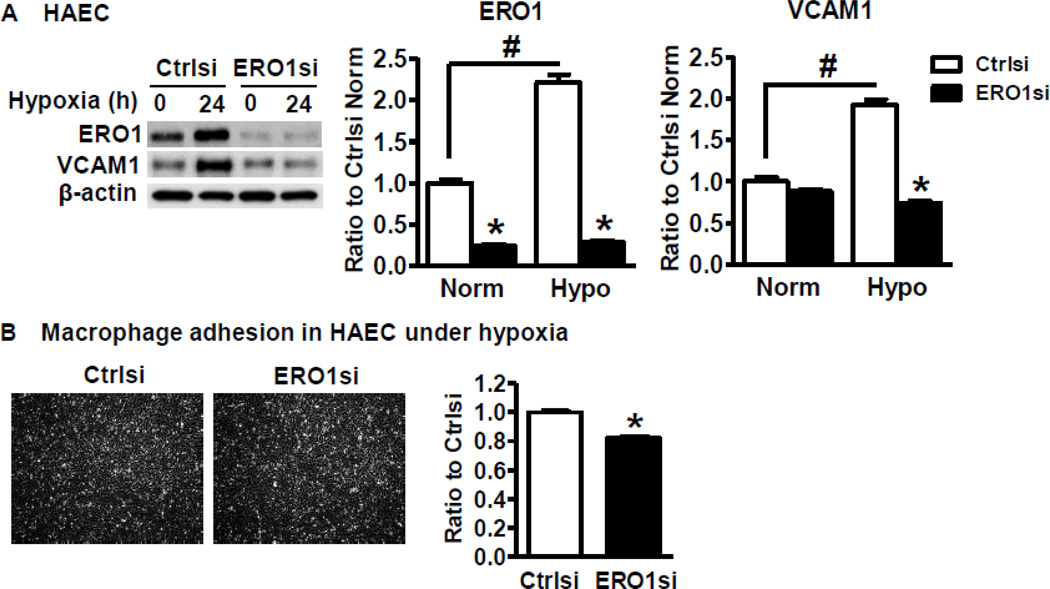
The initial step during ischemia is the infiltration of macrophages to ischemic tissue and the release of growth factors such as VEGF to stimulate angiogenesis. We therefore tested macrophage infiltration in the ischemic muscle at 3 d after HLI, as shown in Figure 3A, macrophage infiltration as determined by F4/80 antibody was significantly lower in the SKI ischemic limb. VCAM1 is the major adhesion molecule expressed in ECs to recruit macrophages. As shown in Figure 3B, ischemia induced robust expression of VCAM1 in the WT ischemic limb but not in SKI at 3 d after HLI. Next, we tested the cellular site of increased VCAM1 under hypoxia. Consistent with in vivo results, hypoxia induced less VCAM1 mRNA expression in SKI ECs compared with WT ECs (Figure 3C), and the VCAM1 expression was very low or undetectable in macrophages (data not shown), indicating that the major cellular site of the increased VCAM1 in ischemic limb was in ECs. In addition, macrophage adhesion to ECs from both WT and SKI after ECs were exposed to hypoxic conditions was compared. As shown in Figure 3D, SKI ECs had less macrophage adhesion compared with WT ECs. To determine if hypoxia mediated VCAM1 expression is regulated by ERO1, we used siRNA to downregulate ERO1 in HAECs because VCAM1 was difficult to detect in cultured mouse ECs from both WT and SKI. In cultured HAECs, hypoxia increased VCAM1 expression level along with that of ERO1, and downregulation of ERO1 significantly decreased both hypoxia-induced VCAM1 expression (Figure 4A) and macrophage adhesion (Figure 4B). This indicates that the ERO1 induced in ECs under hypoxic conditions is at least in part responsible for increased VCAM1 and macrophage adhesion.
Figure 3. SERCA 2 C674 regulates macrophage infiltration under ischemic conditions in vivo and macrophage adhesion under hypoxic conditions in vitro.
A. Macrophage infiltration in WT and SKI ischemic muscle at 3 d after HLI. Red fluorescence represents macrophage marker F4/80, blue represents DAPI. *P<0.05, vs. WT, n=5. B. VCAM1 protein expression at 3 d in the non-ischemic muscle (N) and ischemic muscle (I) of WT and SKI mice after femoral artery ligation. HSP90 acts as a loading control. *P<0.05, vs. WT, n=5. C. VCAM1 mRNA expression in WT and SKI ECs after 24 h under hypoxic conditions. *P<0.05, vs. WT, n=3. D. Macrophage adhesion to WT or SKI ECs that have been exposed to hypoxia for 24 h. The number of macrophages attached to WT or SKI ECs was counted with Image J. *P<0.05, vs. WT, n=3.
Figure 4. ERO1 is required for EC VCAM1 expression and macrophage adhesion to ECs under hypoxic conditions.
A. ERO1 and VCAM1 expression in HAECs transfected with control siRNA (Ctrlsi) and ERO1 siRNA (ERO1si) and exposed to hypoxia for 24 h. For the densitometric analysis, ERO1 and VCAM1 were corrected by β-actin. *P<0.05, vs. Ctrlsi, #P<0.05 vs. normoxia, n=3. B. Macrophage adhesion to WT ECs, which have been transfected with control siRNA (Ctrlsi) or ERO1 siRNA (ERO1si) and exposed to hypoxia for 24 h. The number of macrophages attached to ECs was counted with Image J. *P<0.05, vs. Ctrlsi, n=3.
SERCA 2 C674 mediates macrophage VEGF production under hypoxia
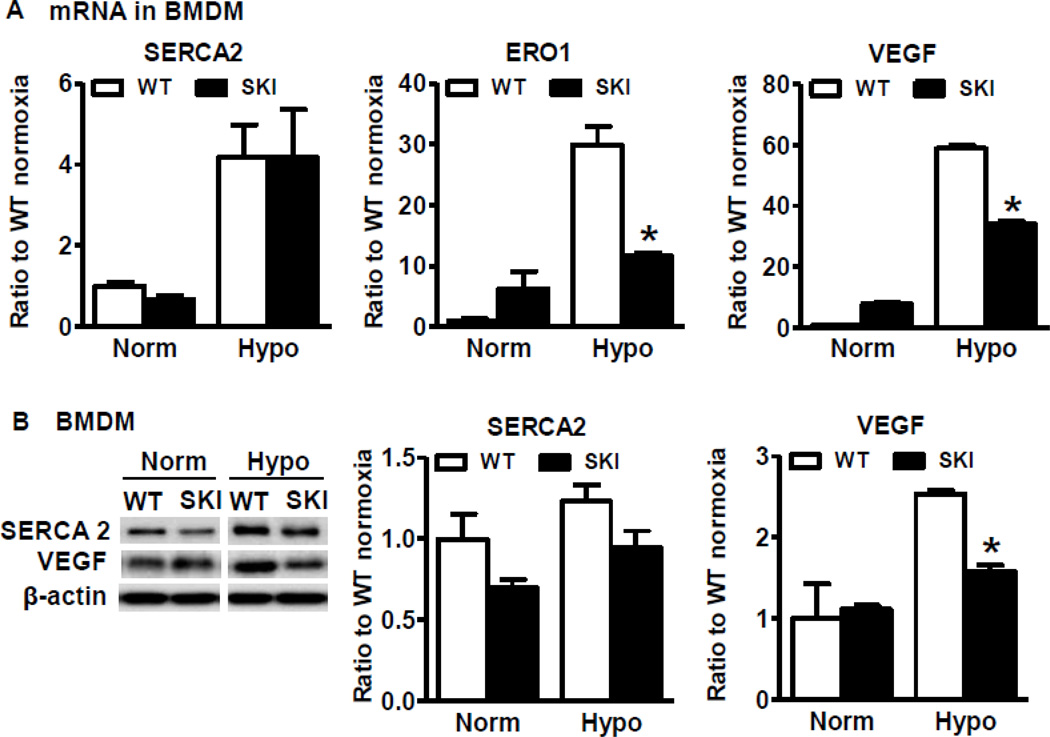
To determine if SERCA 2 C674 regulates VEGF production in macrophages under hypoxia, we compared VEGF mRNA level in BMDM from both WT and SKI mice. As shown in Figure 5A, though hypoxia induced comparable increases in SERCA 2 mRNA levels between WT and SKI, hypoxia induced much higher mRNA levels of both VEGF and ERO1 in WT compared with SKI. SERCA 2 protein levels tended to increase after hypoxia, but there was no significant difference between WT and SKI (Figure 5B). Similar to its mRNA levels under hypoxia, there was more VEGF protein expression in WT than in SKI macrophages under hypoxia (Figure 5B).
Figure 5. SERCA 2 C674 regulates macrophage VEGF production under hypoxic conditions.
A. SERCA 2, ERO1 and VEGF mRNA expression in WT or SKI macrophages exposed to hypoxia for 24 h. *P<0.05, vs. WT, n=3. B. SERCA 2 and VEGF protein expression in WT or SKI macrophages exposed to hypoxia for 24 h. For the densitometric analysis, SERCA 2 and VEGF protein were corrected by β-actin. *P<0.05, vs. WT, n=3.
SERCA 2 C674-mediated VEGF production in hypoxic macrophage conditioned media induces EC proliferation and network formation
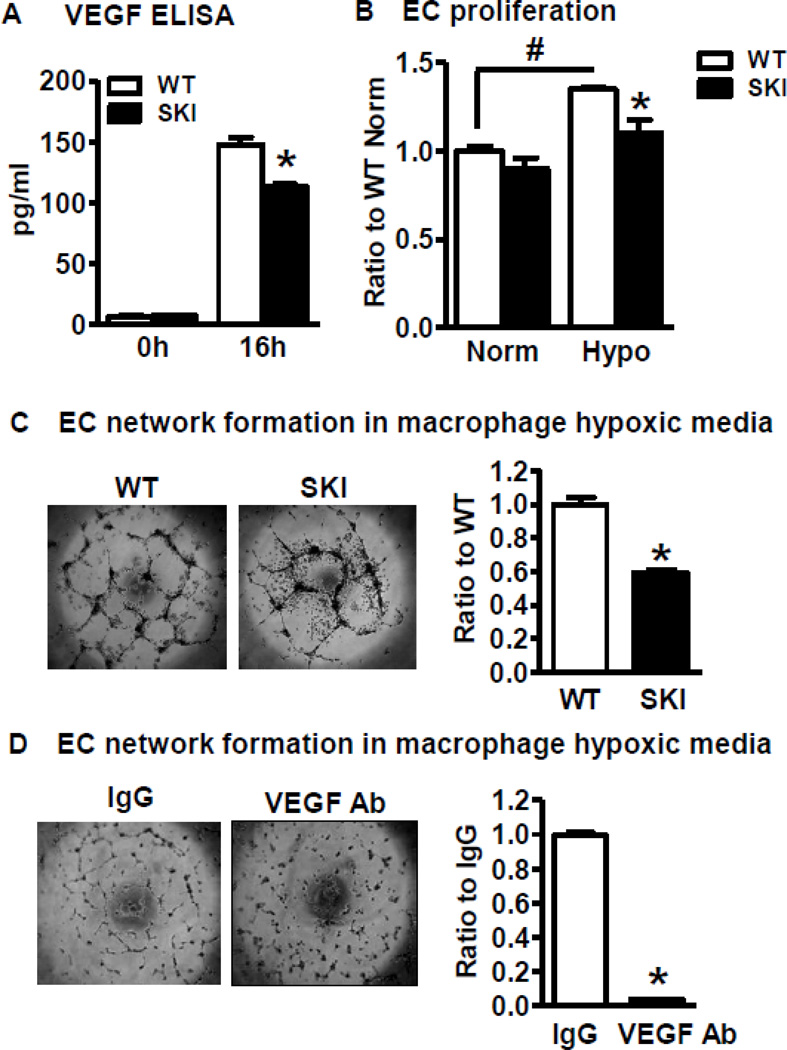
Next, we tested the effects of media from hypoxic macrophages from both WT and SKI mice on EC functions. As shown in Figure 6A, the secretion of VEGF into the supernatant under hypoxic conditions was more in WT compared to SKI macrophages (Figure 6A). Conditioned media from both WT and SKI hypoxic macrophages increased EC proliferation, but the media from hypoxic SKI macrophages had less mitogenic effects in EC proliferation compared with that from WT macrophages (Figure 6B). Though media from normoxic macrophages had no effect on EC network formation (not shown), hypoxic WT macrophage conditioned media dramatically increased EC network formation. Compared with hypoxic WT macrophage media, fewer EC networks formed when treated with conditioned media from hypoxic SKI macrophages (Figure 6C). To determine if the angiogenic effects of hypoxic macrophage conditioned media is mainly due to VEGF, a VEGF neutralizing antibody was used. As shown in Figure 6D, VEGF neutralizing antibody totally blocked the EC network formation induced by hypoxic macrophage conditioned media, indicating that the major angiogenic factor released by mouse macrophages under hypoxic conditions was VEGF.
Figure 6. SERCA 2 C674 regulated VEGF production by hypoxic macrophages induces endothelial cell angiogenic function.
A. VEGF concentration measured by ELISA in the culture media of WT or SKI macrophages exposed to hypoxia for 16 h. *P<0.05, vs. WT, n=3. B. Proliferation assay of ECs exposed to conditioned media of hypoxic macrophages. ECs were seeded into 96-well plates and treated with media from WT or SKI macrophages exposed to normoxia (Norm) or hypoxia (Hypo) for 72 h. Proliferation of ECs was analyzed with water soluble tetrazolium salt assay. *p<0.05, vs. WT, n=3. C. Network formation by ECs exposed to media of hypoxic macrophages. ECs were seeded into BD Matrigel™ coated 96-well plates and treated with conditioned media from WT or SKI macrophages exposed to hypoxia for 16 h. EC network formation was determined by network length measured by Image J. *P<0.05, vs. WT, n=3. D. Effect of neutralizing anti-VEGF antibody on network formation by ECs exposed to media of hypoxic macrophages. ECs were seeded in BD Matrigel™ coated 96-well plates and treated with conditioned media from hypoxic macrophages together with rabbit IgG or VEGF neutralizing antibody (VEGF Ab). Network formation was compared after 16 h treatment. *P<0.05, vs. IgG, n=3.
Discussion
Low oxidant levels including those during hypoxia increase SERCA 2 activity by increasing C674 glutathione adducts, but higher and prolonged oxidant levels cause irreversible oxidation and inactivation of SERCA 2 [23, 24]. We have identified SERCA 2 C674 as the major cysteine to be S-glutathiolated which increases SERCA 2 Ca2+ uptake activity [1]. The irreversible oxidation of C674 occurs in atherosclerosis [1], restenosis [25, 26], aortic stiffness [27] and cardiac dysfunction [26, 28]. All these previous studies suggest that the redox status of SERCA 2 C674 is involved in cardiovascular homeostasis.
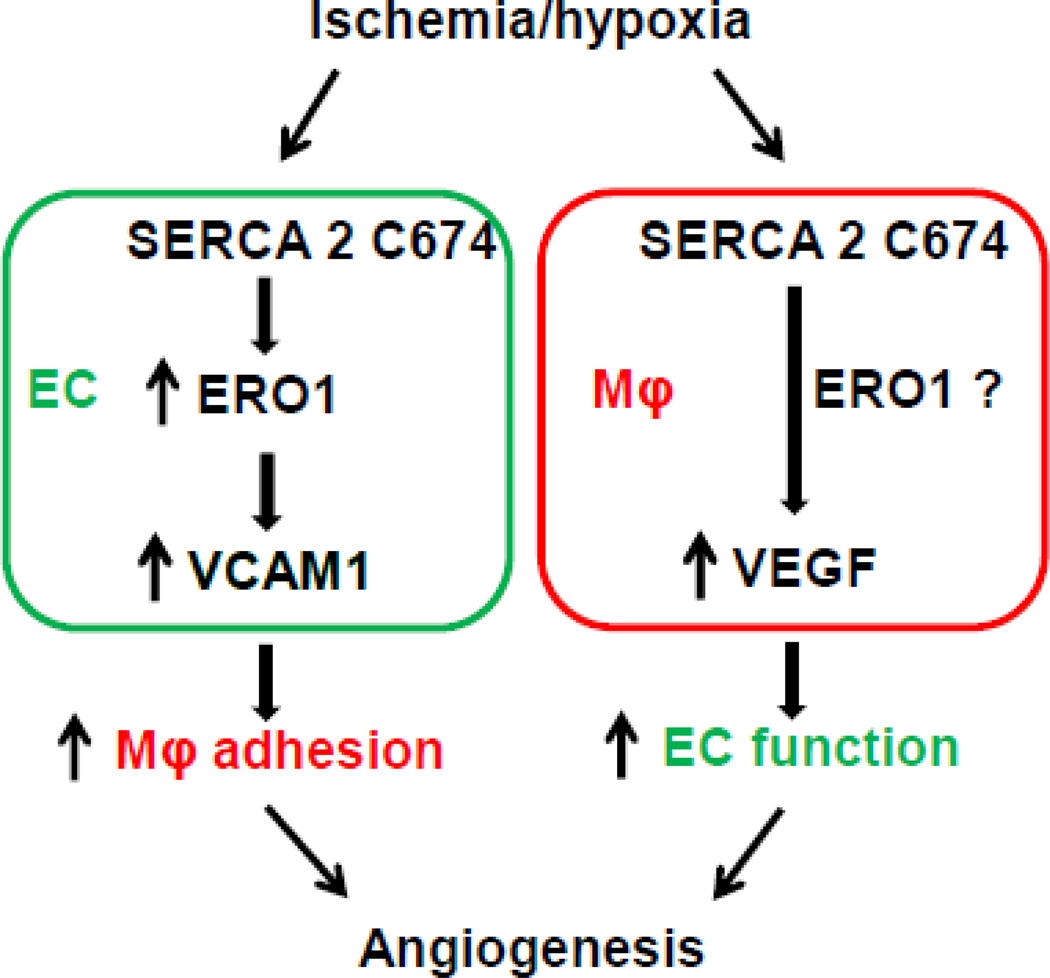
Using the C674S heterozygous mouse to mimic the partial irreversible oxidation of SERCA 2 C674 that occurs under disease conditions, we recently reported that this SKI mouse exhibits impaired hind limb revascularization and its ECs have impaired angiogenic behavior [4]. Here our novel results demonstrate the dual roles of SERCA 2 C674 in both ECs and macrophages, the two major cells involved in ischemic revascularization. Both EC-mediated macrophage adhesion and macrophage-mediated VEGF secretion in response to hypoxia are involved. Our hypothesis as shown in Figure 7, is that on one hand, SERCA 2 C674 regulates ERO1 induction in EC under hypoxia, which in turn promotes macrophage adhesion by elevating VCAM1 expression. On the other hand, SERCA 2 C674 regulates VEGF production in macrophages under hypoxic conditions to stimulate EC angiogenesis. As we showed that Ca2+ stores are partially depleted in SKI ECs [4], the importance of SERCA 2 C674 on EC adhesion and macrophage VEGF production shown here is likely through dysregulation of ER Ca2+ homeostasis under ischemia/hypoxia conditions, though we were unable to study Ca2+ under hypoxic conditions to show this directly.
Figure 7. SERCA 2 C674 regulates ischemia-induced angiogenesis by effects on EC ERO1, VCAM1 and adhesion of macrophages, as well as on macrophage production of VEGF.
Formation of intra- and intermolecular disulfide bonds is an essential step in the synthesis of secretory proteins. This process occurs in the ER and requires an oxidative environment with the action of several chaperones and folding catalysts. During protein folding, ERO1 oxidizes protein disulfide isomerase, which then directly catalyzes the formation of disulfide bonds in folding proteins [29]. Oxygen is the major source of oxidizing power for disulfide bond formation to maintain protein correct folding. Under hypoxia, the limited availability of oxygen halts the whole cascade, which results in the accumulation of misfolded proteins in the ER that triggers a process known as the unfolded protein response marked by increased transcription of chaperones, apoptosis and ER associated degradation [7]. We found ERO1 was strongly upregulated in WT ischemic limbs and this induction was attenuated in SKI mice. In addition, ischemia induced ERO1 expression in both ECs and macrophages. The impaired angiogenic outcome in SKI mice associated with less ERO1 induction [4], suggests that ERO1 may serve as a protective ER stress marker, perhaps to mediate proper protein folding under limited oxygen conditions.
The important contribution of macrophage infiltration to angiogenesis has been confirmed not only in animals [18] but also in patients [30]. CD4+ T cells control the arteriogenic response to acute HLI by recruiting macrophages, which trigger the development of collaterals through VEGF synthesis [31] and enhance smooth muscle cell recruitment and growth to promote collateral vessel formation in the ischemic limb [18]. In addition, macrophages protect tissues from necrosis and promote tissue repair by increasing post-ischemic angiogenesis and muscle regeneration during HLI [32]. Indeed, local injection of macrophages into ischemic muscle stimulates capillary formation and arteriogenesis in ischemic limbs through upregulation of basic fibroblast growth factor and VEGF [33]. Here we found that macrophage infiltration and VCAM1 expression in ischemic muscle were both SERCA C674-dependent. To determine the connection between EC and macrophage infiltration, we examined adhesion molecules in ECs and their effects on macrophage behavior under hypoxia. We found that hypoxia induced VCAM1 expression in cultured ECs as well as macrophage adhesion, both of which were also ERO1- and SERCA C674-dependent. The regulation by ERO1 of VCAM1 expression in ECs shown here is supported by other reports showing that RONS generated during the transfer of electrons from protein thiols to molecular oxygen by ERO1 [34] upregulate VCAM1 expression in ECs [35–37]. Our results further demonstrate an important role in ECs of SERCA 2 C674 on hypoxia-induced ERO1 induction, VCAM1 expression, and macrophage adhesion.
We found that SERCA 2 C674 is required for hypoxia-induced VEGF production by macrophages but that total SERCA 2 mRNA and protein levels under hypoxia were comparable between WT and SKI macrophages. In addition, we found a strong causal association between macrophage VEGF production during hypoxia and EC angiogenic behavior, which is also SERCA 2 C674-dependent. Similar to ECs, we found the induction of ERO1 was less in SKI macrophages. Another study showed that hypoxia induced VEGF production in macrophages, and that ERO1 mRNA was upregulated under hypoxic conditions [38]. ERO1 is also required for hypoxia-induced VEGF production in mouse embryonic fibroblasts [39]. It is possible that ERO1 is involved in SERCA 2 C674 mediated VEGF secretion in macrophages under hypoxic conditions. Because we lacked the tools to manipulate ERO1 in macrophages to decipher its role in VEGF production, we can only conclude that the induced ERO1 in macrophages under hypoxia is SERCA C674-dependent, and that the induced ERO1 is a potential mediator of VEGF production by hypoxic macrophages.
In summary, the availability of redox-modifiable SERCA 2 C674 promotes ischemic revascularization as we showed previously in part by the fact that glutathione adducts increase SERCA activity, EC Ca2+ stores, and Ca2+ entry. Here we show that the redox active SERCA thiol also promotes angiogenic functions during hypoxia in ECs through ERO1 and increasing macrophage adhesion, as well as in macrophages through secretion of VEGF. The significance of this contribution could be in identifying specific molecular markers, which can serve as predictors of EC and macrophage status during the angiogenic response to ischemia, and its potential predictive ability of a beneficial or detrimental outcome of tissue ischemia. The knowledge attained could enable the development of new therapies that would be applicable not only for peripheral artery disease, but also broadly for ischemia in heart, brain, kidney and other tissues.
Highlights.
This study highlights the unrecognized interaction of two ER proteins, SERCA 2 C674 and ERO1, in angiogenic response to ischemia/hypoxia.
In endothelial cells, SERCA 2 C674 regulates ERO1 induction under hypoxia, which in turn promotes macrophage adhesion by elevating VCAM1 expression.
In macrophages, SERCA 2 C674 regulates VEGF production under hypoxic conditions to stimulate endothelial cell angiogenesis.
Acknowledgements
This work was supported by American Diabetes Association award 7-09-JF-69 (XT), the National Institutes of Health grants HL031607-30 (RAC, XT), R37 HL104017 (RAC), NHLBI T32 training grant HL07969 (MDT) and a Martin Luther King, Jr. Fellowship from Boston University (MDT), as well as the NHLBI-sponsored Boston University Cardiovascular Proteomics Center (Contract No. N01-HV-28178, RAC).
Abbreviations
- BMDM
bone marrow derived macrophage
- EC
endothelial cell
- ELISA
enzyme linked immunosorbent assay
- ER
endoplasmic reticulum
- ERO1
endoplasmic reticulum oxidoreductin-1
- GSH
glutathione
- HAECs
human aortic endothelial cells
- HLI
hind limb ischemia
- HSP90
heat shock protein 90
- Redox
reduction/oxidation
- RONS
reactive oxygen and nitrogen species
- SERCA 2
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- SKI
SERCA 2 C674S knockin
- VCAM1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista AM, Thompson MD, Bolotina VM, Tong X, Cohen RA. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med. 2012;53:2327–2334. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evangelista AM, Thompson MD, Weisbrod RM, Pimental DR, Tong X, Bolotina VM, Cohen RA. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid Redox Signal. 2012;17:1099–1108. doi: 10.1089/ars.2011.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson MD, Mei Y, Weisbrod RM, Silver M, Shukla PC, Bolotina VM, Cohen RA, Tong X. Glutathione adducts on sarcoplasmic/endoplasmic reticulum Ca2+ ATPase C674 regulate endothelial cell calcium stores and angiogenic function as well as promote ischemic blood flow recovery. J Biol Chem. 2014;289:19907–19916. doi: 10.1074/jbc.M114.554451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med. 2010;16:396–399. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei Y, Thompson MD, Cohen RA, Tong X. Endoplasmic reticulum stress and related pathological processes. J Pharmacol Biomed Anal. 2013;1:1000107. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. Mitofusin 2 in POMC Neurons Connects ER Stress with Leptin Resistance and Energy Imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, Hilton B, Wolkowicz R, Sussman MA, Glembotski CC. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ Res. 2010;106:307–316. doi: 10.1161/CIRCRESAHA.109.203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang CP, Han S, Li G, Tabas I, Tall AR. Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment. Diabetes. 2012;61:2609–2620. doi: 10.2337/db11-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Fernandez-Hernando C, Suarez Y, Schleicher M, Hao Z, Wright PL, DiLorenzo A, Kyriakides TR, Sessa WC. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc Natl Acad Sci U S A. 2009;106:17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, Bode C, Liao JK. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation. 2012;126:2236–2247. doi: 10.1161/CIRCULATIONAHA.111.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology. 2011;54:1777–1789. doi: 10.1002/hep.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoneich C, Sharov VS. Mass spectrometry of protein modifications by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;41:1507–1520. doi: 10.1016/j.freeradbiomed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem J. 2006;394:605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong X, Hou X, Jourd'heuil D, Weisbrod RM, Cohen RA. Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ Re. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin F, Siwik DA, Pimentel DR, Morgan RJ, Biolo A, Tu VH, Kang YJ, Cohen RA, Colucci WS. Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am J Physiol Heart Circ Physiol. 2014;306:H1453–1463. doi: 10.1152/ajpheart.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2012;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Re. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari DM, Soling HD. The protein disulphide-isomerase family: unravelling a string of folds. Biochem J. 1999;339(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 30.Patel AS, Smith A, Nucera S, Biziato D, Saha P, Attia RQ, Humphries J, Mattock K, Grover SP, Lyons OT, Guidotti LG, Siow R, Ivetic A, Egginton S, Waltham M, Naldini L, De Palma M, Modarai B. TIE2-expressing monocytes/macrophages regulate revascularization of the ischemic limb. EMBO Mol Med. 2013;5:858–869. doi: 10.1002/emmm.201302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 32.Brechot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Brechot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirose N, Maeda H, Yamamoto M, Hayashi Y, Lee GH, Chen L, Radhakrishnan G, Rao P, Sasaguri S. The local injection of peritoneal macrophages induces neovascularization in rat ischemic hind limb muscles. Cell Transplant. 2008;17:211–222. doi: 10.3727/000000008783906919. [DOI] [PubMed] [Google Scholar]
- 34.Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012;24:1548–1555. doi: 10.1016/j.cellsig.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Chung J, Ha IS, Yi K, Lee JE, Kang HG, Choi I, Oh KH, Kim JY, Surh CD, Ahn C. Hydrogen peroxide increases human leukocyte adhesion to porcine aortic endothelial cells via NFkappaB-dependent up-regulation of VCAM-1. Int Immunol. 2007;19:1349–1359. doi: 10.1093/intimm/dxm104. [DOI] [PubMed] [Google Scholar]
- 37.Lee YW, Kuhn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001;33:83–94. doi: 10.1006/jmcc.2000.1278. [DOI] [PubMed] [Google Scholar]
- 38.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, Greten FR, Rius J, Lewis CE. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene. 2005;24:1011–1020. doi: 10.1038/sj.onc.1208325. [DOI] [PubMed] [Google Scholar]