Abstract
The incretin hormone glucagon-like peptide-1 (Glp1) is cardioprotective in models of ischemia-reperfusion injury, myocardial infarction and gluco/lipotoxicity. Inflammation is a factor in these models, yet it is unknown whether Glp1 receptor (Glp1r) agonists are protective against cardiac inflammation. We tested the hypothesis that the Glp1r agonist Exendin-4 (Ex4) is cardioprotective in mice with cardiac-specific monocyte chemoattractant protein-1 overexpression. These MHC-MCP1 mice exhibit increased cardiac monocyte infiltration, endoplasmic reticulum (ER) stress, apoptosis, fibrosis and left ventricular dysfunction. Ex4 treatment for 8 weeks improved cardiac function and reduced monocyte infiltration, fibrosis and apoptosis in MHC-MCP1 mice. Ex4 enhanced expression of the ER chaperone glucose-regulated protein-78 (GRP78), decreased expression of the pro-apoptotic ER stress marker CCAAT/-enhancer-binding protein homologous protein (CHOP) and increased expression of the ER calcium regulator Sarco/Endoplasmic Reticulum Calcium ATPase-2a (SERCA2a). These findings suggest that the Glp1r is a viable target for treating cardiomyopathies associated with stimulation of pro-inflammatory factors.
Keywords: Glucagon-like peptide-1, Exendin-4, Monocyte chemoattractant protein-1, Inflammation, Cardiomyopathy
1. Introduction
Glucagon-like peptide-1 (Glp1) is gut hormone that stimulates insulin secretion via a pancreatic Glp1 receptor (Glp1r) [1]. Glp1r agonists also improve cardiac function during ischemia-reperfusion injury, myocardial infarction and diabetic cardiomyopathy [2]. Inflammation is common to many cardiomyopathies [3], and Glp1r agonists display anti-inflammatory properties including inhibition of monocyte adhesion to aortic endothelial cells [4], polarization of macrophages towards the anti-inflammatory M2 population [5] and reduction of cardiac expression of inflammatory cytokines in insulin resistant mice [6]. Thus, Glp1r agonists may provide cardioprotective effects via their anti-inflammatory actions.
The present studies directly address whether Glp1r activation is cardioprotective in mice with cardiac-specific overexpression of monocyte chemoattractant protein-1 (MCP1). MCP1 overexpression stimulates recruitment of monocytes to the myocardium. Interestingly, these monocytes do not appear to become activated and instead exhibit increased apoptosis. This, in turn, leads to a late-onset inflammatory state characterized by elevated cytokine levels, fibrosis, endoplasmic reticulum (ER) stress, cardiomyocyte apoptosis and subsequent left ventricular (LV) dysfunction [3]. We show that the Glp1r agonist Exendin-4 (Ex4) ameliorates LV dysfunction, reduces macrophage infiltration, cardiac fibrosis, ER stress and monocyte/cardiomyocyte apoptosis in this model. Ex4 treatment also stimulates expression of the Sarco/Endoplasmic Reticulum Calcium ATPase-2a (SERCA2a), a key enzyme for the regulation of cardiomyocyte calcium flux and contractility. These results suggest that Glp1r agonists can be used therapeutically to treat cardiomyopathies associated with the stimulation of pro-inflammatory factors.
2. Materials and Methods
2.1. Animals
Three month old male MHC-MCP1 mice were implanted with osmotic minipumps delivering PBS or Ex4 (24 nmol·kg-1·day-1 (100 μg·kg-1·day-1), a dose chosen based on therapeutic efficacy in previous mouse experiments [7]) for 8 weeks and were compared to FVB/N mice receiving PBS (WT). Cardiac function was assessed via echocardiography. Hearts were then harvested for additional analyses. Procedures were approved by the Animal Care and Use Committee of the University of Central Florida.
2.2. Echocardiography
Mice were anesthetized with 0.5–2.0% isoflurane (AErrane, Baxter, USA) mixed with oxygen. Echocardiography was performed with a 15-MHz high-frequency transducer (Agilent Technologies SONOS 4500, Philips Medical System). A two-dimensional short-axis view of the left ventricle was obtained at the level of the papillary muscles, and two-dimensionally targeted M-mode tracings were recorded at a sweep speed of 100 mm/s. Fractional shortening (FS) was calculated as: FS (%) = [(LVEDD − LVESD) / LVEDD] × 100, where LVEDD and LVESD indicate LV end-diastolic and end-systolic dimension, respectively. Data from three to five consecutive selected cardiac cycles were analyzed and averaged.
2.3. Histology
Ventricular sections were fixed in 10% phosphate-buffered formaldehyde and were paraffin embedded. Sections (5-μm) were stained with hematoxylin and eosin (H&E), and Masson's trichrome for histopathological analysis. Samples were imaged and quantitatively assessed for myocardial fibrosis by taking 2-3 sections in 4 randomly selected fields per section and determining the interstitial collagen volume fraction. The collagen volume fraction was calculated as a percentage of the sum of all blue-stained areas to the total ventricular areas by using the Aperio image analysis program.
2.4. TUNEL
Ventricular sections were assessed for cell death using CardioTACS in situ Apoptosis Detection Kit (Trevigen) per manufacturer's instructions. Cells with clear striations were scored as cardiomyocytes. TUNEL-positive infiltrating mononuclear cells were manually counted and expressed as a percentage of the total amount of infiltrating cells in five randomly selected fields of view.
2.5. Quantitative PCR
RNA was extracted from ventricles using TRIzol. First-strand cDNA was synthesized using 1 μg of total RNA (DNase-treated) using a Reverse Transcription System (Promega). Real-time PCR was performed using SYBR Green Master mix (Applied Biosystems). Primers were as follows: MCP1 forward, 5′-TTAAAAACCTGGATCGGAACCAA-3′ and reverse, 5′-GCATTAGCTTCAGATTTACGGG-3′; MCP1 induced protein (MCPIP) forward, 5′-TGTGCCTATCACAGACCAGCACAT-3′ and reverse, 5′-TCGGATTCATAGGCCAGGTTCACA-3′; and L32 forward, 5′-ACATTTGCCCTGAATGTGGT-3′ and reverse, 5′-ATCCTCTTGCCCTGATCCTT-3′. Gene expression was normalized to L32.
2.6. Immunoblots
Snap-frozen ventricles were pulverized in liquid nitrogen and treated with lysis buffer (0.5% NP40, 20mmol/L Tris-HCL, 150mmol/L NaCl, 1mmol/L EDTA, 1mmol/L EGTA, 2.5mmol/L Sodium Pyrophosphate, 1mmol/L Sodium Orthovanadate, 10μL/1mL Protease Inhibitor Cocktail (Sigma P8340), and 10μL/1mL Phosphatase Inhibitor Cocktail (Sigma)). Immunoblots were performed using antibodies for MCPIP (1:500), glucose-regulated protein-78 (GRP78; 1:1000), CCAAT/-enhancer-binding protein homologous protein (CHOP; 1:250), SERCA2 (1:1000), cysteine-aspartic protease 3 (Caspase 3; 1:500), and GAPDH (1:2000). MCPIP antibody was from Santa Cruz Biotechnology. All other antibodies were from Cell Signaling. Immunoblots were normalized to GAPDH expression.
2.7. Immunohistochemistry
Immunohistochemistry was performed with a rabbit primary antibody for macrophage F4/80 (ab74383, Abcam). Ventricular sections were heat retrieved with a 10mM Na-citrate buffer (pH 8.5) and incubated with the primary antibody overnight at 4°C. Anti-rabbit secondary antibodies were used for detection with 3,3′-diaminobenzidine chromagen (Vector Labs).
2.8. Cell Viability
HL-1 atrial cardiomyocytes (from Dr. William Claycomb, Louisiana State University Health Sciences Center) were grown in Claycomb complete media supplemented with 10% FBS, 0.1mM Norepinephrine, 2mM L-glutamine, and 1% Penicillin/Streptomycin. Cells were seeded on 96-well white clear bottom plates at a density of 2.0×105 cells/well and were treated with vehicle (PBS), 100nM MCP1 or 100nM MCP1+100nM Ex4. After 24 hours, cell viability was assessed using an MTT assay (Promega) according to manufacturer's instructions.
2.9. Statistical Analysis
Data were analyzed using Graphpad statistical software (SPSS Inc.) and are presented as mean ± SEM. Groups were compared using ANOVA followed by either Bonferroni or Tukey's post-test. Differences were significant at P<0.05.
3. Results
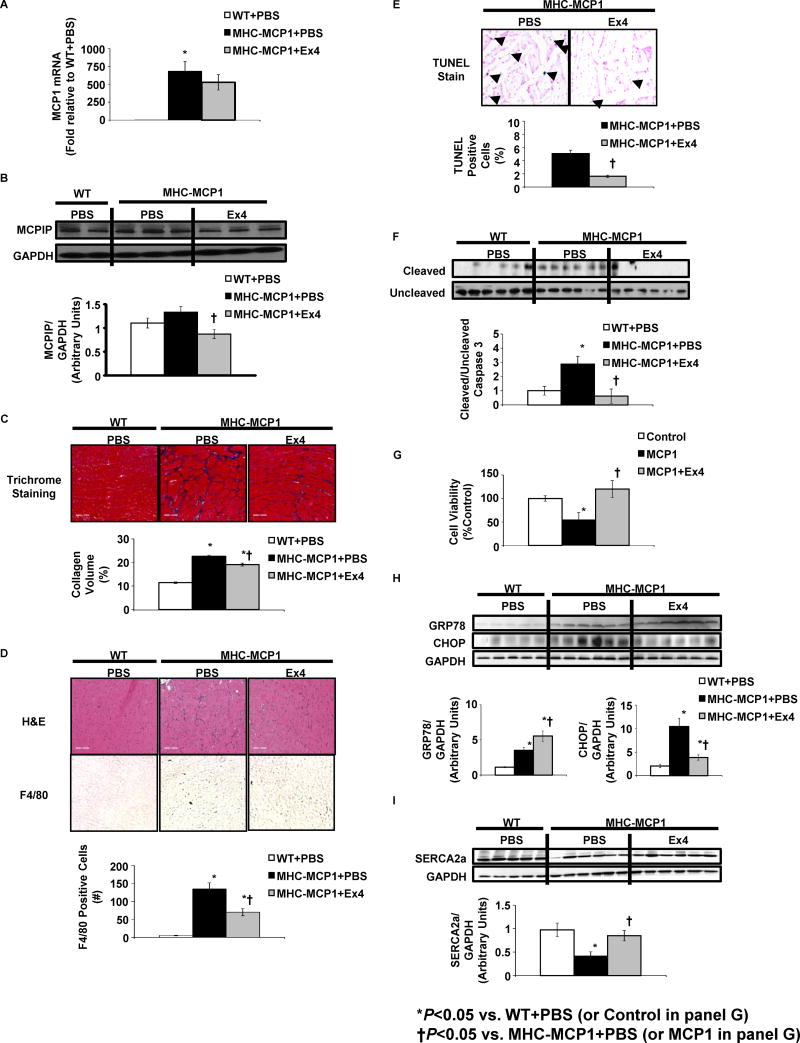
MHC-MCP1 mice exhibited decreased fractional shortening (FS) and ejection fraction (EF) compared to controls (Table 1). These functional parameters were markedly restored with Ex4 (Table 1). MHC-MCP1 mice also exhibited LV hypertrophy, and this was either partially or fully restored to control values by Ex4 (Table 1). Cardiac MCP1 expression was markedly increased in MHC-MCP1 mice treated with PBS, and this was not reduced by Ex4 (Fig. 1A). Levels of the MCP1-induced protein (MCPIP) were only modestly increased in MHC-MCP1 mice (Fig. 1B). MCPIP levels are markedly elevated in young MHC-MCP1 mice, but they decrease with age (P. Kolattukudy, personal communication). Interestingly, Ex4 treatment reduced MCPIP expression (Fig. 1B).
Table 1. Echocardiography parameters.
Cardiac function and morphology were assessed via echocardiography in wild-type (WT) mice infused with PBS and in MHC-MCP1 mice infused with either PBS or Ex4 for 8 weeks. FS: Fractional Shortening; EF: Ejection Fraction; IVSd: Interventricular Septum, diastole; LVIDd: LV Internal Dimension, diastole; LVPWd: LV Posterior Wall, diastole; IVSs: Interventricular Septum, systole; LVIDs: LV Internal Dimension, systole; LVPWs: LV Posterior Wall, systole. N=7 mice/group.
| WT | MHC-MCP1 | ||
|---|---|---|---|
| PBS | PBS | Ex4 | |
| FS (%) | 49.3 ± 0.7 | 35.7 ± 1.9* | 43.8 ± 1.4*† |
| EF (%) | 86.0 ± 0.6 | 71.9 ± 1.0* | 79.8 ± 0.8*† |
| IVSd (mm) | 0.70 ± 0.02 | 0.66 ± 0.03 | 0.70 ± 0.03 |
| LVIDd (mm) | 3.68 ± 0.02 | 3.94 ± 0.02* | 3.73 ± 0.03† |
| LVPWd (mm) | 0.75 ± 0.03 | 0.75 ± 0.02 | 0.75 ± 0.01 |
| IVSs (mm) | 1.39 ± 0.03 | 1.12 ± 0.03* | 1.26 ± 0.02*† |
| LVIDs (mm) | 1.87 ± 0.03 | 2.54 ± 0.03* | 2.15 ± 0.04*† |
| LVPWs (mm) | 1.38 ± 0.02 | 1.17 ± 0.02* | 1.25 ± 0.02* |
| LV mass (mg) | 89.2 ± 2.2 | 96.7 ± 2.2* | 91.3 ± 3.3 |
P<0.05 vs. WT+PBS;
P<0.05 vs. MHC-MCP1+PBS.
Figure 1. Ex4 treatment reduces fibrosis, inflammation, apoptosis and ER stress and stimulates SERCA2a expression in MHC-MCP1 mice.
Three month-old MHC-MCP1 mice were implanted with osmotic minipumps delivering either Ex4 (24 nmol/(kg·day)) or PBS and were compared to FVB/N wild-type (WT) mice receiving PBS. Hearts were harvested after 8 weeks of treatment. (A) MCP1 mRNA levels were quantified using reverse transcriptase PCR. (B) Protein extracts were subjected to immunoblot analysis with an antibody specific to MCPIP. Results were quantified as the ratio of MCPIP to the GAPDH loading control. A representative immunoblot image for 5-6 mice per treatment group is shown. (C) Fibrosis was assessed using trichrome staining. Collagen volume was calculated as a percentage of blue stain for the field of view. (D) Hematoxylin and eosin (H&E) staining was used to account for morphology and visualization of infiltrating cell nuclei. Also shown are representative photomicrographs demonstrating immunohistochemical staining with anti-F4/80 antibody. Positive-stained cells were visualized with diaminobenzidine (brown) and quantified. (E) Apoptosis was assessed using TUNEL analysis. Results were quantified as a percentage of TUNEL positive nuclei per total nuclei in the field of view. (F) Protein extracts were subjected to immunoblot analysis with antibodies specific to uncleaved and cleaved caspase 3. Results are presented as the ratio of cleaved to uncleaved caspase 3. (G) Cell viability was assessed using a MTT assay in HL-1 atrial cardiomyocytes treated with PBS (Control), 100nM MCP1 or 100nM MCP1 + 100nM Ex4 for 24h. (H) Protein extracts subjected to immunoblot analysis with antibodies specific to GRP78 and CHOP. Results were quantified as the ratio of GRP78 or CHOP to the GAPDH loading control. (I) Protein extracts were subjected to immunoblot analysis with antibodies specific to SERCA2a. Results were quantified as the ratio of SERCA2a to the GAPDH loading control. Data are presented as mean ± SEM for N=5-6 mice/group and 3 separate experiments for cell viability studies. *P<0.05 vs. WT+PBS (or Control for G); †P<0.05 vs.MHC-MCP1+PBS
Hearts from MHC-MCP1 mice displayed substantial fibrosis (Fig. 1C). Treatment with Ex4 attenuated this effect (Fig. 1C). Monocyte recruitment was also elevated in MHC-MCP1 mice, demonstrated by increased nuclear staining in non-cardiomyocyte regions (Fig. 1D). This was also markedly attenuated by Ex4 (Fig. 1D). Immunohistochemical analysis of cardiac sections from the same mice showed reduced expression of the inflammatory cell marker F4/80 in MHC-MCP1 treated with Ex4 compared to MHC-MCP1 mice receiving PBS (Fig. 1D).
Cardiac-specific MCP1 overexpression induced apoptosis primarily in inflitrating mononuclear cells but also in scattered cardiomyocytes as determined by TUNEL analysis. Treatment with Ex4 markedly attenuated apoptosis in both mononuclear cells and cardiomyocytes (Fig. 1E). Confirming these results, MHC-MCP1 mice displayed increased caspase 3 cleavage (a marker of apoptosis), and treatment with Ex4 reduced this effect (Fig. 1E). We next assessed whether Ex4 exerts direct cytoprotective effects on cardiomyocytes. Ex4 enhanced cell viability in HL-1 atrial cardiomyocytes treated with 100nM MCP1 for 24 hours (Fig. 1G).
ER stress was elevated in the hearts of MHC-MCP1 mice as shown by increased expression of the ER chaperone GRP78 and the pro-apoptotic factor CHOP (Fig. 1H). Interestingly, Ex4 further stimulated GRP78 levels these mice (Fig. 1H). Since GRP78 initiates a program to resolve ER stress [8], the stimulation of GRP78 expression by Ex4 may suggest a protective mechanism. Ex4 treatment reduced CHOP levels in MHC-MCP1 mice (Fig. 1H) further supporting the anti-apoptotic effects of Glp1r activation.
Inflammatory states such as sepsis and diabetes are associated with reduced cardiac SERCA2a function, resulting in impaired contractility and cardiomyocyte viability [9, 10]. We demonstrate that SERCA2a protein levels are significantly reduced in MHC-MCP1 mice, and Ex4 infusion restored SERCA2a expression (Fig. 1I).
4. Discussion
The present studies demonstrate that Ex4 improves cardiac function in mice with cardiac-specific overexpression of MCP1. This protective effect is accompanied by reduced apoptosis of monocytes and cardiomyocytes, and reduced fibrosis, ER stress and macrophage infiltration. These data suggest that cardioprotective effects of Glp1r agonists are mediated at least in part via attenuation of inflammatory damage.
Cardiac overexpression of MCP1 is characterized by increased recruitment of monocytes to the myocardium. A significant proportion of these infiltrating monocytes undergo apoptosis, as confirmed in the present studies. This monocyte apoptosis stimulates localized cardiac inflammation, resulting in ER stress, fibrosis and cardiomyocyte apoptosis. Our findings show that Ex4 reduces monocyte infiltration, suggesting that the cardioprotective effects of Ex4 in this model are attributed at least in part to the attenuation of this primary insult. Reduced infiltration could be due to a direct effect on monocytes. Ex4 inhibits monocyte adhesion to aortic endothelial cells and reduces lipopolysaccharide-induced MCP1 and TNFα expression and NfκB nuclear translocation in isolated macrophages [4]. Glp1r activation also promotes polarization of macrophages towards the anti-inflammatory M2 population [5] and prevents cellular migration in isolated human monocytes in response to MCP1 [11]. However, it is unclear whether Glp1r agonists directly target macrophages since a recent report suggests that the Glp1r is not expressed in these cells [12]. Anti-inflammatory effects of Ex4 may be mediated via other immune cells such as lymphocytes and T cells [13]. Our results showing that Ex4 enhances viability of MCP1-treated HL-1 cells also suggest a direct effect on cardiomyocytes. The Glp1r is also expressed in the heart, specifically in the atrium [14]. Therefore, the cardioprotective effects of Ex4 in MHC-MCP1 mice are likely mediated via a combination of reduced monocyte infiltration and direct effects on cardiomyocytes. Future studies will address the mechanisms by which Ex4 reduces monocyte infiltration into MCP1-expressing tissues and will elucidate the signaling pathways regulated by Glp1r activation in cardiomyocytes. MHC-MCP1 mice are characterized by a late-onset inflammatory state, with localized increases in inflammatory markers (e.g. TNFα and IL-6) observed at ∼6 months of age [3]. Ex4 treatment decreased MCPIP expression, suggesting that Glp1r activation modulates targets downstream of MCP1.
ER stress contributes to the pro-apoptotic effects of MCP1 [3]. We demonstrate that Ex4 stimulates the ER chaperone GRP78 and inhibits the pro-apoptotic ER stress marker CHOP. We previously reported a similar effect of Ex4 on CHOP but no stimulation of GRP78 in cardiomyocytes exposed to high glucose stress [15]. In those studies Ex4 was added prior to the glycemic stress, so we hypothesize that Ex4 prevented the initiation of ER stress and circumvented the need to stimulate GRP78 expression. In the present studies Ex4 was administered after the inflammatory stress, so Ex4 likely stimulates GRP78 expression as a means to resolve ER stress. The nature of the insult (high glucose versus inflammation) and the context of the experiments (isolated cardiomyocytes versus in vivo) may also contribute to the differences between our present and previous findings.
Decreased SERCA2a function in inflammatory states induces ER stress and impairs cardiac function [9, 10]. The present studies confirm our previous findings that Ex4 induces cardiac SERCA2a expression [15] and provide a potential mechanism for the cardioprotective effects of Ex4.
In summary, our findings show that the Glp1r agonist Ex4 is cardioprotective in the setting of MCP1-induced cardiomyopathy. In these studies, Ex4 was administered prior to overt systolic dysfunction or heart failure in MHC-MCP1 mice [3]. Additional studies will determine whether Ex4 continues to provide cardioprotection beyond the treatment window tested here. Furthermore, future studies using targeted disruption of the Glp1r will identify the tissues and mechanisms by which Glp1r activation prevents the deterioration of cardiac function in MHC-MCP1 mice.
Acknowledgments
We thank John Shelley (Histology Core) and Humberto Ibarra-Avila (Cell Imaging Core) of the Sanford-Burnham Medical Research Institute at Lake Nona for their assistance in generating tissue section images. Current address for Craig Younce: Department of Biological Sciences, Howard Payne University, Brownwood, TX.
Grants: This work was supported by institutional funding from the Sanford|Burnham Medical Research Institute (Craig Younce and Julio Ayala) and by National Institutes of Health grant R01 HL69458 (Pappachan Kolattukudy and Jianli Niu).
Abbreviations
- Glp1
Glucagon-like peptide-1
- Glp1r
Glp1 receptor
- Ex4
Exendin-4
- MCP1
monocyte chemoattractant protein-1
- MCPIP
MCP1-induced protein
- ER
endoplasmic reticulum
- GRP78
glucose-regulated protein 78 kDa
- CHOP
CCAAT/-enhancer-binding protein homologous protein
- SERCA2a
sarco/endoplasmic reticulum calcium ATPase-2a
Footnotes
Disclosures: The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocrine reviews. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174–89. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–7. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425:304–8. doi: 10.1016/j.bbrc.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 6.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, et al. A Glucagon-Like Peptide-1 Analogue Reverses the Molecular Pathology and Cardiac Dysfunction of a Mouse Model of Obesity. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 7.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 9.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53:3201–8. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 10.He HB, Yu F, Dai DZ, Dai Y. Down-regulation of FKBP12.6 and SERCA2a contributes to acute heart failure in septic shock and is related to an up-regulated endothelin signaling pathway. The Journal of pharmacy and pharmacology. 2007;59:977–84. doi: 10.1211/jpp.59.7.0010. [DOI] [PubMed] [Google Scholar]
- 11.Vittone F, Liberman A, Vasic D, Ostertag R, Esser M, Walcher D, et al. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe (-/-) mice. Diabetologia. 2012;55:2267–75. doi: 10.1007/s00125-012-2582-5. [DOI] [PubMed] [Google Scholar]
- 12.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154:127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 13.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53:730–40. doi: 10.1007/s00125-009-1643-x. [DOI] [PubMed] [Google Scholar]
- 14.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterisation of glucagon-like peptide-1 receptor expressing cells using a new transgenic mouse model. Diabetes. 2013;63:1224–33. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younce C, Burmeister MA, Ayala JE. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. American journal of physiology Cell physiology. 2013;304:C508–18. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]