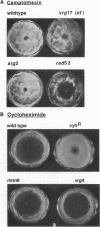
Abstract
Aminoglycosides are a therapeutically important class of antibiotics that inhibit bacterial protein synthesis and a number of viral and eukaryotic functions by blocking RNA-protein interactions. Vanadate-resistant Saccharomyces cerevisiae mutants with defects in Golgi-specific glycosylation processes exhibit growth sensitivity to hygromycin B, an aminoglycoside [Ballou, L., Hitzeman, R. A., Lewis, M. S. & Ballou, C. E. (1991) Proc. Natl. Acad. Sci. USA 88, 3209-3212]. Here, evidence is presented that glycosylation is, in and of itself, a key factor mediating aminoglycoside sensitivity in yeast. Examination of mutants with a wide range of glycosylation abnormalities reveals that all are sensitive to aminoglycosides. This effect is specific to aminoglycosides and not merely a consequence of increased permeability of the yeast mutants to drugs. Furthermore, inhibition of glycosylation in wild-type cells leads to a marked increase in their sensitivity to aminoglycosides. These results establish that a defect in glycosylation is sufficient to render yeast cells susceptible to these clinically important drugs. Further, they suggest that a molecule which prevents the uptake or mediates removal of aminoglycosides requires glycosylation for its activity. Perhaps more importantly, this finding on drug sensitivity provides the most powerful screen to date to identify mutants and thereby to isolate genes involved in all aspects of N-linked glycosylation.
Full text
PDF
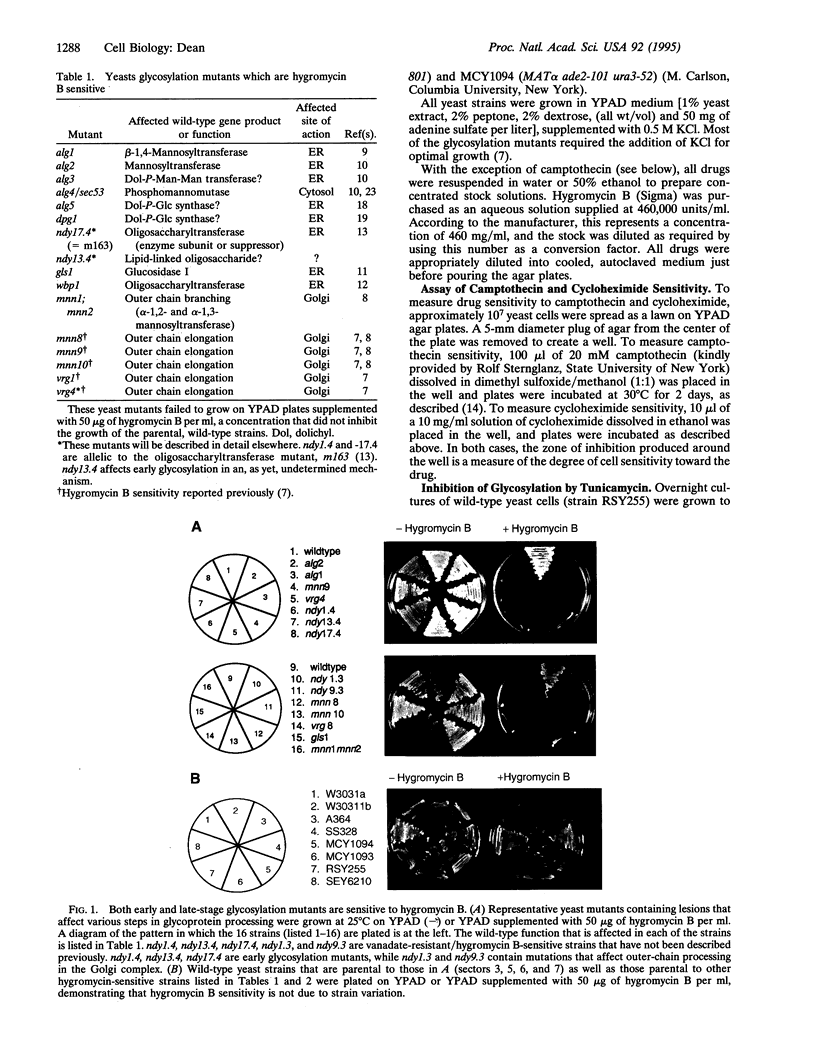
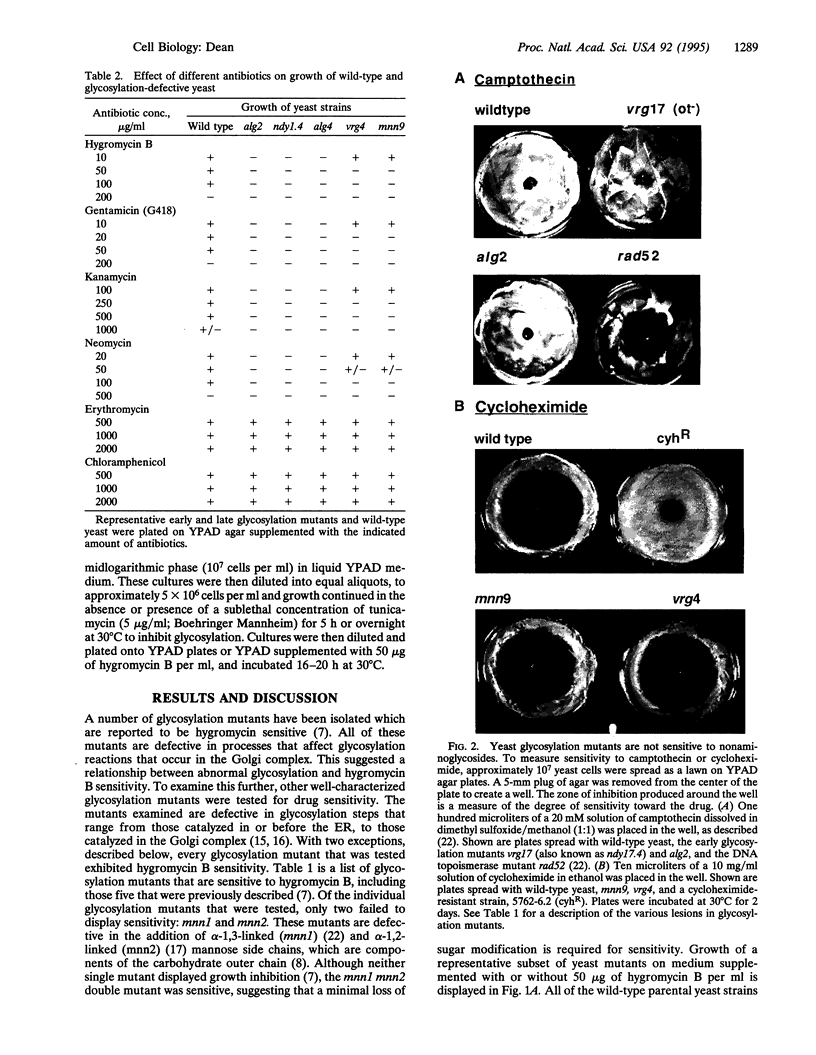


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. doi: 10.1016/0076-6879(90)85038-p. [DOI] [PubMed] [Google Scholar]
- Ballou L., Gopal P., Krummel B., Tammi M., Ballou C. E. A mutation that prevents glucosylation of the lipid-linked oligosaccharide precursor leads to underglycosylation of secreted yeast invertase. Proc Natl Acad Sci U S A. 1986 May;83(10):3081–3085. doi: 10.1073/pnas.83.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L., Hitzeman R. A., Lewis M. S., Ballou C. E. Vanadate-resistant yeast mutants are defective in protein glycosylation. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3209–3212. doi: 10.1073/pnas.88.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. On the nature of antibiotic binding sites in ribosomes. Biochimie. 1987 Aug;69(8):863–869. doi: 10.1016/0300-9084(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Devlin C., Ballou C. E. Identification and characterization of a gene and protein required for glycosylation in the yeast Golgi. Mol Microbiol. 1990 Nov;4(11):1993–2001. doi: 10.1111/j.1365-2958.1990.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Eng W. K., Faucette L., Johnson R. K., Sternglanz R. Evidence that DNA topoisomerase I is necessary for the cytotoxic effects of camptothecin. Mol Pharmacol. 1988 Dec;34(6):755–760. [PubMed] [Google Scholar]
- Esmon B., Esmon P. C., Schekman R. Early steps in processing of yeast glycoproteins. J Biol Chem. 1984 Aug 25;259(16):10322–10327. [PubMed] [Google Scholar]
- González A., Jiménez A., Vázquez D., Davies J. E., Schindler D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim Biophys Acta. 1978 Dec 21;521(2):459–469. doi: 10.1016/0005-2787(78)90287-3. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982 Mar 25;257(6):3203–3210. [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes F., Schekman R. The yeast SEC53 gene encodes phosphomannomutase. J Biol Chem. 1988 Jul 5;263(19):9155–9161. [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Roos J., Sternglanz R., Lennarz W. J. A screen for yeast mutants with defects in the dolichol-mediated pathway for N-glycosylation. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1485–1489. doi: 10.1073/pnas.91.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge K. W., Huffaker T. C., Robbins P. W. Two yeast mutations in glucosylation steps of the asparagine glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):412–417. [PubMed] [Google Scholar]
- Zapp M. L., Stern S., Green M. R. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- te Heesen S., Janetzky B., Lehle L., Aebi M. The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J. 1992 Jun;11(6):2071–2075. doi: 10.1002/j.1460-2075.1992.tb05265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ahsen U., Davies J., Schroeder R. Antibiotic inhibition of group I ribozyme function. Nature. 1991 Sep 26;353(6342):368–370. doi: 10.1038/353368a0. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Noller H. F. Footprinting the sites of interaction of antibiotics with catalytic group I intron RNA. Science. 1993 Jun 4;260(5113):1500–1503. doi: 10.1126/science.8502993. [DOI] [PubMed] [Google Scholar]