Abstract
We have shown that Goto-Kakizaki (GK) rats, a lean model of type 2 diabetes, develop significant cerebrovascular remodeling by 18 weeks of age, which is characterized by increased media thickness and matrix deposition. While early glycemic control prevents diabetes-mediated remodeling of the cerebrovasculature, whether the remodeling can be reversed is unknown. Given that angiotensin II Type 1 receptor blockers (ARBs) reverse pathological vascular remodeling and function independent of changes in blood pressure in other vascular beds, we hypothesized that azilsartan medoxomil, a new ARB, is vasculoprotective by preventing and reversing cerebrovascular remodeling in diabetes. Control Wistar and diabetic GK rats (n=6–8/group), were treated with vehicle (water) or azilsartan medoxomil (3 mg/kg/day) from 14 to 18 or 18 to 22 weeks of age before or after vascular remodeling is established, respectively. Blood glucose and blood pressure were monitored and middle cerebral artery structure and function were evaluated using pressurized arteriography. Blood glucose was higher in GK rats compared to Wistar rats. Azilsartan treatment lowered blood glucose in diabetes with no effect on blood pressure. Diabetic animals exhibited lower myogenic tone, increased wall thickness, and cross sectional area compared to controls, which were corrected by azilsartan treatment when started at the onset of diabetes or later after vascular remodeling is established. Azilsartan medoxomil offers preventive and therapeutic vasculoprotection in diabetes-induced cerebrovascular remodeling and myogenic dysfunction and this is independent of blood pressure.
Introduction
Type 2 diabetes, a disease that affects more than 20 million Americans, increases the risk and worsens outcomes of cerebrovascular disease and stroke.1 Mounting evidence suggests that inhibition of the renin-angiotensin-aldosterone system reduces cardiovascular and cerebral complications including the primary and secondary stroke risk, and angiotensin II Type 1 (AT1) receptor blockers (ARBs) are as effective as angiotensin converting enzyme inhibitors.2–4 Regulation of cerebrovascular function and structure is critical for the maintenance of cerebral blood flow, which ultimately is the most important determinant of stroke.5 Several studies suggested that in experimental models of hypertension, a common confounding factor in patients with diabetes and stroke, ARBs reverse pathological vascular remodeling and function independent of changes in blood pressure.6–9 The effect of ARBs on cerebrovascular function and structure in diabetes remains unknown. We have recently demonstrated hypertrophic cerebrovascular remodeling in GK rats, a non-obese and non-hypertensive model of type 2 diabetes.10 We also showed that inhibition of vascular remodeling reduces neurovascular injury if stroke is induced in this model.11 Given that ARBs are one of the most commonly prescribed drugs in patients with diabetes, we used both a preventive and a therapeutic approach to test the overall hypothesis that azilsartan medoxomil, a new ARB, is vasculoprotective in diabetes by improving vascular function and structure.
METHODS
Animals
All experiments were performed using male Wistar rats (Harlan; Indianapolis, ID) and age-matched diabetic GK rats (In-house bred, derived from the Tampa colony or purchased from the Tampa colony, Taconic; Hudson, NY). The animals were housed at the Georgia Regents University animal care facility that is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the institutional animal care and use committee. Animals were fed standard rat chow and tap water ad libitum. Body weights and blood glucose measurements were taken biweekly. Blood glucose measurements were taken from tail vein samples using a commercially available glucometer (Freestyle, Abbott Diabetes Care, Inc; Alameda, CA). Mean arterial pressure (MAP, mmHg) was measured using the tail-cuff method. All animals were anesthetized with pentobarbital sodium (Fatal-Plus, Vortech Pharmaceuticals Ltd; Dearborn, MI), exsanguinated via cardiac puncture, and decapitated to extract the brain. Middle cerebral arteries (MCA) were isolated for structure and function studies as described below.
Experiment 1
To determine whether angiotensin II antagonisms prevents the progression or reverses established cerebrovascular remodeling and dysfunction, 4 groups were included: 1) Control vehicle, 2) Control + azilsartan medoxomil, 3) GK vehicle and 4) GK+ azilsartan medoxomil. Starting at 18 weeks of age after the development of diabetes-induced cerebrovascular remodeling, rats were treated with azilsartan medoxomil (3 mg/kg/day by gavage) for 4 weeks.
Experiment 2
To determine whether angiotensin II antagonisms prevents the development of cerebrovascular remodeling and dysfunction, 4 groups were included: 1) Control vehicle, 2) Control + azilsartan medoxomil, 3) GK vehicle and 4) GK+ azilsartan medoxomil. Starting at 14 weeks of age before the development of diabetes-induced cerebrovascular remodeling, rats were treated with azilsartan medoxomil (3 mg/kg/day by gavage) for 4 weeks. GK rats showed elevated blood glucose levels (Experiment 1: mean t sem and Experiment 2: mean + sem) at the onset of treatment. Metabolic parameters including body weight, blood glucose and blood pressure at the end of treatment are given in Table 1.
Table 1.
Animal characteristics
| Wistar | Wistar-Azil | GK | GK-Azil | ||
|---|---|---|---|---|---|
| Experiment 1 (18–22 Week) | No./group | 8 | 8 | 8 | 6 |
| Body weight (g) | 505±20 | 528±16 | 375±6* | 393±7* | |
| Blood glucose (mg/dl) | 73±2.6 | 76±3.7 | 246±50* | 153±15**, # | |
| Mean arterial pressure (mm-Hg) | 99±5 | 99±3 | 87±3 | 91±3 | |
| Experiment 2 (12–18 Week) | No./group | 8 | 9 | 8 | 6 |
| Body weight (g) | 454±22 | 468±27 | 341±12* | 345±21* | |
| Blood glucose (mg/dl) | 86±5 | 76±5 | 188±27 | 114±9**, # | |
| Mean arterial pressure (mm-Hg) | 89±2 | 86±3 | 97±3 | 101±3 |
p<0.001 vs Wistar,
p<0.05 vs Wistar,
p<0.05 vs untreated GK
MCA function and morphometry
MCAs were quickly excised and used within 45 minutes of isolation to ensure viability of the vessels. A pressure arteriograph system (Living Systems; Burlington, VT) was used to evaluate MCA structure, myogenic tone, and mechanical properties. For these studies, MCA segments approximately 200–250 μm in diameter and proximal to the junction between the MCA and the inferior cerebral vein were used exclusively. The vessels were first mounted onto glass cannulas in an arteriograph chamber and HEPES bicarbonate buffer (in mM: 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.18 KH2PO4, 5.5 glucose, 1.8 CaCl2) was circulated and maintained at 37±0.5°C. The MCA segments were then pressurized at 60 mmHg for 45 min to generate spontaneous tone. A video dimension analyzer connected to the arteriograph system was used to measure media thickness (MT) and lumen diameter (LD) at pressures ranging from 0–180 mmHg, in 20 mmHg increments. The first measurement was taken at 5 mmHg because negative pressure is generated at 0 mmHg, causing the vessel to collapse. All vessels were exposed to each pressure point for 5 min before readings were recorded. Pressure-diameter curves were obtained, first in the presence of Ca2+ to observe the vessels’ contractile properties, and then in Ca2+-free buffer with the addition of 10−7 M papaverine hydrochloride to evaluate the vessels’ passive properties.
Data calculations
Using the MT and LD measurements obtained in active conditions (in the presence of Ca2+) and in passive conditions (in the absence of Ca2+), the following parameters related to MCA structure, myogenic tone, and mechanical properties can be calculated: Media Thickness (MT, μm) = Left Wall + Right Wall; Outer Diameter (OD, μm) = LD + MT; Medial/Lumen ratio (M/L) = MT/LD, and Percent Myogenic Tone (% tone) = 1 − (Active OD/Passive OD) × 100; Circumferential stress (σ) = (Intraluminal pressure x passive Lumen diameter)/2 X Wall thickness; and circumferential strain (ε) = (passive Lumen diameter − passive Lumen diameter at 5 mmHg)/ passive Lumen diameter at 5 mmHg. The β coefficient is the slope of the stress-strain curve (a higher β value is indicative of a stiffer vessel). Results are shown across the pressure range (20–160 mmHg) and also at 100 mmHg pressure point, which is a midpoint in the autoregulatory range.
Statistical analysis
% tone, lumen diameter, wall thickness, and wall:lumen ratio across the pressure range were analyzed by one-way ANOVA with Tukey post-hoc analysis. The study had a 2 × 2 disease (control vs diabetes) by treatment (vehicle vs azilsartam medoxomil) design. As such, % tone, lumen diameter, wall thickness, and wall:lumen ratio at 100 mm-Hg pressure point were analyzed using 2 way ANOVA to assess disease and treatment effects and interactions. A Bonferroni adjustment for multiple comparisons was used for mean comparisons for significant effects from all analyses. Data was expressed as Mean ± SEM and p<0.05 was considered significant.
RESULTS
Effect of azilsartan medoxomil on metabolic profile
Body weight was slightly lower and blood glucose was higher in diabetic animals as compared to control. There was no difference in mean arterial blood pressure (MAP). There was a disease and treatment interaction with treatment with azilsartan medoxomil not affecting body weight or blood pressure in either experiment but significantly decreasing blood glucose in diabetic animals (Table 1).
Azilsartan medoxomil reverses established cerebrovascular remodeling and dysfunction
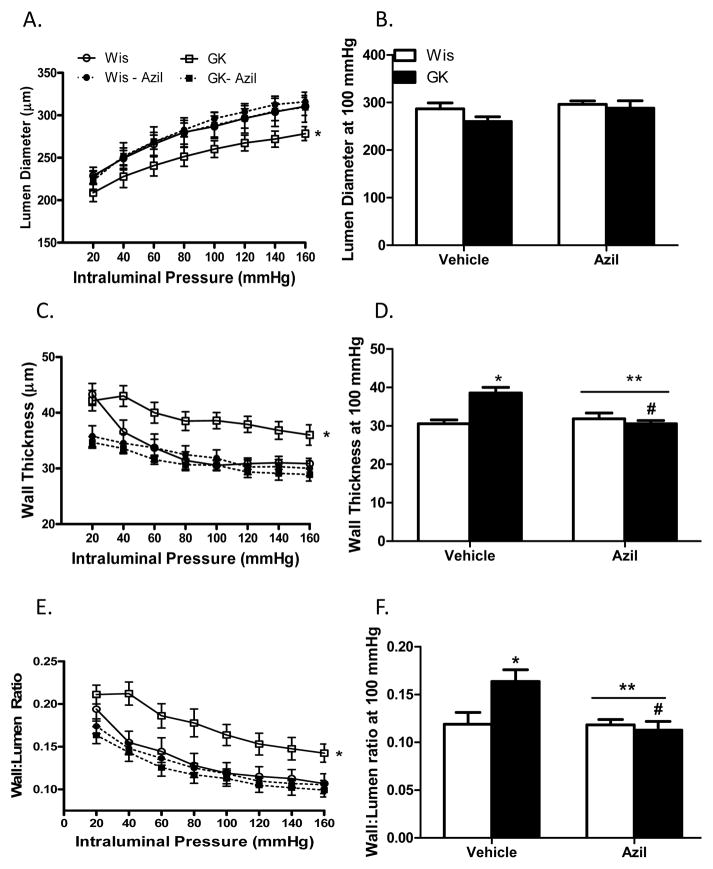
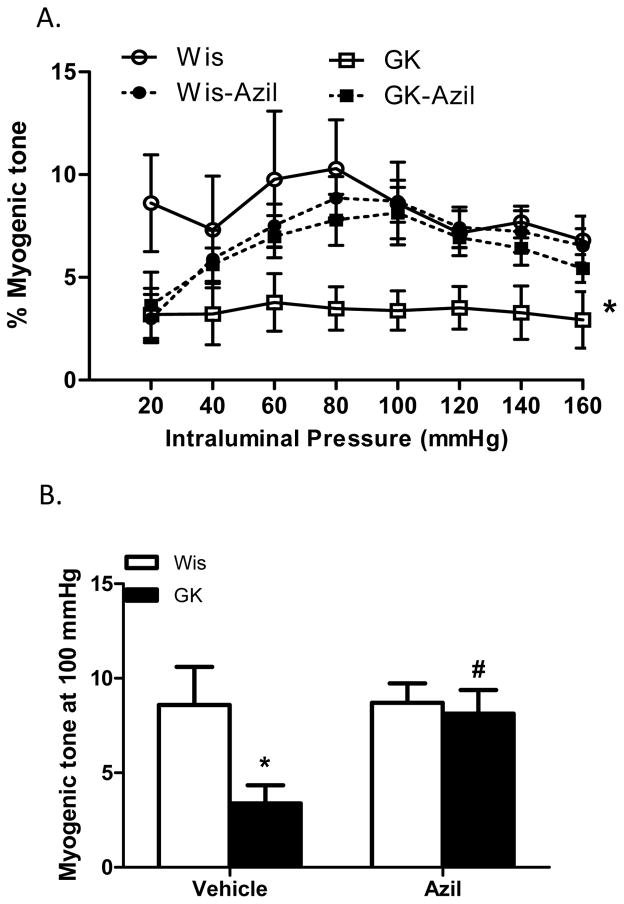
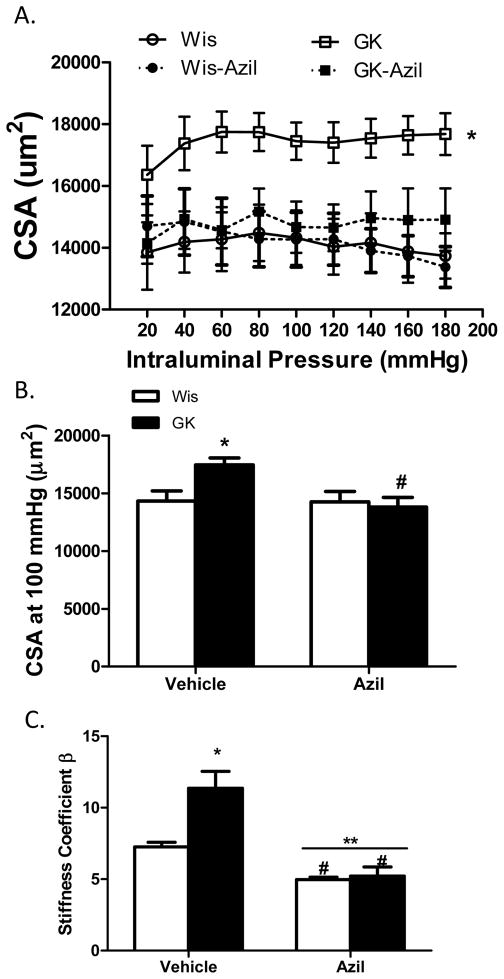
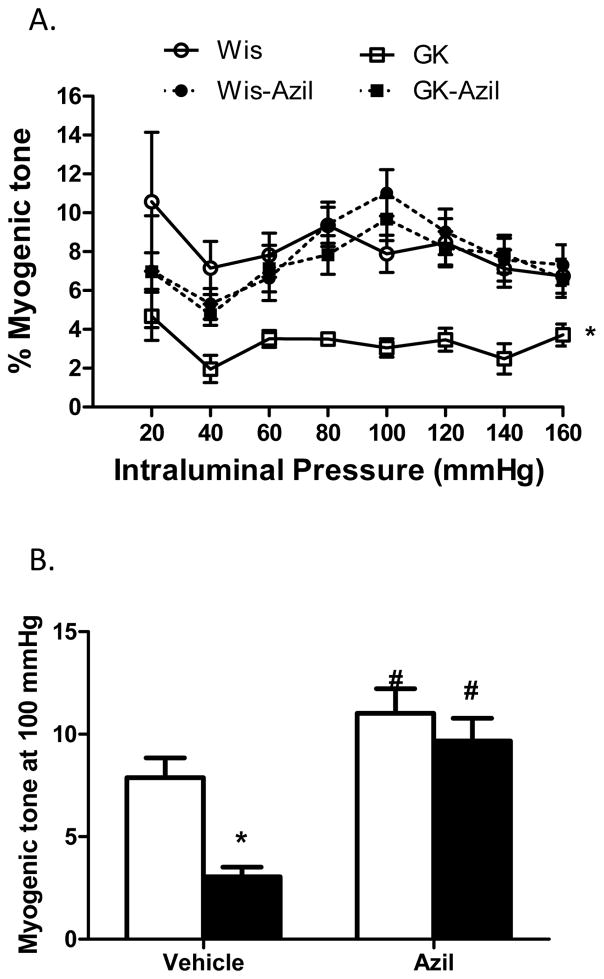
Several indices of remodeling were measured. At 22 weeks, 16 weeks after the onset of diabetes, lumen diameter was decreased and wall thickness, cross sectional area and wall:lumen ratios were all increased in diabetic animals across the pressure range as compared to control Wistar rats (Fig 1A–C and Fig 2A). At 100 mmHg, which is the physiological pressure for these vessels,12, 13 there was no difference in lumen diameter among control and diabetic vehicle or azilsartam-treated groups (1B). However, there was a disease and treatment interaction such that wall thickness and wall:lumen ratio were normalized to control levels with azilsartan medoxomil treatment in GK rats but had no effect on control animals (Fig 1E and F). Cross sectional area (CSA) was greater in diabetic animals and azilsartan medoxomil decreased CSA in both groups (Fig 2A and B). β coefficient, which is an indicator of vessel stiffness, was greater in diabetic group and treatment reduced the stiffness again in both groups. Disease by treatment interaction indicated that this effect is greater in diabetic rats (Fig 2C). Myogenic reactivity was lost in GK rats and tone was significantly lower than in control rats. Treatment restored myogenic tone in diabetes (Fig 3A and B).
Fig 1.
Azilsartan medoxomil reverses established cerebrovascular remodeling in diabetes. Across the pressure range, lumen diameter is lower while wall thickness and wall: lumen ratio are greater in diabetic GK rats (A–C, *p<0.05). At 100 mmHg pressure point, lumen diameter at is similar across groups (B) but wall thickness (E) and wall:lumen ratio (F) are greater in diabetic rats (*p<0.05) and treatment restores these indices only in diabetic rats with no effect in control animals (E, **p=0.022; F, **p=0.0138; disease treatment interaction, #p<0.05 vs vehicle). Values are mean ± SEM, n=6–8/group.
Fig 2.
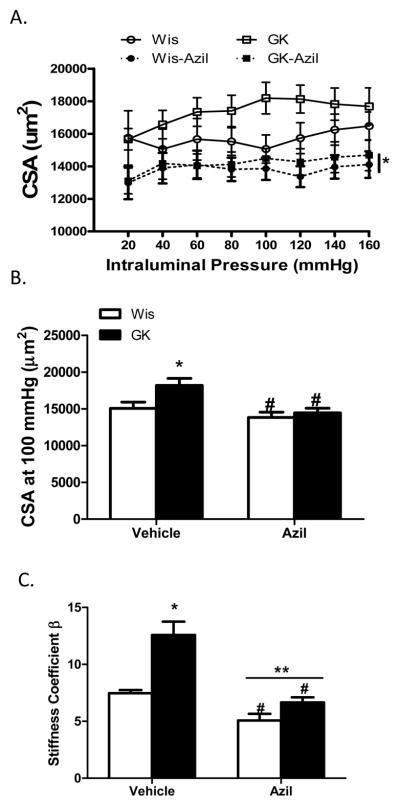
Angiotensin II antagonism reverses increased CSA in diabetes. A. CSA across the pressure range is increased in diabetic GK rats and azilsartan medoxomil treatment lowers CSA in diabetes as well as in control animals (*p<0.05 vs other groups). B. CSA at 100 mmHg pressure point is greater in GK rats and treatment reduces it both control and diabetic animals (*p<0.05 vs Wis, #p<0.05 vs untreated). C. β-coefficient was greater in diabetic animals indicating stiffer vessels (*p<0.05 vs Wis, #p<0.05 vs vehicle, **p=0.0084; disease treatment interaction). Values are mean ± SEM, n=6–8/group.
Fig 3.
Angiotensin II antagonism reverses reduced myogenic tone in diabetes. A. Myogenic reactivity is impaired and myogenic tone across the pressure range is lower in diabetic GK rats and azilsartan medoxomil treatment restores tone (*p<0.05 vs other groups). B. Tone at 100 mmHg pressure point is lower in GK rats and treatment restores it in diabetic animals with no effect in controls (*p<0.05 vs Wis, #p<0.05 vs vehicle). Values are mean ± SEM, n=6–8/group.
Azilsartan medoxomil prevents cerebrovascular remodeling and dysfunction
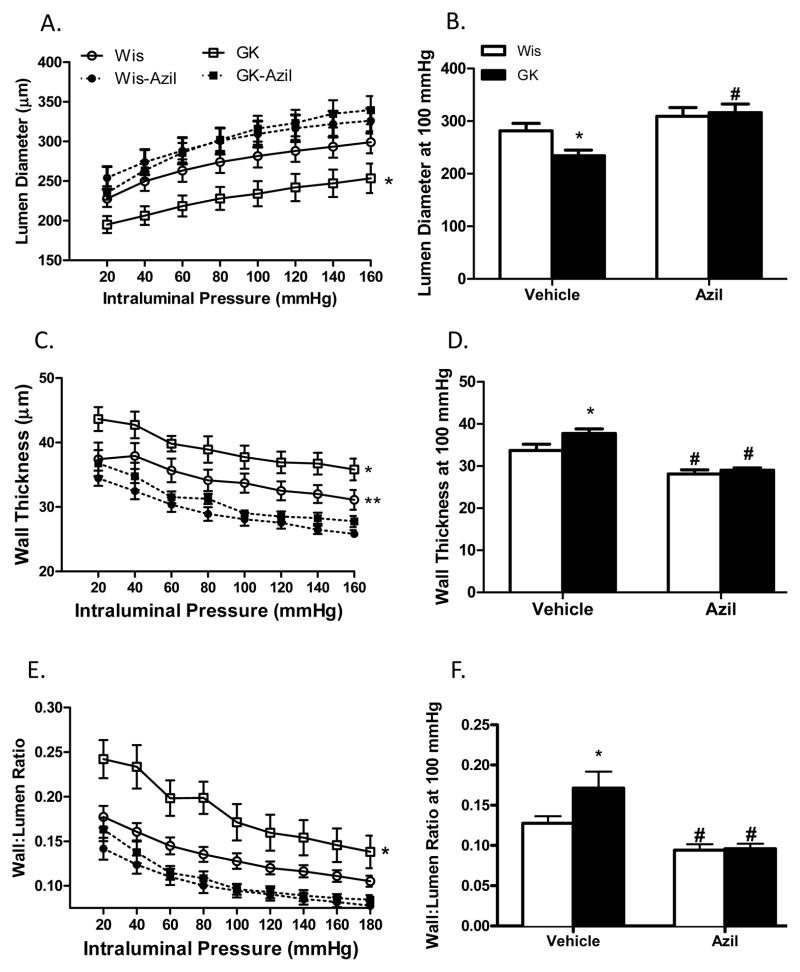
At 18 weeks, 12 weeks after the onset of diabetes, lumen diameter was decreased and wall thickness, cross sectional area and wall: lumen ratios were all increased in diabetic animals across the pressure range as compared to control Wistar rats (Fig 4A–C and Fig 5A). At 100 mmHg pressure point, azilsartan medoxomil treatment improved lumen diameter and CSA only in GK rats but decreased wall thickness and wall: lumen ratio in both control and diabetic groups (Fig 4D–F and Fig 5B). Diabetic vessels were stiffer than control MCAs and treatment reduced stiffness in both group of rats and the effect was greater in the diabetic group (Fig 5C). Myogenic reactivity was lost in diabetes and myogenic tone was significantly lower than in control rats (Fig 6A). Treatment improved myogenic tone in both control and diabetic animals (Fig 6B). A comparison of remodeling indices at 18 w versus 22 weeks showed that established remodeling at 18 weeks did not progress any further.
Fig 4.
Azilsartan medoxomil prevents cerebrovascular remodeling in diabetes. Lumen diameter is lower and wall thickness and wall:lumen ratio are greater across the pressure range in diabetic GK rats (A–C, *p<0.05 vs all other groups). D. Lumen diameter at 100 mmHg pressure point is lower in GK rats and treatment prevents this change in lumen diameter in diabetes (*p<0.05 vs Wis, #p<0.05 vs vehicle). Azilsartan medoxomil reduces wall thickness (E) and wall:lumen ratio (F) in both control and diabetic rats when started at the onset of diabetes (*p<0.05 vs Wis, #p<0.05 vs vehicle). Values are mean ± SEM, n=6–11/group.
Fig 5.
Angiotensin II antagonism prevents an increase in CSA in diabetes. A. CSA across the pressure range is increased in diabetic GK rats and azilsartan medoxomil treatment lowers CSA in diabetes (*p<0.05 vs other groups). B. CSA at 100 mmHg pressure point is greater in GK rats (*p<0.05 vs Wis) and treatment reduces it diabetic animals (#p<0.05 vs vehicle). C. β-coefficient was greater in diabetic animals indicating stiffer vessels (*p<0.05 vs Wis, #p<0.05 vs vehicle, **p=0.016; disease and treatment interaction). Values are mean ± SEM, n=6–11/group.
Fig 6.
Angiotensin II antagonism prevents diabetes-induced decrease in myogenic tone. A. Myogenic reactivity is impaired and myogenic tone across the pressure range is lower in diabetic GK rats and azilsartan medoxomil treatment restores tone (*p<0.05 vs other groups). B. Tone at 100 mmHg pressure point is lower in GK rats and treatment restores it in diabetic animals with no effect in controls (*p<0.05 vs Wis, #p<0.05 vs vehicle). Values are mean ± SEM, n=6–11/group.
DISCUSSION
The current study was designed to address the following important question: Do ARBs prevent and/or reverse diabetes-induced structural and functional changes in the cerebrovasculature independent of their blood pressure lowering effect? Our results demonstrate that there is significant vascular remodeling characterized by increased wall to lumen ratio and CSA in diabetic animals as compared to their age-matched controls if left untreated. This remodeling is associated with impaired myogenic reactivity and decreased tone. When azilsartam medoxomil is started shortly after the onset of diabetes, these changes are prevented. More importantly, angiotensin II type 1 receptor (AT1R) antagonism by azilsartan medoxomil reverses pathological remodeling even if it is started after the development of the cerebrovascular changes and this effect is independent of changes in blood pressure.
Renin-angiotensin-aldosterone (RAAS) plays a key role in cardiovascular homeostasis and as such ARBs are one of the most commonly used therapeutics to prevent/reduce cardiovascular and renal complications associated with diabetes at the microvascular and macrovascular levels.3, 4, 14, 15 In addition to seminal clinical trials including VALUE (The Valsartan Antihypertensive Long-term Use Evaluation) 14 and LIFE (the Losartan Intervention for Endpoint reduction in hypertension)15, BPLTTC (the Blood Pressure Lowering Treatment Trialists’ Collaboration) provided evidence that inhibition of the Angiotensin II system by ARBs or angiotensin converting enzyme inhibitors (ACEi) reduces the relative risk of stroke, coronary artery disease and heart failure. ONTARGET (the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) which compared ARB (telmisartan) and ACEi (ramipril) combination with either agent alone in patients at high risk of cerebral, coronary and peripheral vascular disease including diabetic subjects with target organ damage.16, 17 The study showed that telmisartan was equally effective as ramipril for cardiovascular risk reduction.16, 17 While these clinical studies provided definitive evidence that ARBs reduce cerebrovascular events, they also led to new questions regarding mechanisms underlying these beneficial effects which can be addressed more systematically in preclinical studies.
We have reported diabetes-induced temporal changes in the structure and function of cerebral blood vessels in the GK model of type 2 diabetes.10, 18, 19 Numerous studies have reported that increased blood pressure negatively influences cerebrovasculature and causes eutrophic vascular remodeling.5, 13 GK rats are not hypertensive per se but start developing slightly higher blood pressure beginning around 9–10 weeks of age. In our previous study, we found that endothelin (ET-1) receptor antagonism given during 14–18 weeks also prevents remodeling in this model. However, in that study this treatment also lowered the blood pressure and we could not determine whether the prevention of remodeling was due to lower blood pressure or a direct effect of ET-1 on vasculature. In a follow-up study we showed that metformin treatment prevented the remodeling in the absence of any changes in blood pressure suggesting that these vascular responses are independent of slightly higher pressures in GK rats.19 Several studies suggested that in experimental models of hypertension, a common confounding factor in patients with diabetes and stroke, ARBs reverse pathological vascular remodeling and function independent of changes in blood pressure.6–9 However, the impact of ARBs on diabetes-mediated cerebrovascular changes remained unknown. The current study provides evidence for the first time that angiotensin II type 1 receptor (AT1R) antagonism by azilsartan medoxomil prevents and reverses cerebrovascular remodeling and dysfunction in diabetes.
Cerebral circulation regulates blood flow within a wide pressure range to maintain constant nutrient and oxygen supply to the brain. The myogenic properties of vascular smooth muscle cells (VSMC) contribute to this autoregulatory capacity.18–21 Previous studies reported different myogenic reactivity in different diabetes models. Zimmermann et al reported increased MCA tone in type 1 diabetes.22 Another study reported temporal changes in cerebrovascular function and showed increased tone in the obese BBZDR/Wor model of type 2 diabetes with relatively high (>450 mg/dl) glucose levels.23 We have also shown that GK rats develop more tone early in the disease but when remodeling develops, these vessels lose their ability to hold tone. This would have important consequences because cerebrovascular autoregulation is essential for the protection of downstream microvessels from changes in perfusion pressure. Azilsartan medoxomil prevents this loss of tone if given early in the disease or restores myogenic tone when given after disease is established and thus offers vascular protection from a vascular function point of view as well.
Limitations
We have used this spontaneously diabetic rat strain24 because glucose intolerance can be observed in GK rats as early as 2 weeks of age in these animals and onset of moderate hyperglycemia can be as early as 5–6 weeks of age25. During weeks 6–12, plasma insulin levels are elevated and then decrease to levels lower than observed in Wistar rats24, 26. In contrast to the severely hyperglycemic streptozotocin (STZ) rat model of diabetes, whose glucose levels are typically above 300 mg/d27, 28, GK rats are moderately hyperglycemic with plasma glucose levels around 200 mg/dl. This non-obese model of type 2 diabetes is ideal for these studies because diabetes-induced cerebrovascular alterations can be studied in the absence of confounding comorbidities such as hypertension or hyperlipidemia26, 29.
In the current study, azilsartan medoxomil reduced blood glucose levels in diabetic animals. Azilsartan has been reported to improve insulin sensitivity in different animal models.30–32 Recent studies also indicate that some ARBs that have peroxisome proliferator activated receptor-γ (PPAR-γ) agonistic effects are more effective in inhibiting vascular smooth muscle proliferation as well as improving metabolic parameters in metabolic syndrome patients and experimental models.33, 34 Initial reports on azilsartan medoxomil reported that this new ARB possesses PPAR-γ agonistic properties.32 It is known that PPAR-γ activation provides cerebrovascular protection after stroke35 and in Alzheimer’s disease36 and interference with PPAR-γ signaling mediates cerebrovascular dysfunction.37 While the protective effect we observe may be partly to improvement of blood glucose, it has to be noted that blood glucose levels in treated animals are still higher that control rats suggesting that azilsartan medoxomil has additional direct vasculoprotective effects. However, further studies are needed for direct comparison of cerebrovascular protective effects of azilsartan medoxomil with other ARBs with low or no PPAR-γ agonistic properties such as valsartan as well as with PPAR-γ agonists.
In this translational study we did not include any mechanistic studies. Given that there is a close interaction between the ET and angiotensin systems38 and that ET receptor antagonism also partially prevents remodeling10, 39, 40, whether azilsartan medoxomil modulates the ET system remains to be determined.
In conclusion, AT1 receptor antagonism by azilsartan medoxomil not only prevents cerebrovascular remodeling and dysfunction that develops over time in diabetes but also reverses established disease. Thus, ARBs can be used for both preventive and therapeutic strategies in cerebrovascular complications of diabetes.
Acknowledgments
Adviye Ergul is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award (BX000347), VA Research Career Scientists Award, NIH (R01NS083559) and Takeda Pharmaceuticals, USA, Inc. grant to Adviye Ergul.
References
- 1.American Diabetes Association. National diabetes fact sheet. 2014 http://www.diabetes.org/diabetes-statistics.jsp.
- 2.Diener HC. Preventing stroke: The profess, ontarget, and transcend trial programs. J Hypertens Suppl. 2009;27:S31–36. doi: 10.1097/01.hjh.0000357906.60778.7f. [DOI] [PubMed] [Google Scholar]
- 3.Munger MA. Use of angiotensin receptor blockers in cardiovascular protection: Current evidence and future directions. P & T. 2011;36:22–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Hoogwerf BJ. Renin-angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 2010;105:30A–35A. doi: 10.1016/j.amjcard.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis F, Atkinson J, Liminana P, Chillon JM. Comparative effects of the angiotensin ii receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J Hypertens. 2005;23:1061–1066. doi: 10.1097/01.hjh.0000166848.95592.a5. [DOI] [PubMed] [Google Scholar]
- 7.Dupuis F, Atkinson J, Liminana P, Chillon JM. Captopril improves cerebrovascular structure and function in old hypertensive rats. Br J Pharmacol. 2005;144:349–356. doi: 10.1038/sj.bjp.0706001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumai Y, Ooboshi H, Ago T, Ishikawa E, Takada J, Kamouchi M, Kitazono T, Ibayashi S, Iida M. Protective effects of angiotensin ii type 1 receptor blocker on cerebral circulation independent of blood pressure. Exp Neurol. 2008;210:441–448. doi: 10.1016/j.expneurol.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis F, Vincent JM, Liminana P, Chillon JM, Capdeville-Atkinson C, Atkinson J. Effects of suboptimal doses of the at1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J Hypertens. 2010;28:1566–1573. doi: 10.1097/hjh.0b013e328339f1f3. [DOI] [PubMed] [Google Scholar]
- 10.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: Role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 11.Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, Fagan SC, Ergul A. Vascular protection in diabetic stroke: Role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30:1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011 doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The value randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 15.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, Johnson M, Ergul A. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: Role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther. 2012;342:407–415. doi: 10.1124/jpet.111.191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly-Cobbs A, Elgebaly MM, Li W, Ergul A. Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic goto-kakizaki rat in response to glycaemic control. Acta Physiol. 2011;203:245–251. doi: 10.1111/j.1748-1716.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 21.Coulson RJ, Cipolla MJ, Vitullo L, Chesler NC. Mechanical properties of rat middle cerebral arteries with and without myogenic tone. J Biomech Eng. 2004;126:76–81. doi: 10.1115/1.1645525. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to atp-sensitive k+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 23.Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type ii diabetic bbzdr/wor rat. Eur J Pharmacol. 2008;579:298–307. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa T, Yanagisawa M, Kimura S, Goto K, Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. Am J Physiol. 1988;255:H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- 26.Ergul A, Johansen JS, Stromhaug C, Harris AK, Hutchinson J, Tawfik A, Rahimi A, Rhim E, Wells B, Caldwell RW, Anstadt MP. Vascular dysfunction of venous bypass conduits is mediated by reactive oxygen species in diabetes: Role of endothelin-1. J Pharmacol Exp Ther. 2005;313:70–77. doi: 10.1124/jpet.104.078105. [DOI] [PubMed] [Google Scholar]
- 27.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: Relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: A vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 29.Elgebaly MM, Kelly A, Harris AK, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A. Impaired insulin-mediated vasorelaxation in a nonobese model of type 2 diabetes: Role of endothelin-1. Can J Physiol Pharmacol. 2008;86:358–364. doi: 10.1139/Y08-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusumoto K, Igata H, Ojima M, Tsuboi A, Imanishi M, Yamaguchi F, Sakamoto H, Kuroita T, Kawaguchi N, Nishigaki N, Nagaya H. Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin ii type 1 receptor blocker, azilsartan medoxomil, in rat and dog models. Eur J Pharmacol. 2011;669:84–93. doi: 10.1016/j.ejphar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Lastra G, Santos FR, Hooshmand P, Mugerfeld I, Aroor AR, Demarco VG, Sowers JR, Henriksen EJ. The novel angiotensin ii receptor blocker azilsartan medoxomil ameliorates insulin resistance induced by chronic angiotensin ii treatment in rat skeletal muscle. Cardiorenal Med. 2013;3:154–164. doi: 10.1159/000353155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai M, Chen R, Imura Y, Horiuchi M. Tak-536, a new at1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens. 2007;20:579–586. doi: 10.1016/j.amjhyper.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz TW. Beyond the classic angiotensin-receptor-blocker profile. Nat Clin Pract Cardiovasc Med. 2008;5 (Suppl 1):S19–26. doi: 10.1038/ncpcardio0805. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz TW, Pravenec M. Molecule-specific effects of angiotensin ii-receptor blockers independent of the renin-angiotensin system. Am J Hypertens. 2008;21:852–859. doi: 10.1038/ajh.2008.202. [DOI] [PubMed] [Google Scholar]
- 35.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. Klf11 mediates ppargamma cerebrovascular protection in ischaemic stroke. Brain. 2013;136:1274–1287. doi: 10.1093/brain/awt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with ppargamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubanyi GM, Polokoff MA. Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 39.Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li W, Sachidanandam K, Portik-Dobos V, Johnson M, Ergul A. Endothelial endothelinb receptor-mediated prevention of cerebrovascular remodeling is attenuated in diabetes due to upregulation of smooth muscle endothelin receptors. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol. 2008;294:R1213–1219. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]