Abstract
This study evaluated the efficacy of TENS in reducing pain and hyperalgesia and increasing function following total knee arthroplasty (TKA). We hypothesized participants using TENS during rehabilitation exercises would: 1) report significantly lower pain during range-of-motion (ROM) and fast walking but not at rest; 2) have less hyperalgesia; and, 3) have better function than participants receiving Placebo-TENS or Standard Care. We also hypothesized that change in ROM pain would differ based on psychological characteristics (trait anxiety, pain catastrophizing and depression) and treatment group. This prospective, randomized study used intent-to-treat analyses on 317 subjects after primary, unilateral TKA. Assessors, blinded to treatment allocation, measured pain, function (ROM and gait speed), and hyperalgesia (quantitative sensory tests) postoperatively and 6 weeks after surgery. Analgesic intake, anxiety, depression, and pain catastrophizing were also assessed. TENS participants used it 1–2 times/day at 42 mA (on average) and had less pain postoperatively during active knee extension (p=0.019) and fast walking (p=0.006) than Standard Care participants. TENS and Placebo-TENS were not significantly different. TENS participants who scored low on anxiety and pain catastrophizing had a greater reduction in ROM pain at 6 weeks than those scoring high on these factors (p=0.002 and 0.03). Both TENS and Placebo-TENS participants had less postoperative mechanical hyperalgesia (p=0.03 – 0.01) than Standard Care participants. Supplementing pharmacologic analgesia with TENS during rehabilitation exercises reduces movement pain postoperatively but a placebo influence exists and the effect is gone by 6 weeks. Patients with low anxiety and pain catastrophizing may benefit most from TENS.
Introduction
Total knee arthroplasty (TKA) is a common procedure performed to reduce pain and improve function for patients with degenerative knee osteoarthritis (OA). However, rehabilitation following this procedure can be painful, particularly during movements such as flexion/extension of the knee joint, and severe pain during these activities has been associated with poor functional recovery [8,76]. Patients rarely receive treatment beyond pharmacologic strategies [20] and this approach is not effective for controlling the severe movement pain associated with rehabilitation of the joint.
Transcutaneous electrical nerve stimulation (TENS) is a potentially efficacious pain treatment for use as a supplement to pharmacologic analgesia during rehabilitation exercises to better control this severe pain. Basic science evidence suggests that there are peripheral and central nervous system mechanisms underlying the analgesic action of TENS [24]. TENS activates endogenous inhibitory mechanisms including opioid receptors in the spinal cord and brainstem [22,41,65], reduces central neuron sensitization [47], and reduces primary and secondary mechanical hyperalgesia (i.e. pain sensitivity to force or pressure) induced by knee joint inflammation [56,61,64,72].
TENS reduces postoperative movement pain in human subjects [23,24,57]. However, the few clinical trials that have evaluated the efficacy of TENS for pain following TKA were conducted over 10 years ago with small sample sizes and varying results [4]. used high frequency TENS with a strong amplitude (30–40mA) and reported significant differences compared to placebo-TENS on pain at rest, pain after quadriceps femoris contraction, and muscle contraction ability, while others [6,75] showed no significant difference in analgesic consumption among TENS, placebo-TENS, or no TENS. One of these studies used sensory threshold TENS and the other did not provide specific information on amplitude. Prior work shows that amplitude is critical in providing analgesia with TENS in healthy controls [48] and those with postoperative pain [5], suggesting low amplitude may have contributed to the nonsignificant findings in these studies. Additionally, these two studies used analgesic intake as the primary outcome which may have been influenced by other factors, such as inability to administer medications during sleep or programmed safety intervals. We have shown that TENS reduces movement but not resting pain [19,57] which is not well controlled with analgesics. Finally, all these studies evaluated a one-time application of TENS during the immediate postoperative period. There have been no large, randomized, blinded, placebo-controlled studies evaluating the long-term usage and efficacy of TENS in controlling pain during rehabilitation following TKA.
Purpose of Study
The purpose of this study was to evaluate the efficacy of TENS in reducing pain and hyperalgesia and increasing function following TKA. We hypothesized that participants receiving TENS during rehabilitation after TKA would: 1) report significantly lower pain during range-of-motion (ROM) and walking but not at rest; 2) have less hyperalgesia (i.e. pain sensitivity) around the surgical incision and at a distant site (i.e. anterior tibialis muscle); and, 3) have better function (i.e. faster walking and greater ROM) than participants receiving Placebo-TENS or Standard Care. We also hypothesized that change in movement pain (i.e. ROM pain) would differ based on psychological characteristics (i.e. trait anxiety, pain catastrophizing and depression screening) and treatment group.
Methods and Design
Study Design
This was a prospective, randomized, double-blinded (2 of 3 groups), placebo-controlled study (ClinicalTrials.gov Identifier: NCT01364870). Participants were randomly assigned to receive: 1) TENS; 2) Placebo-TENS; or 3) Standard Care (i.e. no TENS) as a supplement to standard pharmacologic analgesia for the control of pain during rehabilitation exercises following TKA.
Setting/Sample
With approval of the local Institutional Review Board and written informed consent, English speaking patients, aged 30 years or older, with knee OA who were scheduled for a primary, unilateral total knee arthroplasty were recruited from The University of Iowa Hospitals and Clinics and the Iowa City Veteran’s Affairs Medical Center. There were 699 patients who met these inclusion criteria and were approached to participate in this study. Potential participants were excluded if they: 1) had experienced a stroke/CNS disease or had mental impairment affecting their ability to understand tests/measures; 2) had chronic pain other than knee OA that was being treated; 3) had sensory impairment, defined as lack of sharp or dull sensations over any of five dermatomes in their surgical leg; 6) were permanently or indefinitely wheelchair bound; 7) use of TENS by subject in the last 5 years (this criteria was changed to current TENS use due to the number of potential subjects being excluded on this criteria and the determination that the type of placebo-TENS used in this study was different from previous TENS use so as to not influence expectation if receiving placebo-TENS) or it was being used by anyone in their household; 8) had a condition that precluded TENS use, such as pacemaker or allergy to nickel; or 9) a prisoner. The first five participants who passed screening assessments and agreed to sign a consent form served as pilot subjects to test and verify the data collection procedures.
Sample size was determined using pain ratings from six studies that compared TENS plus analgesic medication to placebo and/or analgesic medication alone and measured resting or overall pain intensity [7,13,18,29,63,70]. These studies were used because there were no between subject studies evaluating the effect of TENS on movement pain. This approach was considered the most conservative because the effect on resting pain is smaller than movement pain. Based on these studies, the average effect size was .44 for TENS versus standard treatment and .21 for TENS versus placebo-TENS with maximum variance of 27.57. Using this information, a three group design, an α = .05, and power = .80, a sample size of 255 (85 participants per group) was targeted. To account for an estimated 20% attrition, 317 participants were recruited. Additionally, we recalculated our target sample sizes using the variance in the primary outcome measures from our sample during an interim analysis conducted halfway through the study to detect a smaller effect size between TENS and Placebo-TENS and a larger effect between TENS and Standard Care. This resulted in a target sample of 58 in Standard Care and a change in randomization to the other two groups alone (TENS and Placebo-TENS). This allowed us to detect an effect size of .54 and a difference of 4.9 between TENS and Standard Care and an effect size of .46 with a difference of 4.1 between TENS and placebo-TENS.
Treatments
TENS
TENS was provided using the EMPI-Select TENS unit (DJO, Inc.). Four circular 2” adhesive electrodes (Empi) were placed around the knee incision approximately two inches from the proximal and distal ends of the surgical incision (see Figure 1). The TENS unit produces a balanced, asymmetrical, biphasic waveform with alternating pulses between channels. A continuous frequency of 150 pps with pulse duration of 150 µs was used and participants were instructed to use the highest tolerable intensity. They were advised to adjust the amplitude setting during the TENS application to maintain this intensity level. TENS was applied 20 minutes prior to each exercise session (to reach peak effect) and then continued until the end of the session. These sessions included a combination of flexibility, strength, and endurance exercises with a physical therapist (in the hospital and following discharge) or alone (at home following discharge). TENS was used during every exercise session (1–2 times per day) until the participant’s 6 week follow-up visit in the clinic. The TENS unit used 3 AAA batteries. Participants were instructed to change the batteries once a week after discharge and were sent home with an adequate supply of batteries, electrodes, and wires. An instruction pamphlet and verbal instructions on how to attach the TENS unit and apply the same settings was given to participants prior to discharge. The TENS unit recorded number of sessions, average amplitude, and average session length on both channels. This information was obtained from the TENS unit at the 6 week clinic visit. Participants were told the TENS unit recorded this information to generate an adherence pipeline. Participants were called once a week following discharge to determine adherence with TENS application and address issues as needed.
Figure 1.
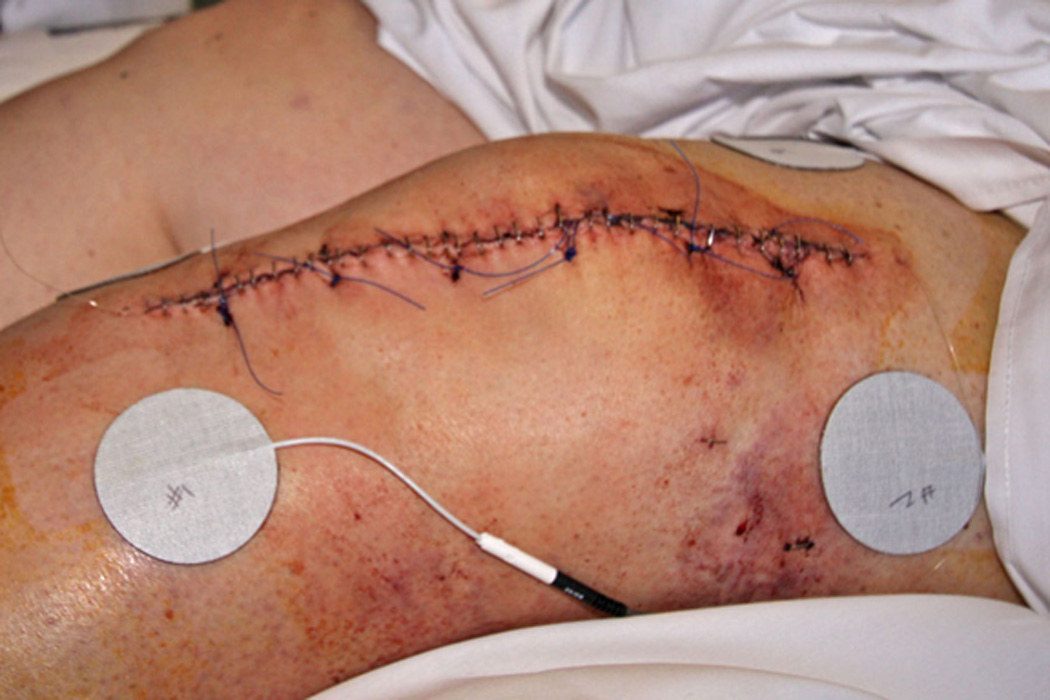
TENS Electrode Application Postoperatively
Placebo-TENS
Placebo-TENS was provided using the same EMPI-Select TENS unit (DJO, Inc.) customized to deliver current for 30 seconds (both channels) and then ramp off over the next 15 seconds so that it was active for a total of 45 seconds. This allowed the participant to feel the TENS sensation while applying the settings. In a study comparing this placebo-TENS approach to standard placebo-TENS methods and active TENS, this transient placebo-TENS was found to improve blinding without providing analgesia [58]. Participants were instructed to report when they first felt a sensation. The amplitude was then decreased by 0.5 and left at this setting throughout the application. Participants were instructed to apply the unit at this same intensity at home and that it was normal to not feel the sensation during the entire application period. This sham unit displayed an active indicator light suggesting to the participant that the unit was actively emitting current even after the 45 seconds. Instructions on electrode placement, battery insertion, and application 20 minutes prior to exercise was the same as for the TENS treatment.
Standard care
Participants randomized to Standard Care received standard pharmacologic analgesia alone (i.e. with no TENS application). This included Oxycontin 10 mg and Celebrex 200 mg orally once prior to surgery and twice a day while hospitalized beginning on postoperative day 1 (POD 1). Intraoperative anesthesia included regional anesthesia with bupivacaine and/or general anesthesia with propofol followed by isoflurane or sevoflurane. Intraoperative analgesia included femoral block using ropivacaine with or without intravenous narcotics. Oxycodone/acetaminophen 5/325 mg 1 to 2 tablets and/or morphine 1 mg intravenously every 30 minutes were used for breakthrough pain as needed after surgery. On discharge from the hospital, participants were given a prescription for Percocet 1 to 2 tabs (325 mg/5 mg), as needed, for breakthrough pain.
All analgesic medications taken by participants before and after surgery were recorded for use as a control variable, if needed. Following discharge, participants self-reported their pain medication in a Home Record Log (HRL). All opioid medications (oral and intravenous) were converted to an equianalgesic dosage of oral morphine [30,43,52,67] and all non-opioid analgesic medications were converted to acetaminophen equivalents using a conversion table [2] as previously described [59].
Outcome Measures
Pain intensity during range of motion of the joint and waking were primary outcome measures. Pain intensity at rest, hyperalgesia and function were secondary outcome measures. All measurements (i.e. pain intensity, hyperalgesia, and function) were obtained prior to surgery (pre-operative), while hospitalized after surgery (resting pain, ROM pain and function on POD 1, gait speed and hyperalgesia on POD 2–3), and 6 weeks following discharge.
Pain intensity (rest and movement)
A 21-point numeric rating scale (0–20 NRS) was used to measure pain intensity in the surgical knee. Participants were asked to rate their pain intensity on this scale where 0 represents “no pain” and 20 represents “the most intense pain imaginable”. A laminated tool was made available for the participants to view with each rating. Previous studies have shown that the use of the Numeric Rating Scale is associated with higher compliance and lower failure rates in older adults when compared to the Visual Analog Scale [35] and has established validity and reliability for assessing acute [12,34,40,50] and postoperative [28] pain. The NRS correlates well with the Visual Analogue Scale during the postoperative period (.90 to .95) [21,28,36]. A 21-point scale was used (vs. an 11-point scale) based on evidence that 21 points provide a sufficient and needed level of discrimination [39]. This assessment was conducted both at rest and with movement. Resting pain intensity was measured prior to any study procedures while the participant was comfortably sitting or lying down without movement of the knee. Movement pain was measured during active flexion and extension of the surgical knee as well as during a gait speed test.
Hyperalgesia (quantitative sensory tests)
Quantitative sensory tests were performed on the surgical leg to assess hyperalgesia (i.e. pain sensitivity). Three test sites were marked 4 cm apart and 4 cm medial to the surgical knee. Three test sites were also marked 4 cm apart and 2 cm lateral to the tibial crest over the anterior tibialis muscle of the surgical leg. A laminated template was used to guide standard placement of the testing sites.
Pressure Pain Threshold (PPT) was used to measure deep mechanical pain sensitivity at the knee (primary hyperalgesia) and the anterior tibialis muscle (secondary hyperalgesia). A hand-held pressure algometer (Somedic AB, Farsta, Sweden) with a 1 cm2 digital probe was used. Prior to PPT testing, a Versaform pillow was placed under the participant’s knees for support. Then a familiarization test with the algometer was performed on the participant’s arm. This was repeated, if needed, to reinforce understanding of the test. To measure PPT, the algometer probe was pressed over the marked test sites perpendicularly to the skin at a rate of 40 kPa/second. The participant was instructed to press a button when the pressure was first perceived as painful. Participants were instructed that if they felt they pressed the button too early or too late, they could let the assessment RA (Research Assistant) know and the test could be repeated. With this method, mean PPT of the knee averages approximately 250 kPa [1,49]. PPT has strong inter-rater reliability across multiple raters (ICC=0.91) and test-retest reliability shows good results (ICC=0.77–0.86) in participants with knee OA [33] and postoperatively (0.70–0.94) [15,27,53,55,74].
Heat pain threshold and tolerance were measured to determine cutaneous thermal pain sensitivity at the knee and the anterior tibialis muscle. A computer-controlled TSA-II Neurosensory Analyzer (Medoc, Israel) and a Peltier thermode, size 16 × 16 mm were used. Minimum temperature was set at 34 °C and maximum at 52 °C. The rate of increase in temperature was 1 °C /s. Participants were informed that the thermode would not reach a temperature that would cause skin damage. A familiarization test was performed on the arm prior to testing. For threshold measures, participants were instructed to concentrate on the stimulus and to press a mouse button when the heat sensation was first perceived as painful. For tolerance measures, participants were instructed to press the mouse button when the heat sensation was no longer tolerable. Once the mouse button was pressed, the probe stopped heating and a temperature registered on the computer screen.
Prior to determining averages at each site (i.e. knee and anterior tibialis muscle), outlier values were identified and removed. A value was identified to be an outlier if the difference between this value and the middle value was greater than the average variance and the difference between the other 2 values was less than 50% of this difference. The remaining readings were averaged to obtain a representative value for each site. Inter-rater reliability estimates were also conducted for each of the measures with Intraclass Correlation Coefficients ranging from 0.82 – 0.97.
Function
Function was determined using flexibility of the knee (i.e. active extension and flexion ROM), strength of the quadriceps femoris muscles (i.e. extensor lag), and endurance (i.e. gait speed).
Range of Motion and Extensor Lag. Range of motion measures of the knee were taken with a hand held goniometer which has been found to be a valid and reliable method for quantifying knee movement [54]. Measures included active extension, extensor lag and flexion. While the participant was lying supine on a table, a towel roll was placed under the ankle to elevate the leg. The participant was asked to straighten the knee as far as possible by pressing the knee toward the table. The assessment RA aligned the stationary aim of the goniometer to the greater trochanter and the moveable arm to the lateral malleolus and measured the maximum extension reached in degrees from straight (e.g. −4 indicates 4 degrees from straight). The participant then raised their leg off the towel roll while keeping the knee as straight as possible. Keeping the goniometer in alignment, the degrees from straight were measured and recorded. This measure was subtracted from the extension degrees to obtain the degrees of “lag”. Lastly, the towel roll was removed and the participant was asked to bend the knee as far as possible. Degrees of active flexion were measured with the same goniometer and landmarks.
Gait Speed Test. Walking function was measured using a gait speed test where participants were instructed to walk as fast as they were safely able to for 15 seconds in a well-lit, unobstructed hallway. Participants began at a predetermined start line. The RA said, “Ready, set, go”, and the participant began walking as the digital stopwatch was started. The assessment RA walked beside the participant and provided assistance as needed. When 15 seconds elapsed, the assessment RA instructed the participant to stop and placed a piece of tape on the floor at the point of contact of the participant’s back heel. The distance from the start line to the piece of tape was then measured with a measuring tape. Participants were permitted to use a walking aid such as a cane or walker, if necessary, for safety and use of an assistive device was recorded for treatment comparisons.
Secondary Variables
Variables that could influence the efficacy of TENS were measured preoperatively or prior to treatment allocation on POD 1 to determine group equivalency. These variables included demographics (i.e. age, sex, ethnicity, race, marital status, and education), medical information (i.e. Kellgren-Lawrence OA grade, height and weight, pain duration, secondary diagnoses, length of pain in the affected knee, current use of pain medication, and previous surgeries), psychological dimensions (i.e. state and trait anxiety, pain catastrophizing, depression), and perception of overall knee pain and function (Knee Injury and Osteoarthritis Outcome Score - KOOS).
Demographic and medical information questionnaire
The demographic and medical information questionnaire was a five page form in which participants were asked demographic and medical information. This form had been used by the research team in prior studies and asked questions in a standardized manner.
State and trait anxiety
The State-Trait Anxiety Inventory (STAI) was used to measure state and trait anxiety prior to surgery. The S-Anxiety scale (STAI Form Y-1) consists of twenty statements that evaluate how respondents feel “right now, at this moment” on a 4-point scale. The T-Anxiety scale (STAI Form Y-2) consists of twenty statements that assess how people generally feel on the same 4-point scale [66]. High scores on their respective scales mean more trait or state anxiety and low scores mean less. This tool has been used successfully in older adults after hip and knee surgery [26].
Pain catastrophizing
The Pain Catastrophizing Scale (PCS) was used to measure pain catastrophizing prior to surgery. It is a 13–item survey designed to measure the extent that individuals ruminate, magnify, or feel helpless about their pain. It uses a 5-point scale ranging from “not at all” to “all the time”. Pain castrophizing has been shown to correlate positively with many aspects of the pain experience, including pain intensity, emotional distress, pain-related disability, heath services use, pain behavior and reliance on medication [31,32,68,69].
Geriatric depression scale
The Geriatric Depression Scale (GDS) is a five item screening tool for depression in the older population. The five item version was chosen over the 15-item GDS because it had better sensitivities, specificities, predictive values and accuracies for outpatients [37,60]. Individuals who have ≥ 2 positive answers are considered to have a positive depression screen [37,78].
Knee injury and osteoarthritis outcome score
The Knee Injury and Osteoarthritis Outcome Score (KOOS) is a knee-specific instrument, developed to assess both short-term and long-term consequences of knee injury using 42 items in 5 separately scored subscales:(1) Pain; (2) Other Symptoms; (3) Activity in Daily Living (ADL); (4) Function in Sport and Recreation (Sport/Rec); and, (5) Knee-related Quality of Life (QOL). Participants respond using a 5-point scale from no difficulty (0) to extreme difficulty (4). Scores are then transformed to a 0–100 scale (0 = extreme knee problems to 100 = no knee problems). The KOOS is an extension of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and has demonstrated adequate reliability (test-retest, ICC=0.6–0.94) and validity (Internal Consistency, Cronbach’s alpha = 0.56–0.98) in patients with knee OA [16].
Data Collection Procedures
Preoperative
At the preoperative clinic visit approximately 1 week prior to surgery, participants were given the demographic and medical information form, STAI, PCS, GDS and KOOS to fill out. They were familiarized with the 0–20 NRS and, while seated comfortably on an exam table, were asked to rate the intensity of pain (at rest) in their surgical knee. Then participants were asked to lie supine on the exam table and a Versaform pillow was placed under their knees for support and comfort. Testing spots were marked and quantitative sensory tests were performed as described above. Active extension and flexion ROM were obtained and the participant rated the pain during each of these movements. Then the participant walked to a straight hallway immediately outside the exam room and the gait speed test was performed. At the end of this test, the participant was asked to rate the maximum pain experienced during this test in their surgical knee. Participants were then reminded of the postoperative protocol including randomization into treatment group on POD 1. A package of 4 sterile electrodes was provided to the surgeon the morning of surgery for application in the OR.
Postoperative
Immediately following skin closure and prior to application of the postoperative dressing, the sterile electrodes were applied at the top and bottom of the knee incision, 2 inches from the incision on either side. The electrodes were attached to wires that extended beyond the dressing. On the morning of POD 1, the assessment RA determined the participant’s continued eligibility and measured resting pain, flexion and extension ROM, and pain during these movements. The assessment RA then left the room and the treatment allocation RA randomized participants to treatment group and applied the appropriate TENS unit. After 20 minutes, the assessment RA re-entered the room and measured pain at rest and again during active flexion and extension of the knee. TENS was applied prior to each physical therapy session until discharge.
On the morning of POD 2, the assessment RA determined continued eligibility and sensation in the surgical leg using dull and sharp stimuli across five dermatomes. The allocation RA applied the TENS and quantitative sensory tests were performed by the assessment RA after a 20 minute wait period. The participant then performed the gait speed test, rated their pain during that test and completed their routine physical therapy session. If sensation was not normal, quantitative sensory and gait speed tests were performed on POD 3.
Prior to discharge from the hospital, participants were given an instruction pamphlet and TENS supplies and taught to apply the TENS at home with a return demonstration. Participants were given a HRL to record on a daily basis: exercise sessions, application of TENS 20 minutes prior to exercise sessions, and pain medication.
Following discharge
Physical therapy sessions were scheduled 2 to 3 times per week. Additionally, participants performed exercise sessions that included flexibility, strength and endurance exercises twice a day at home. To help ensure accuracy and facilitate adherence, participants were called the day after discharge and weekly by the allocation RA to address any issues with TENS application or HRL documentation.
Participants returned to the clinic 6 weeks after surgery. At this visit, they were asked to apply their TENS unit according to how they had been using it at home. Participants were reminded not to discuss the function of the unit with the assessment RA so that the RA remained blinded to treatment group. After 20 minutes, the assessment RA measured resting pain in the surgical knee. Quantitative sensory tests were performed followed by the gait speed test. At the conclusion of testing, the TENS unit was removed and data on the number of sessions, average session length, and average intensity were downloaded from the TENS unit. These data were used to determine TENS dose.
Randomization and Blinding
A randomization sequence using SPSS was generated with randomization occurring in permuted blocks of 3 and 6, stratified by gender. Allocation to treatment groups remained concealed in a central office until POD 1 when the allocation RA made a phone call to the Project Director to receive the participant’s assigned treatment. The allocation RA then verified the serial number on the TENS unit corresponded with the appropriate TENS treatment. After verification, the allocation RA attached the TENS unit around the knee incision at the participant’s bedside. The participant was instructed to not disclose any information regarding TENS settings or TENS-related sensations to the assessment RA. This approach was done to minimize potential sources of experimenter and participant bias by protecting the randomization sequence in a central office, maintaining concealment of treatment allocation until the last possible moment, and keeping participants and the RA who collects outcome data blinded to whether the TENS unit was set to active or placebo mode. Given the nature of the design, it was not possible to blind participants assigned to Standard Care. At the end of the study, participants in the TENS and Placebo-TENS groups were asked if they thought they received an active or placebo treatment and this information was recorded.
Data Analyses
Data were described using percentages for categorical variables, and mean ± SD or median and 25th to 75th percentiles (IQR) for continuous variables. Data from the TENS unit were converted to mA using the calibrated amplitudes determined for each unit. Intent-to-treat analyses were performed including all participants randomized to treatment groups, regardless of adherence to treatment protocols. Outcomes were analyzed at POD 1 (POD 2 for gait speed and pain sensitivity measures) and at 6 weeks following surgery. The distribution of continuous variables was evaluated for normality using histograms. Analgesic intake (opioids and non-opioids) was normally distributed so was compared using linear mixed model analyses. The fixed effects in the model included treatment group, time, and treatment by time interaction. Pain, pain sensitivity and function data at each time point were not normally distributed. Treatment comparisons at each time point were performed using Kruskal-Wallis non-parametric tests. Pairwise comparisons were performed for significant tests using ordered rankings. To test for a treatment by time interaction, linear mixed model analyses were used on difference scores (which were normally distributed) for these measures. The p values for all tests comparing the three treatment groups were adjusted using Bonferroni’s method to account for the multiple comparisons. Associations between pain and function were evaluated using Spearman rho correlations. For analyses of pain intensity, a p < 0.025 was considered statistically significant. This was adjusted from a p < 0.05 due to the interim analysis on these variables that was conducted halfway through the study. For all other analyses, a p < 0.05 was considered statistically significant. Analyses were conducted using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
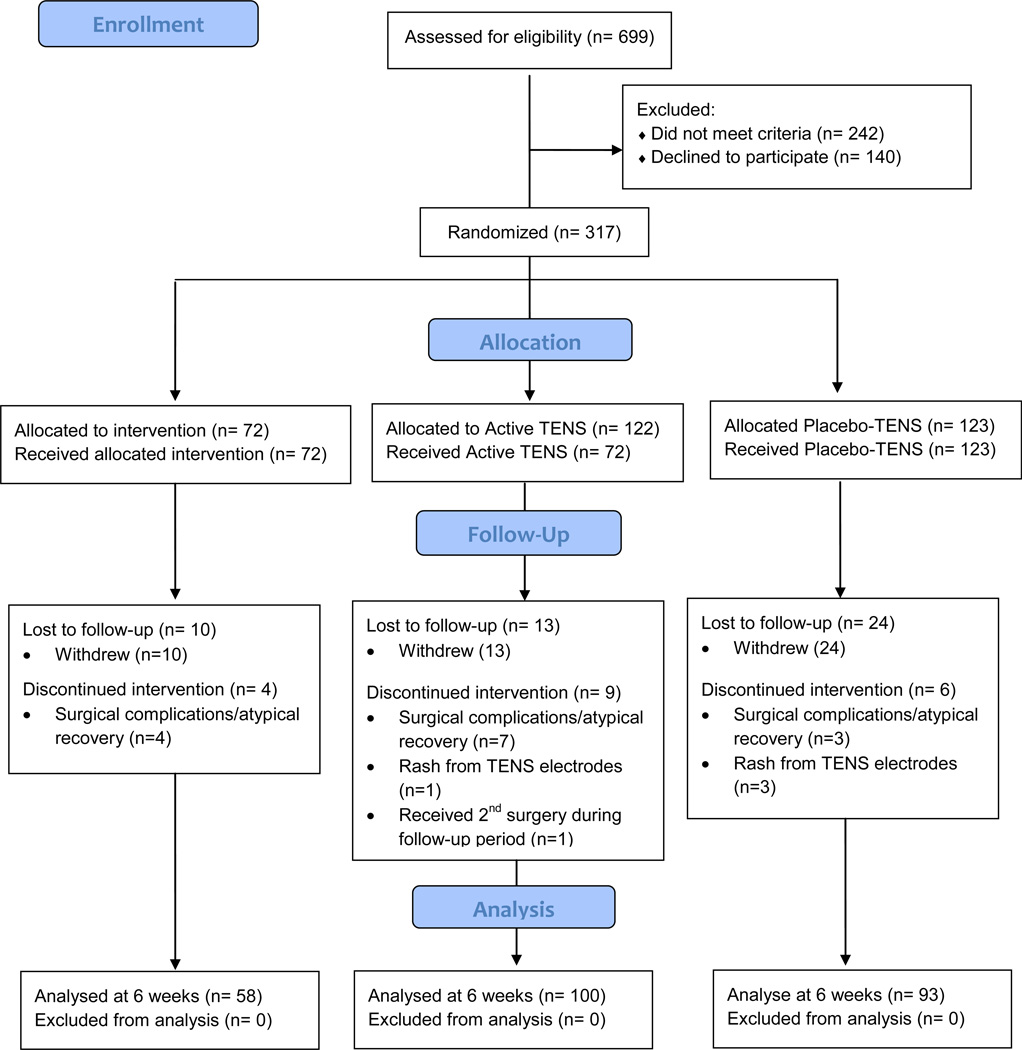
Between June 26, 2008 and December 20, 2012, 699 patients met inclusion criteria. Of these, 242 were ineligible (35%) and 140 declined to participate (20%), leaving 317 randomized to treatment on POD 1 (see Figure 2, CONSORT Diagram). Of the 242 patients who were excluded, 40% had current TENS use, 18% had a condition that precluded TENS use, 17% were being treated for a chronic pain condition, 11% had a CNS disease or mental impairment affecting their ability to understand tests/measures, 7% were prisoners, 5% had sensory impairment, and 2% were permanently or indefinitely wheelchair bound.
Figure 2.
CONSORT Diagram
The 317 participants ranged in age from 40 to 90 years with a mean of 62 ± 9.5 years (± SD). There were slightly more women (54.4%) than men (45.6%), most were non-Hispanic (97.8%), white (94.3%), married or living with someone (59.1%), had at least some college education (60.1%), a household income < $60,000 (55.7%), an OA grade of 4 (73%), pain in their affected knee for 3 or more years (65.7%) and were taking either a non-opioid or nothing for this pain (68.9%). Most had not undergone a previous knee arthroplasty in their opposite knee or hip arthroplasty (64.8%). There were no significant differences across treatment groups on these variables (see Table 1).
Table 1.
Demographic and Preoperative Characteristics of Enrolled Subjects
| Standard Care (n=72) |
Placebo-TENS (n=124) |
TENS (n=122) | Total (N=318) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Data | n | data | n | data | n | data | p | ||
| Age | 72 | 62 (9.1) | 124 | 62 (9.7) | 122 | 63 (9.7) | 318 | 62 (9.5) | 0.79 | |
| BMI | 72 | 35.5 (7.8) | 124 | 34 (6.7) | 122 | 34 (6.7) | 318 | 34 (6.9) | 0.30 | |
| Gender | Female | 42 | 58.3% | 66 | 53.2% | 65 | 53.3% | 173 | 54.4% | 0.73 |
| Male | 30 | 41.7% | 58 | 46.8% | 57 | 46.7% | 145 | 45.6% | ||
| Ethnicity | Non-Hispanic | 71 | 98.6% | 122 | 98.4% | 118 | 96.7% | 311 | 97.8% | |
| Hispanic/Latino | 1 | 1.4% | 1 | 0.8% | 1 | 0.8% | 3 | 0.9% | 0.61 | |
| Declined/Missing | 0 | 0.0% | 1 | 0.8% | 3 | 2.5% | 4 | 1.3% | ||
| Racial Heritage |
White | 68 | 94.4% | 118 | 95.2% | 114 | 93.4% | 300 | 94.3% | |
| Other | 3 | 4.2% | 4 | 3.2% | 5 | 4.1% | 12 | 3.8% | 0.94 | |
| More than one | 1 | 1.4% | 2 | 1.6% | 2 | 1.6% | 5 | 1.6% | ||
| Declined/Missing | 0 | 0.0% | 0 | 0.0% | 1 | 0.8% | 1 | 0.3% | ||
| Marital Status |
Married/SO | 44 | 61.1% | 73 | 58.9% | 71 | 58.2% | 188 | 59.1% | |
| Divorced/Widow | 15 | 20.8% | 36 | 29.0% | 36 | 29.5% | 87 | 27.4% | 0.24 | |
| Never married | 8 | 11.1% | 4 | 3.2% | 5 | 4.1% | 17 | 5.3% | ||
| Declined/Missing | 5 | 6.9% | 11 | 8.9% | 10 | 8.2% | 26 | 8.2% | ||
| Education | ≤ High School | 24 | 33.3% | 40 | 32.3% | 35 | 28.7% | 99 | 31.1% | |
| ≥ Some College | 41 | 56.9% | 73 | 58.9% | 77 | 63.1% | 191 | 60.1% | 0.27 | |
| Declined/Missing | 7 | 9.7% | 11 | 8.9% | 10 | 8.2% | 28 | 8.8% | ||
| Household Income |
< $60,000 | 47 | 65.3% | 62 | 50.0% | 68 | 55.7% | 177 | 55.7% | |
| ≥ $60,000 | 15 | 20.8% | 38 | 30.6% | 34 | 27.9% | 87 | 27.4% | 0.49 | |
| Declined/Missing | 10 | 13.9% | 24 | 19.4% | 20 | 16.4% | 54 | 17.0% | ||
| OA Grade | 2 | 2 | 2.8% | 1 | 0.8% | 2 | 1.6% | 5 | 1.6% | |
| 3 | 22 | 30.6% | 28 | 22.6% | 31 | 25.4% | 81 | 25.5% | 0.31 | |
| 4 | 48 | 66.7% | 95 | 76.6% | 89 | 73.0% | 232 | 73.0% | ||
| Pain Duration |
<1 yr | 4 | 5.6% | 6 | 4.8% | 5 | 4.1% | 15 | 4.7% | |
| 1-<3yrs | 16 | 22.2% | 25 | 20.2% | 32 | 26.2% | 73 | 23.0% | 0.80 | |
| 3–5 yrs | 18 | 25.0% | 28 | 22.6% | 30 | 24.6% | 76 | 23.9% | ||
| >5 yrs | 31 | 43.1% | 55 | 44.4% | 47 | 38.5% | 133 | 41.8% | ||
| Pain Medication |
Opioid | 3 | 4.2% | 7 | 5.6% | 2 | 1.6% | 12 | 3.8% | |
| Non-opioid | 38 | 52.8% | 64 | 51.6% | 54 | 44.3% | 156 | 49.1% | 0.40 | |
| Combo | 18 | 25.0% | 29 | 23.4% | 40 | 32.8% | 87 | 27.4% | ||
| None | 13 | 18.1% | 24 | 19.4% | 26 | 21.3% | 63 | 19.8% | ||
| Prior Joint | Opposite Knee | 24 | 33.3% | 32 | 25.8% | 36 | 29.5% | 92 | 28.9% | |
| Arthroplasty | Hip (one or both) | 2 | 2.8% | 9 | 7.3% | 9 | 7.4% | 20 | 6.3% | 0.17 |
SO=Significant Other. No significant differences across treatment groups (p<0.05). Data are means (SD) or numbers and percents.
Of the 317 participants randomized, 251 (79%) completed the study (i.e. continued to be enrolled at their 6 week postoperative visit). The 70 participants who did not complete the study either withdrew due to study burden (73%) or were excluded due to surgical complications (22%) or rash from TENS electrodes (5%), most during the immediate postoperative period. These participants were not significantly different than those who remained in the study in sex, race/ethnicity, marital status, education, income, BMI, OA grade, pain duration, resting or movement pain, pain medication, or physical function. Those who participated, however, were significantly younger (average age of 61.5 versus 65 years). Retention of participants was not significantly different by treatment group.
Participants took an average of 22.4 ± 12.2 mEq of morphine per hour (i.e. opioids) and 1077.84 ± 724.82 mEq of acetaminophen per hour (i.e. non-opioids) between surgery and testing on POD #2. These intakes were not significantly different across the three treatment groups (p=0.67 and 0.35, respectively). During the first week following discharge, participants took an average of 45.37 ± 4.01 mEq of morphine per day and this decreased to 10.96 ± 1.61 mEq of morphine per day on average during week 6. For non-opioids, participants took an average of 1914.13 ± 171.4 mEq of acetaminophen per day during the first week and this decreased to an average of 1007.37 ± 136.63 mEq of acetaminophen per day during week 6. Participants in all three groups reduced their opioid and non-opioid intake significantly over the 6 week period (linear mixed model analysis, p<0.001 for both opioid and non-opioid) but these reductions were not significantly different across treatment groups (opioids p=0.90; non-opioids p=0.88) and the average intake during this time period was not significantly different across groups (opioids p=0.33; non-opioids p=0.16) (see Figure 3).
Figure 3.
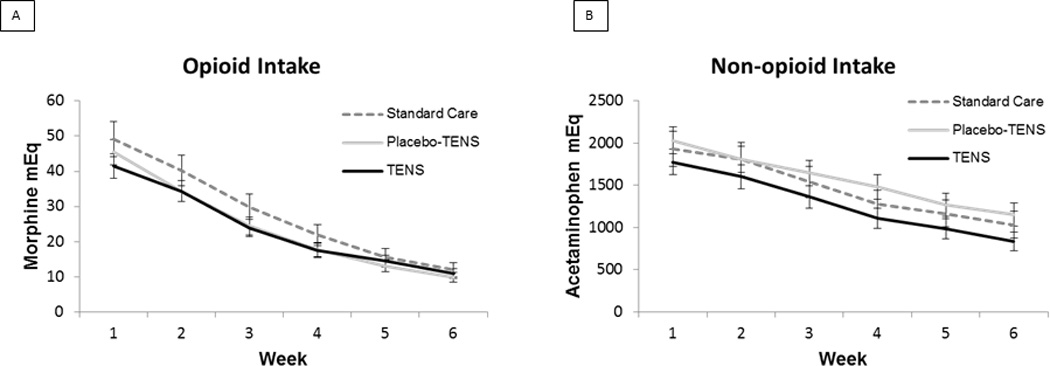
Analgesia Intake Over 6 Weeks by Treatment Group
Of the participants who received active TENS, 16 % were blinded (i.e. thought they received Placebo-TENS or did not know which treatment they received) and 84 % knew they had received an active treatment. Of the participants who received Placebo-TENS, 45 % were blinded and 55 % indicated they had received a placebo treatment.
Data retrieved from the active TENS units at the 6 week visit was normally distributed and showed an average number of sessions of 54.03 ± 34.05 (consistent with application 1–2 times per day), average amplitude of 42.04 ± 8.12 mA, and average session length of 55.2 ± 25.8 minutes (suggesting the TENS was applied 20 minutes before exercise and left on during exercise sessions that lasted an average of 35.2 minutes, which is consistent with instructions). Data from the weekly phone calls showed that the weekly average amplitudes stayed at 42 mA throughout the 6 week period (i.e. averaged 42 mA at week 1, 2, 3, 4, 5, and 6). There were no significant associations between TENS dose or TENS amplitude and outcome variables. Data could not be reliably retrieved from the sham TENS units due to amplitudes that were below the recording cut off of 3mA for some participants.
Pain Intensity
Prior to surgery, participants rated their movement pain intensity mild to moderate (i.e. active extension 6/20, 2–10; active flexion 8/20, 3–13, and gait speed 5/20, 2–10) and their resting pain intensity as mild (i.e. median 2/20, IQR 0–5). Immediately prior to treatment on POD 1, movement pain intensity during active extension and flexion of the knee was moderate to severe (i.e. 12, 7–17 and 15, 10–19, respectively) and resting pain intensity was moderate (i.e. 9, 5–15). Participants randomized to Standard Care reported significantly higher pain during active flexion of the knee prior to treatment than participants randomized to Placebo-TENS (pairwise comparison, p=0.03). Due to the need for nonparametric analyses, this variable could not be controlled in the analyses. However, no significant differences were found between groups on flexion pain postoperatively or at 6 weeks (see below). Therefore, this difference did not affect the results. There were no other significant differences between treatment groups.
Following surgery, the treatment by time interaction using difference scores was significant for extension pain (p=0.035) but not for flexion or gait pain (p=0.91 and 0.77, respectively). There was a suggested treatment by time interaction for resting pain (p=0.063) but it did not reach significance at the 0.05 level.
During the immediate postoperative period, pain during active extension of the knee was significantly different between groups (Kruskal-Wallis, p=0.016) with pairwise comparisons showing participants receiving TENS reported significantly lower pain than participants receiving Standard Care (median 9.5 versus 14, p=0.019, effect size = 0.5). There was no significant difference between Placebo-TENS and Standard Care (p=0.05), although the effect size was also 0.5, suggesting a possible effect exists even though differences did not reach significance at p<0.025. There was no significant difference between Placebo-TENS and TENS (p=1.0, effect size = 0). Pain during the gait speed test postoperatively was also significantly different between groups (p=0.008) with pairwise comparisons showing participants receiving TENS had significantly lower pain than participants receiving Standard Care (median 8 versus 10, p=0.006, effect size = 0.24). There were no significant differences between Placebo-TENS and Standard Care (p=0.24, effect size = 0) or Placebo-TENS and TENS (p=0.3, effect size = 0.25). Pain during active flexion and at rest were not significantly different across the 3 treatment groups (p=0.19 and p=0.13, respectively) postoperatively (see Figure 4).
Figure 4.
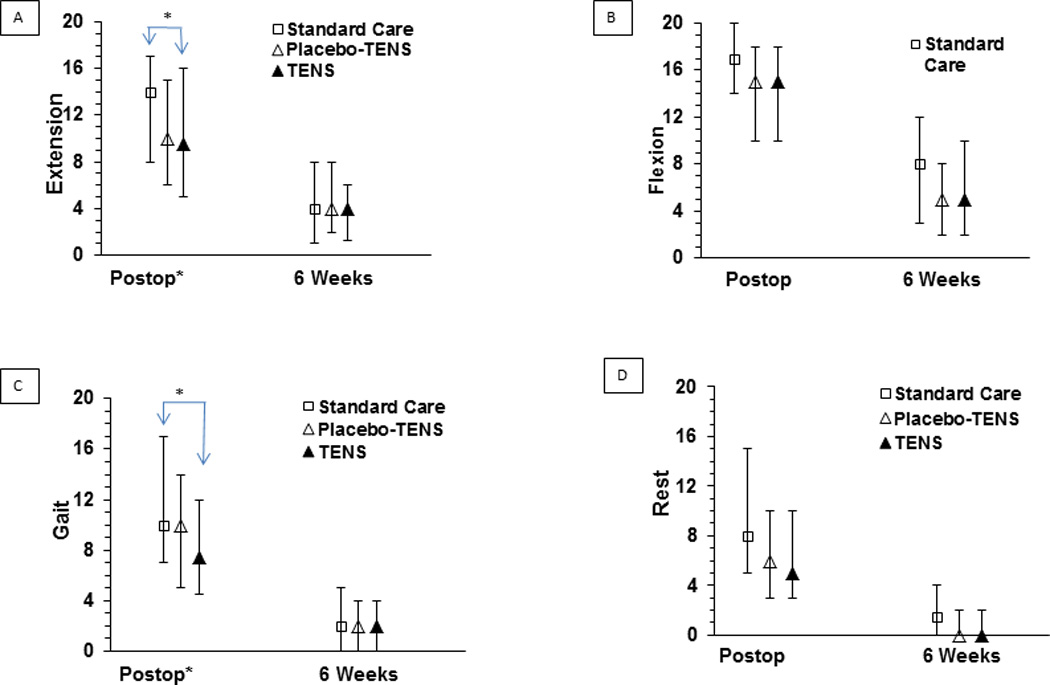
Pain Intensity (on 0–20 NRS) by Treatment Group
At 6 weeks following surgery, pain at rest was significantly different between groups (Kruskal-Wallis, p=0.019). However, even though participants receiving TENS and Placebo-TENS reported lower resting pain than participants receiving Standard Care (0 versus 1.5), pairwise comparisons did not reach significance at p<0.025 (TENS versus Standard Care p=0.036, effect size = 0.5; Placebo-TENS versus Standard Care p=0.13, effect size = 0.5) (see Figure 4). Pairwise comparisons for TENS and Placebo-TENS were not significant (p=1.0, effect size = 0). There were no significant differences in extension pain (p=0.89), flexion pain (p=0.18), or gait speed pain (p=0.90) between treatment groups at 6 weeks following surgery. In keeping with the intent-to-treat analysis, missing data at 6 weeks were imputed using a predictive mean matching imputation method. This method allows for discrete target variables and imputations are based on values observed elsewhere so are realistic and meaningful [71]. Postoperative pain, age, preoperative trait anxiety, depression and pain catastrophizing were used to calculate predicted values at 6 weeks. Five imputation scenarios were conducted and all results were similar to the original findings.
Based on literature that anxiety, pain catastrophizing and depression are predictive of pain following surgery [25,38,59], it was hypothesized that change in movement pain (i.e. average extension and flexion pain from POD 1 to 6 weeks) would differ based on these psychological characteristics and treatment group. As hypothesized, change in ROM pain was significantly different across treatment groups depending on how individuals scored on the Trait Anxiety Inventory, Pain Catastrophizing Scale, and Geriatric Depression Scale preoperatively (p=0.012, 0.046, and 0.032, respectively). For TENS participants, decrease in pain was significantly larger for those with lower trait anxiety (p=0.002) and lower pain catastrophizing (p=0.03). A similar trend was seen for participants receiving Standard Care but, while the results for trait anxiety suggest a possible difference, these trends did not reach significance (trait anxiety p=0.05 and pain catastrophizing p=0.10). In contrast, Placebo-TENS participants had little change in their ROM pain based on trait anxiety (p=0.45) or pain catastrophizing (p=0.36). Additionally, TENS and Standard Care participants who screened negative for depression had a larger decrease in ROM pain than those who screened positive for depression but Placebo-TENS participants who screened negative for depression had a smaller decrease in ROM pain than those who screened positive for depression. These trends did not reach significance (TENS p=0.12, Standard Care p=0.11, and Placebo-TENS p=0.10) (see Figure 5).
Figure 5.
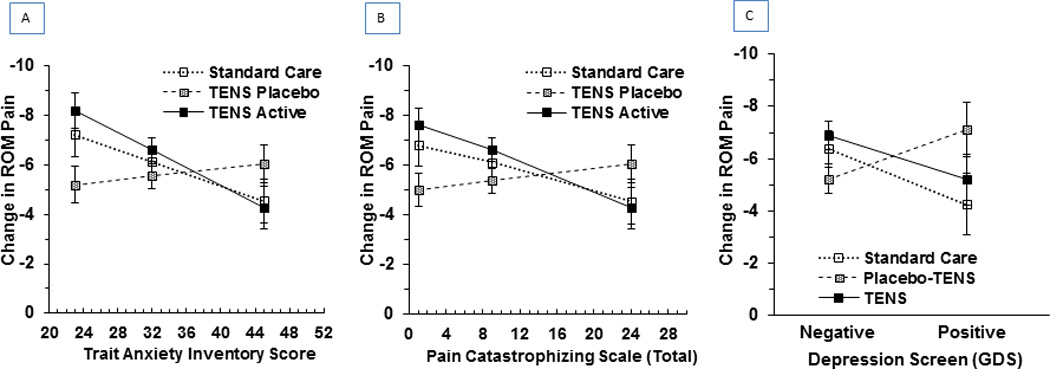
Change in Pain Intensity from Postop to 6 weeks by Preoperative Psychological Characteristics and Treatment Group
Pain Sensitivity
Prior to surgery, median algometer pressure pain thresholds (PPTs) were 215 kPa (IQR 155–320) at the knee and 251 kPa (IQR 183–357) at the anterior tibialis muscle. Median heat pain thresholds were 43.74 °C (IQR 41.3–45.8) at the knee and 44.57 °C (IQR 43–46.6) at the anterior tibialis muscle. Heat pain tolerances were 47.4 °C (IQR 46.1–48.6) and 47.8 °C (IQR 46.6–49.1) at the knee and anterior tibialis muscle, respectively. There were no significant differences in any of these measures between groups prior to surgery.
Following surgery, the treatment by time interaction was non-significant for all pain sensitivity measures (p=0.12 to 0.625). During the immediate postoperative period, PPTs at the knee were significantly different across treatment groups (Kruskal-Wallis, p=.009), with pairwise comparisons showing participants receiving TENS and Placebo-TENS had significantly higher PPTs than participants receiving Standard Care (i.e. median 156 kPa for Standard Care versus 194 kPa for TENS, p=.01, effect size = 0.3 and 187kPa for Placebo-TENS, p=.03, effect size = 0.2). PPTs at the anterior tibialis muscle were also significantly different across treatment groups (Kruskal-Wallis, p=.02), with pairwise comparisons showing participants receiving Placebo-TENS had significantly higher PPTs than those receiving Standard Care (median 310 versus 247, p=0.023, effect size = 0.3). Participants receiving TENS had a median PPT of 288 but this did not reach significance at p<0.05 compared to Standard Care (p=0.083, effect size = 0.2) or Placebo-TENS (p=1.0). Heat pain thresholds and tolerances were similar across groups with no significant differences at the knee or at the anterior tibialis muscle postoperatively. At 6 weeks, there were no significant differences in PPTs, heat pain thresholds, or heat pain tolerances between groups (see Figure 6).
Figure 6.
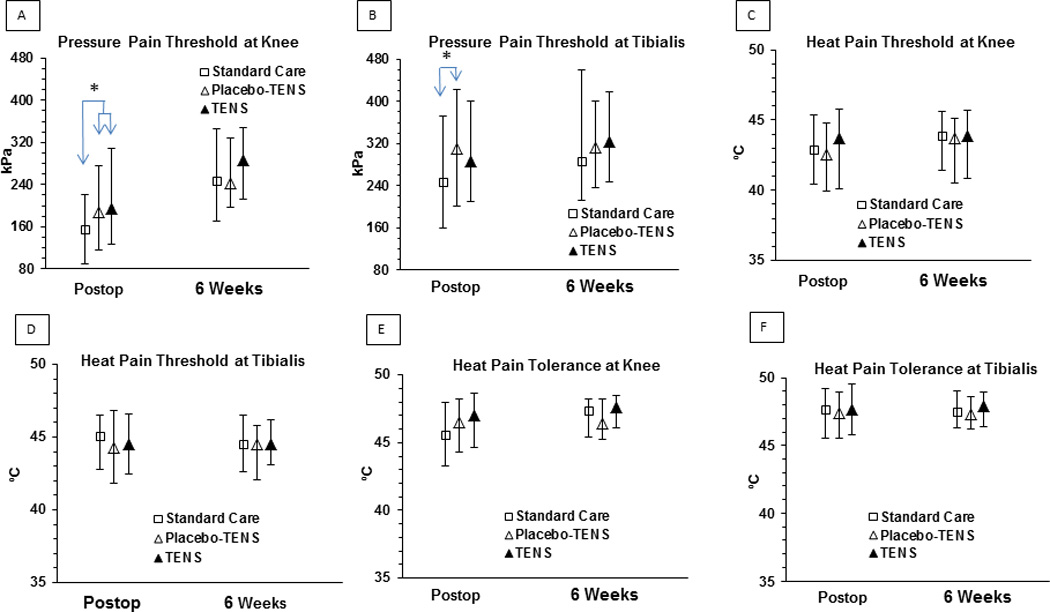
Quantitative Sensory Tests
Function
Prior to surgery, knee extension for all participants was −4 (median degrees, IQR −9 to −.75), knee flexion was 115.5 (median, IQR105–125), and extensor lag (minus extension degrees) was −1 (median, IQR −3 to 0). Immediately prior to treatment postoperatively, extension was −14 (median, IQR −19 to −10), flexion was 52 (median, IQR 41 to 65), and extensor lag was −5 worse than extension (median, IQR −10 to −2). There were no significant differences across treatment groups prior to treatment allocation on any of these measures.
Following surgery, the treatment by time interaction was non-significant for all the function measures (p ≥ 0.11). Postoperatively, there was no significant difference across treatment groups for extension (p=0.17), flexion (p=0.71) or extensor lag (p=0.92). At 6 weeks following surgery, there were also no significant differences across treatment groups (extension, p=0.99, flexion, p=0.58, extensor lag, p=0.24).
The distance walked prior to surgery during the gait speed test was 62.7 ft (median, IQR 49–74.6 ft). Postoperatively, the distance walked reduced to 11.2 ft (median, IQR 6.7–18.8 ft) but increased back to 60.8 ft (median, IQR 50–72.3 ft) at 6 weeks. There was no significant difference across treatment groups in distance walked postoperatively or at 6 weeks (Kruskal-Wallis p=.60 and .24, respectively) (see Figure 7).
Figure 7.
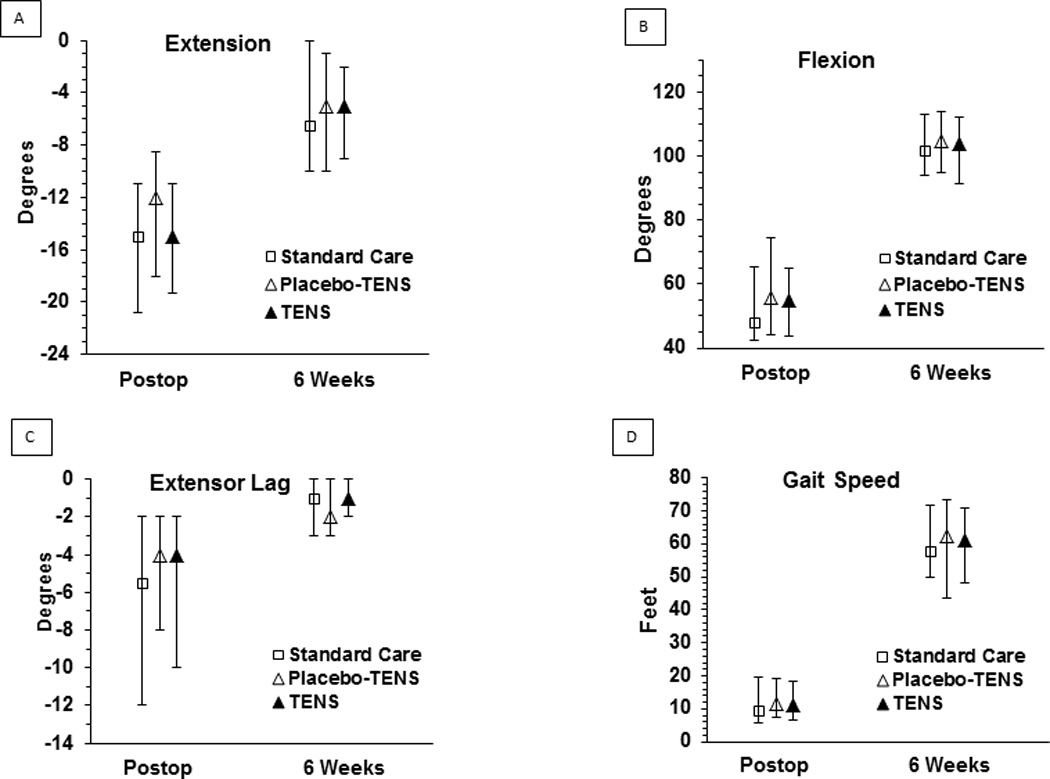
Function Measures (ROM, Extensor Lag, and Gait Speed)
Correlations between pain and function were performed to determine if increased pain was associated with decreased function (i.e. poorer extension and flexion of the knee and shorter walking distance). All correlations were significant and negative demonstrating that increased pain was associated with decreased function (see Table 2).
Table 2.
Correlations Between Pain and Function
| Spearman Correlation Statistics (Fisher's z Transformation) | ||||||
|---|---|---|---|---|---|---|
| Variable | With Variable | N | Correlation Estimate |
95% Confidence Limits |
p Value for H0:Rho=0 |
|
| POD1/2 | ||||||
| Extension Pain | Extension Degrees | 307 | −0.184 | −0.290 | −0.073 | 0.0012 |
| Flexion Pain | Flexion Degrees | 307 | −0.229 | −0.333 | −0.120 | <0.0001 |
| Gait Speed Pain | Gait Speed Distance | 287 | −0.151 | −0.262 | −0.036 | 0.0101 |
| 6 Weeks | ||||||
| Extension Pain | Extension Degrees | 249 | −0.366 | −0.469 | −0.254 | <0.0001 |
| Flexion Pain | Flexion Degrees | 248 | −0.342 | −0.447 | −0.227 | <0.0001 |
| Gait Speed Pain | Gait Speed Distance | 247 | −0.168 | −0.286 | −0.043 | 0.0082 |
Discussion
This study demonstrated that adding TENS to routine pharmacologic analgesia significantly reduces movement pain (i.e. pain during active joint range of motion and walking) during the immediate postoperative period following TKA compared to pharmacologic analgesia alone. There were no significant differences between TENS and Placebo-TENS, suggesting that the expectation of receiving additional pain relief through TENS had comparable effects to actually receiving TENS. Participants receiving Placebo-TENS, however, did not report significantly less pain than participants receiving Standard Care.
A potentially important finding in this study was that participants receiving TENS who scored low on trait anxiety and pain catastrophizing had a significantly larger decrease in ROM pain than participants receiving TENS who scored high on these factors. This finding is consistent with a prospective, double-blind, controlled trial conducted by Lim, et al. [46] evaluating the influence of psychological factors and TENS in determining the intensity of pain after abdominal surgery. They found that when the contribution of neuroticism was included in the analysis, subjects receiving TENS had significantly less morphine requirements than subjects receiving placebo-TENS. This connection between TENS effect and psychological factors on pain suggests that patients scoring high on these factors may not benefit from TENS and that this treatment should be targeted to those who are not anxious or catastrophizing their pain. Further investigation is needed to confirm these results.
There were no significant differences between TENS and Placebo-TENS in this study suggesting a placebo effect occurs when applying TENS. This may be due to the large percentage of participants who were blinded to the Placebo-TENS treatment (45%). Blinding of participants was higher using the transient sham unit in this study than blinding reported with conventional sham units that do not turn on but use active indicator lights to stimulate a placebo effect (i.e. 13%) [58]. The blinding achieved in this study was similar to other studies using this same transient sham unit [48,51,58,73] and, consistent with these studies, is a lack of significance between TENS and Placebo-TENS when this transient sham unit is compared to TENS in clinical populations [73]. The lack of significance in subjective pain intensities between TENS and Placebo-TENS is also consistent with a meta-analysis conducted by AHRQ in 1992 evaluating TENS for postoperative pain [9]. Placebo treatments can have a powerful and real effect on pain scores [17]. Prior studies show that this effect can be manipulated and mimic the effect of morphine [3,44]. Thus, when delivered so that people believe they are receiving an active treatment, the differences between an active treatment and a placebo treatment can be mitigated.
Additionally, PPTs at the surgical knee were significantly higher for participants receiving both TENS and Placebo-TENS compared to those receiving Standard Care and significantly higher for Placebo-TENS then Standard Care at the anterior tibialis muscle postoperatively, suggesting the effect of TENS on deep mechanical primary and secondary hyperalgesia also involves placebo influences. This finding is in contrast to studies showing that high frequency TENS reduces primary and secondary hyperalgesia when compared to placebo-TENS [42,56,61,64,72] and our prior study in patients with OA using the same placebo-TENS treatment [73]. The lack of significant differences in heat pain thresholds and tolerances is similar to prior studies in healthy controls and in people with OA [58,73]. These studies suggest that TENS is more effective for mechanical deep tissue pain than thermal cutaneous pain. In healthy controls we and others routinely show that TENS reduces PPTs [45,48,51].
Bjordal, et al. [5] found that TENS intensity was a variable that improved the effect of TENS for postoperative pain. All participants receiving TENS in this study used the treatment well above Bjordal’s cut off of 15mA [5]. Additionally, there was no significant correlation between TENS amplitude (or dose) and outcomes in this study. This finding suggests that the effect of TENS was similar across amplitudes and that all participants used a potentially effective amplitude.
This is the first clinical trial to evaluate the effect of long term TENS on postoperative pain following TKA. The lack of significance on movement pain at 6 weeks is consistent with other studies that have evaluated the effect of repeated TENS application beyond a few days [11,77]. This is in contrast to significance found with one-time TENS applications [4,19]. It is possible that the reduced effect over time is due to the development of tolerance to the TENS stimulation. Studies that have evaluated the effect of repeated TENS use have demonstrated tolerance to TENS after five days of repeated application when given at the same dose (frequency and intensity) in healthy human subjects and in animal studies [10,45]. Factors that prolong this tolerance include alternating between low and high frequency [23] and increasing the amplitude over time [62]. While the amplitude was high for all participants in this study, it did not increase over time and high frequency was used throughout. Future studies should incorporate these strategies to determine their influence on the efficacy of TENS over time following TKA. Another possible explanation for this lack of significance at 6 weeks following surgery is that pain improved in all participants by 6 weeks making significant differences between groups more difficult to detect. Future studies evaluating the effect of pain interventions may want to consider an earlier target for this evaluation.
Finally, while pain and function were significantly correlated across all participants, this correlation was small and the moderate effect of TENS on pain was not enough to significantly improve function beyond the other treatment groups. These results suggest that other factors contribute to the functional limitations experienced following TKA, such as concomitant low back pain [14] or differences in maximum muscle strength and torque development deficits [79]. Future studies should consider other variables that may contribute to functional ability when making these comparisons.
A limitation of this study was the lack of ethnic diversity in the sample. A large majority (95%) of the sample was non-Hispanic and white. This limits generalization to similar patients and it is therefore, unknown if the efficacy of TENS would be different for other ethnicities or races. Further studies are needed that evaluate the effect of TENS in a more ethnically diverse population. In addition, while participants in the Placebo-TENS groups were blinded to treatment, the majority of participants receiving TENS knew they received an active treatment, and the Standard Care group could not be blinded. This lack of blinding of the TENS participants is similar to other studies where TENS is given at strong intensities [19,58,73]. This is a problem with all non-pharmacologic clinical trials and illustrates the importance of including a placebo-TENS treatment that can be appropriately blinded.
In summary, this large, blinded, randomized, placebo-controlled trial with intent-to-treat analyses demonstrated that adding TENS during rehabilitation exercises (i.e. as a supplement to routine pharmacologic analgesia) resulted in significantly reduced movement pain during the immediate postoperative period compared to Standard Care. However, no significant differences were found between TENS and Placebo-TENS suggesting a placebo influence. Additionally, there were no group differences at 6 weeks following TKR suggesting that TENS is not beneficial beyond the immediate postoperative period. The benefit of adding TENS may be greatest for patients who are not anxious or catastrophizing their pain.
Supplementary Material
Summary.
TENS significantly decreases movement pain postoperatively but not by 6 weeks following TKA compared to Standard Care. TENS and placebo-TENS were not significantly different.
Acknowledgments
The authors would like to thank Linda Fink, RN, MSN, Susan Kloos, RN, Carolyn Rourke, RN, Tamera Lansing, PT, Jeff Adcock, PT and all the other nurses and physical therapists at the University of Iowa Hospitals and Clinics, the Veterans Administration Medical Center, and surrounding clinics for their support. We would also like the thank Nyla Logsden-Sacket, MSN for her help on the project. This study was funded by the National Institute of Nursing Research, National Institute of Health (R01 NR009844), the University of Iowa College of Nursing, and DJO, Inc. Dr. Sluka serves as a consultant for DJO, Inc., the manufacturer of the TENS units used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no other conflicts of interest.
ClinicalTrials.gov Identifier: NCT01364870
References
- 1.Agresti A. An introduction to categorical data analysis. New York: Wiley; 1996. [Google Scholar]
- 2.Allen RS, Thorn BE, Fisher SE, Gerstle J, Quarles K, Bourgeois MS, Dijkstra K, Burgio LD. Prescription and dosage of analgesic medication in relation to resident behaviors in the nursing home. J Am Geriatr Soc. 2003;51:534–538. doi: 10.1046/j.1532-5415.2003.51164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 4.Arvidsson IEE. Postoperative TENS pain relief after knee surgery: objective evaluation. Orthopedics. 1986;9:1346–1351. doi: 10.3928/0147-7447-19861001-06. [DOI] [PubMed] [Google Scholar]
- 5.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Breit R, Van der Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. J Arthroplasty. 2004;19:45–48. doi: 10.1016/s0883-5403(03)00458-3. [DOI] [PubMed] [Google Scholar]
- 7.Britt JA. Use of transcutaneous electrical nerve stimulation in control of postoperative cesarean section pain. 1983 [Google Scholar]
- 8.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Carr DB, Jacox AK, Chapman CR, Ferrell B, Fields HL, Heidrich G, Hester NK, Hill CS, Lipman AG, McGarvey CL, Miaskowski C, Mulder DS, Payne R, Schecter N, Shapiro BS, Smith RS, Tsou CV, Vecchiarelli L. Acute pain management: operative or medical procedures and trauma. Clinical Practice Guideline. Rockville: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, AHCPR Pub. No. 92-0032; 1992. [Google Scholar]
- 10.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 11.Chesterton LS, Lewis AM, Sim J, Mallen CD, Mason EE, Hay EM, van der Windt DA. Transcutaneous electrical nerve stimulation as adjunct to primary care management for tennis elbow: pragmatic randomised controlled trial (TATE trial) BMJ. 2013;347:f5160. doi: 10.1136/bmj.f5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain. 2001;92:173–186. doi: 10.1016/s0304-3959(00)00485-1. [DOI] [PubMed] [Google Scholar]
- 13.Christoph SS. A comparison of patient-controlled transcutaneous electrical nerve stimulation with traditional analgesics for relief of postoperative pain. Washington, DC: Catholic University of America; 1984. [Google Scholar]
- 14.Clement ND, MacDonald D, Simpson AH, Burnett R. Total knee replacement in patients with concomitant back pain results in a worse functional outcome and a lower rate of satisfaction. Bone Joint J. 2013;95-B:1632–1639. doi: 10.1302/0301-620X.95B12.31684. [DOI] [PubMed] [Google Scholar]
- 15.Collins N, Teyes P, Vicenzino B. The initial effects of a Mulligan's mobilization with movement technique on dosiflexion and pain in subacute ankle sprains. Man Ther. 2004;9:77–82. doi: 10.1016/S1356-689X(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 16.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. Pain. 2013;154:511–514. doi: 10.1016/j.pain.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn IG, Marshall AH, Yadav SN, Daly JC, Jaffer M. Transcutaneous electrical nerve stimulation following appendicectomy: the placebo effect. Ann R Coll Surg Engl. 1986;68:191–192. [PMC free article] [PubMed] [Google Scholar]
- 19.Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, Lee KS, Lee JE, Sluka KA. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–2562. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalury DF, Lieberman JR, Macdonald SJ. Current and innovative pain management techniques in total knee arthroplasty. Instr Course Lect. 2012;61:383–388. [PubMed] [Google Scholar]
- 21.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86:102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 22.DeSantana JM, Da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163:1233–1241. doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desantana JM, Sluka KA, Lauretti GR. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clin J Pain. 2009;25:12–19. doi: 10.1097/AJP.0b013e31817d1070. [DOI] [PubMed] [Google Scholar]
- 24.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14:307–311. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feeney SL. The relationship between pain and negative affect in older adults: anxiety as a predictor of pain. J Anxiety Disord. 2004;18:733–744. doi: 10.1016/j.janxdis.2001.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson L, Alstergren P, Kopp S. Pressure pain thresholds in the craniofacial region of female patients with rheumatoid arthritis. J Orofac Pain. 2003;17:326–332. [PubMed] [Google Scholar]
- 28.Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Galloway DJ, Boyle P, Burns HJ, Davidson PM, George WD. A clinical assessment of electroanalgesia following abdominal operations. Surg Gynecol Obstet. 1984;159:453–456. [PubMed] [Google Scholar]
- 30.Gordon DB, Stevenson KK, Griffie J, Muchka S, Rapp C, Ford-Roberts K. Opioid equianalgesic calculations. J Palliat Med. 1999;2:209–218. doi: 10.1089/jpm.1999.2.209. [DOI] [PubMed] [Google Scholar]
- 31.Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: a structural equations approach. Pain. 2004;107:234–241. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Goubert L, Francken G, Crombez G, Vansteenwegen D, Lysens R. Exposure to physical movement in chronic back pain patients: no evidence for generalization across different movements. Behav Res Ther. 2002;40:415–429. doi: 10.1016/s0005-7967(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 33.Hastie BA, Riley JL, 3rd, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Herr KA, Mobily PR, Kohout FJ, Wagenaar D. Evaluation of the Faces Pain Scale for use with the elderly. Clin J Pain. 1998;14:29–38. doi: 10.1097/00002508-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Herr KA, Spratt K, Mobily PR, Richardson G. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain. 2004;20:207–219. doi: 10.1097/00002508-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S European Palliative Care Research Collaborative (EPCRC) Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Hoyl MT, Alessi CA, Harker JO, Josephson KR, Pietruszka FM, Koelfgen M, Mervis JR, Fitten LJ, Rubenstein LZ. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47:873–878. doi: 10.1111/j.1532-5415.1999.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 38.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657–677. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58:387–392. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 41.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmaco Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 42.King EW, Audette K, Athman GA, Nguyen HO, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115:364–373. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38:426–439. doi: 10.1016/j.jpainsymman.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Levine J, Gordon N, Fields H. The mechanism of placebo analgesia. Lancet. 1978;312:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 45.Liebano RE, Vance CG, Rakel BA, Lee JE, Cooper NA, Marchand S, Walsh DM, Sluka KA. Transcutaneous electrical nerve stimulation and conditioned pain modulation influence the perception of pain in humans. Eur J Pain. 2013;17:1539–1546. doi: 10.1002/j.1532-2149.2013.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim AT, Edis G, Kranz H, Mendelson G, Selwood T, Scott DF. Postoperative pain control: contribution of psychological factors and transcutaneous electrical stimulation. Pain. 1983;17:179–188. doi: 10.1016/0304-3959(83)90141-0. [DOI] [PubMed] [Google Scholar]
- 47.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137:94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- 48.Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, Rakel BA, Sluka KA, Walsh DM. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12:929–935. doi: 10.1016/j.jpain.2011.02.352. [DOI] [PubMed] [Google Scholar]
- 49.Moss P, Sluka KA, Wright A. Does accessory knee joint mobilisation reduce osteoarthritic hyperalgesia? J Manipulative Physiol Ther in press [Google Scholar]
- 50.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20:88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Pantaleao MA, Laurino MF, Gallego NL, Cabral CM, Rakel B, Vance C, Sluka KA, Walsh DM, Liebano RE. Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain. 2011;12:581–590. doi: 10.1016/j.jpain.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute care. Ann Pharmacother. 2007;41:255–266. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 53.Persson AL, Brogardh C, Sjolund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil Med. 2004;36:17–27. doi: 10.1080/16501970310015218. [DOI] [PubMed] [Google Scholar]
- 54.Piriyaprasarth P, Morris ME. Psychometric properties of measurement tools for quantifying knee joint position and movement: a systematic review. Knee. 2007;14:2–8. doi: 10.1016/j.knee.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Prushansky T, Dvir Z, Defrin-Assa R. Reproducibility indices applied to cervical pressure pain threshold measurements in healthy subjects. Clin J Pain. 2004;20:341–347. doi: 10.1097/00002508-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain. 2005;6:673–680. doi: 10.1016/j.jpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 58.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, Miller CA, Polehna AC, Ruggle RC, Vance CG, Walsh DM, Sluka KA. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakel BA, Blodgett NP, Bridget Zimmerman M, Logsden-Sackett N, Clark C, Noiseux N, Callaghan J, Herr K, Geasland K, Yang X, Sluka KA. Predictors of postoperative movement and resting pain following total knee replacement. Pain. 2012;153:2192–2203. doi: 10.1016/j.pain.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rinaldi P, Mecocci P, Benedetti C, Ercolani S, Bregnocchi M, Menculini G, Catani M, Senin U, Cherubini A. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694–698. doi: 10.1034/j.1600-0579.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 61.Sabino GS, Santos CM, Francischi JN, de Resende MA. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain. 2008;9:157–163. doi: 10.1016/j.jpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Sato KL, Sanada LS, Rakel BA, Sluka KA. Increasing intensity of TENS prevents analgesic tolerance in rats. J Pain. 2012;13:884–890. doi: 10.1016/j.jpain.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sim DT. Effectiveness of transcutaneous electrical nerve stimulation following cholecystectomy. Physiotherapy. 1991;77:715–722. [Google Scholar]
- 64.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77:97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 65.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 66.Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- 67.Strassels SA, McNicol E, Suleman R. Postoperative pain management: a practical review, part 1. Am J Health Syst Pharm. 2005;62:1904–1916. doi: 10.2146/ajhp040490.p1. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan MJ, Neish N. The effects of disclosure on pain during dental hygiene treatment: the moderating role of catastrophizing. Pain. 1999;79:155–163. doi: 10.1016/s0304-3959(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 69.Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91:147–154. doi: 10.1016/s0304-3959(00)00430-9. [DOI] [PubMed] [Google Scholar]
- 70.Taylor AG, West BA, Simon B, Skelton J, Rowlingson JC. How effective is TENS for acute pain? Am J Nurs. 1983;83:1171–1174. [PubMed] [Google Scholar]
- 71.van Buuren S. Chapman & Hall/CRC interdisciplinary statistics series. Boca Raton, FL: CRC Press; 2012. Flexible imputation of missing data. [Google Scholar]
- 72.Vance CG, Radhakrishnan R, Skyba DA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 73.Vance CG, Rakel BA, Blodgett NP, DeSantana JM, Amendola A, Zimmerman MB, Walsh DM, Sluka KA. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92:898–910. doi: 10.2522/ptj.20110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6:205–212. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- 75.Walker RH, Morris BA, Angulo DL, Schneider J, Colwell CW., Jr Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty. J Arthroplasty. 1991;6:151–156. doi: 10.1016/s0883-5403(11)80010-0. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med. 2002;27:139–144. doi: 10.1053/rapm.2002.29253. [DOI] [PubMed] [Google Scholar]
- 77.Warke K, Al-Smadi J, Baxter D, Walsh DM, Lowe-Strong AS. Efficacy of transcutaneous electrical nerve stimulation (tens) for chronic low-back pain in a multiple sclerosis population: a randomized, placebo-controlled clinical trial. Clin J Pain. 2006;22:812–819. doi: 10.1097/01.ajp.0000210935.73686.79. [DOI] [PubMed] [Google Scholar]
- 78.Weeks SK, McGann PE, Michaels TK, Penninx BW. Comparing various short-form Geriatric Depression Scales leads to the GDS-5/15. J Nurs Sch. 2003;35:133–137. doi: 10.1111/j.1547-5069.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 79.Winters JD, Christiansen CL, Stevens-Lapsley JE. Preliminary investigation of rate of torque development deficits following total knee arthroplasty. Knee. 2013 doi: 10.1016/j.knee.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.