Abstract
Background
Generating myocyte grafts that bridge across infarcts could maximize their functional impact and best utilize small numbers of stem cells. To date, however, graft survival within acute infarcts has not been feasible. To enhance intra-infarct graft viability, human embryonic-derived cardiomyocytes (hESC-CMs) were pretreated prior to implantation with cobalt protoporphyrin (CoPP), a pharmacologic inducer of cytoprotective heme oxygenase-1.
Methods
After pre-culturing with CoPP (vs. PBS), hESC-CMs were injected intramyocardially into acutely infarcted rat hearts, using directed injections to span the infarct. A further group received CoPP-pretreated hESC-CMs plus 4 weekly doses of systemic CoPP to prolong exposure to cytoprotectants. Two control groups with infarcts received vehicle-only intramyocardial injections or weekly systemic CoPP without cell therapy. Post-infarct ventricular function was gauged by echocardiography and graft size quantified at 8 weeks by histomorphometry.
Results
CoPP pre-conditioned hESC-CMs formed stable grafts deep within infarcted myocardium, while grafts without CoPP exposure survived mainly at the infarct periphery. Fractional shortening was improved at 4 and 8 weeks in all hearts receiving cell therapies (P < 0.01 vs. vehicle-only injections). CoPP treatment of both graft hESC-CMs and recipient animals resulted in the largest grafts, highest fractional shortening, preserved wall thickness, and reduced infarct dimensions.
Conclusions
Cellular therapy delivered acutely after infarction significantly improved post-infarct ventricular function at 1 and 2 months. CoPP pretreatment of cells resulted in stable hESC-CM grafts within infarcted myocardium. This design enables construction of directionally-oriented, infarct-spanning bands of new cardiomyocytes that might further improve functional restoration as engrafted myocytes proliferate and mature.
Keywords: cell therapy, human embryonic stem cells, heme oxygenase-1, myocardial infarct repair, preconditioning
Introduction
In the normal heart, ventricular cardiomyocytes are organized into macroscopic helical myocardial bands that produce the twisting systolic movement, or torsional deformation, of the left ventricle during contraction.1-4 Myocardial infarction (MI) destroys these oriented myocardial fibers, damaging the structural architecture of the ventricle. Current intramyocardial cell delivery techniques produce disconnected clusters of randomly-oriented grafts. We examined whether it was instead possible to generate band-like cardiomyocyte grafts to span the infarct area. Such graft bridges could reconnect the inherent ventricular myocardial bands still present at the infarct borders, thus maximizing the functional contribution of the new cells.
However, a major limitation for cell-based therapies is that only 10-20% of injected cells survive implantation into ischemic infarcts, with most cell loss occurring within the first week. Graft cell survival within an acute infarct has not previously been a realistic expectation5,6,9,10 and, consequently, cell injections are commonly delivered into peri-infarct areas.7,11,12 The goal in this study was to determine whether pharmacologic induction of survival factors could improve graft survival within the infarct which, in turn, would allow construction of infarct-spanning cellular bridges.
Our group has previously shown that pretreating cardiomyocyte grafts with cobalt protoporphyrin (CoPP) markedly improves their survival following implantation into an ischemic environment.12,13 CoPP activates the transcription factor, nrf2, which initiates transcription of cytoprotective heme oxygenase-1 (HO-1) and additional anti-oxidative factors.14,15 Pre-emptive HO-1 induction by gene therapy has been shown to reduce infarct size, myocyte apoptosis, and remodeling in hearts subjected to myocardial infarction.16-19 The downstream endproducts of HO-1, bilirubin and carbon monoxide, are anti-apoptotic, anti-oxidative, pro-angiogenic, and antifibrotic20,21--all desirable attributes for cell therapy in ischemic beds. In these experiments, we are applying ex vivo CoPP treatment to human embryonic-derived cardiomyocytes (hESC-CMs), a cell population with potential use in clinical cell therapy. Our laboratory has found that exposing hESC- CMs to a single dose of CoPP produces sustained HO-1 upregulation for at least 4 days. Thus, ex vivo CoPP pretreatment of cells prior to implantation would, potentially, provide grafted cells with a survival advantage over the critical first few days following implantation. Besides CoPP pretreatment of graft cells, a month-long course of systemic CoPP was administered to some recipient groups as a means to extend the pharmacologic effects during early infarct maturation.
Materials and Methods
Preparation and Characterization of hESC-CMs
HESC-CMs were generated from the H7 human embryonic stem cell line22 by serial application of activin A (R&D Systems, Minneapolis, MN) and BMP4 (R&D Systems),10 omitting the “pro-survival” cocktail and Percoll gradient centrifugation. Spontaneous contraction was observed after further culture in RPMI-B27 serum-free medium (SFM) (Invitrogen, Carlsbad, CA). For HO-1 induction, the cell culture medium was supplemented with 25 μM CoPP (Frontier Scientific Inc., Logan, UT) in phosphate buffered saline (PBS, Invitrogen). Control hESC-CMs were cultured in media supplemented with PBS alone. Cells were then enzymatically dispersed and cryopreserved until implantation.
To characterize cells just prior to implantation, aliquots from each thawed cell batch (Supplementary Data, Video 1) were plated and fixed with methanol for immunocytochemical profiling. Nascent cardiomyocytes were identified with antibodies to cardiac troponin I (cTnI) (Abcam, San Francisco, CA) and human Nkx2.5 (R&D Systems), an early cardiac-specific transcription factor; mitotic cells with antibody to Ki67 (Abcam); and endothelial cells with antibody to human CD31 (hCD31) (Dako Inc., Carpinteria, CA). Nuclei were counterstained with Hoechst 33342 dye (Invitrogen).
Permanent Myocardial Infarction Model
Animal protocols were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC 2011). Rats were placed under isoflurane anesthesia and mechanically ventilated for MI surgery. Through a left thoracotomy, the left anterior descending coronary artery (LAD) was permanently ligated without reperfusion. Five minutes after LAD ligation, microspheres, cells, or media were injected into the infarct, followed by chest closure and recovery from anesthesia.
Microsphere Retention after Intramyocardial Injection
Microspheres, equivalent in size to hESC-CMs, were injected into acute MIs to model immediate cell loss from leakage and washout after direct intramyocardial injection. In 5 Fischer 344 rats (Charles River Labs, Portage, MI), 5×106 Hydro-Coated Yellow E-Z Trac Ultraspheres [15μm diameter, Interactive Medical Technology (IMT), Irvine, CA] suspended in 70μL RPMI were injected into infarct centers; the heart, lung, and spleen were excised 15 minutes later. In heart specimens, yellow microspheres were imaged by dark field epi-illumination under fluorescence stereomicroscopy (Leica Microsystems, Wetzlar, Germany). Whole hearts, lungs, and spleens were placed into individual centrifuge tubes to which equal numbers of Pink E-Z Trac Ultraspheres (5×106 in 70 μL RPMI) were added as the standard for 100% recovery. After microsphere extraction, the relative proportion of injected (yellow) to standard (pink) microspheres in each organ was quantified by flow cytometry.
Implantation of hESC-CMs into Infarcted Hearts
1×107 hESC-CMs, suspended in 100 RPMI SFM, were loaded into a 0.3 mL syringe with a 29G needle for intra-infarct injection in athymic male rats (NIH-Whn, 240-280 g, Taconic Farms, Cambridge City, IN). To produce a band of cells across the infarct, the needle was inserted from each of the two infarct margins, converging at the infarct center, creating a track that spanned the infarct from one uninjured edge to the other (Supplementary Data, Video 2). Cells were delivered as the needle was withdrawn from the myocardium,23 rather than during needle insertion, to facilitate even distribution along the needle track.
Treatment Groups
Athymic rats, all with acute infarcts, were randomized into 5 treatment groups (n = 8/group). Three cell treatment groups received intra-infarct injections of hESC-CMs: (1) hESC-CMs pretreated ex vivo with PBS; (2) hESC-CMs pretreated ex vivo with 25 μM CoPP for 24 hours before implantation; and (3) hESC-CMs pretreated with CoPP plus recipient rat treatment with a 4 week course of systemic CoPP (5 mg/kg/dose intraperitoneally, delivered 24 hours before surgery and once weekly). Two animals dying post-surgery (one each from Groups 1 and 2) were replaced; all were included in baseline data. Two control groups did not receive cell therapy. Of these, one control group (4) received intra-infarct injections of vehicle (SFM) alone, while a second control group (5) received the 4 week course of systemic CoPP, but without cell therapy. This intraperitoneal dose of CoPP,24-26 given weekly27, produces sustained HO-1 upregulation in heart tissue.
Echocardiography
Two-dimensional M-mode echocardiograms were performed under isofluorane anesthesia 24 hours prior to surgery (pre-infarction baseline) and at 48 hours, 4, and 8 weeks after surgery (SonoSite® M-Turbo, Bothell, WA). Percent fractional shortening (FS) was calculated from left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) dimensions as [(LVEDD-LVEDS)/LVEDD]×100.
Histologic Evaluation of hESC-CM Grafts
At 8 weeks following surgery, hearts were excised, fixed in methyl Carnoy's solution, paraffin embedded, and sectioned at 1-mm intervals between the coronary ligation site and ventricular apex. Serial 5 μm thick sections within each macro-section were treated with hematoxylin and eosin (H&E) and Masson's trichrome stains. Human cellular grafts were identified with antibodies to the β-myosin heavy chain isoform (ATCC, Manassas, VA), which is preferentially expressed in human, but not rat, myocardium6,10 and with human-specific antibody to lamin A/C (Millipore, Billerica, MA).28 Gap junctions were labeled with antibodies to connexin 43 (Cell Signaling, Danvers, MA). Nuclei were counterstained with Hoechst dye.
Morphometric Assessments
Morphometry was performed on four transverse macro-sections per heart encompassing the entire infarct. Planimetry was done in ImageJ (Bethesda, MD), using Masson's trichrome staining, which delineates fibrillar collagen, to define the infarct. Infarct size was quantified as the mean percentage of left ventricular circumference occupied by the trichrome blue-positive infarct zone over all sections per heart. Infarct wall thickness was calculated as the mean of three measurements taken across the infarct, divided by the mean of two measurements of non-infarcted adjacent ventricular wall.32
Graft size was measured as the cumulative sum of βMHC+ areas on these same standardized sections. The percentage of grafted area within the infarct was quantified as the sum of grafted areas surrounded by collagen-rich infarct tissue on each section divided by the total graft area. The extent of infarct replacement by grafted cells was determined as the percentage of the total infarct area occupied by human lamin A/C+ graft cells.
Statistical Analysis
Data are expressed as means ± standard deviations. One-way analyses of variance (ANOVAs) with post-hoc Bonferroni corrections were used to assess single outcome measures among treatment groups at the 8 week time point. Significance levels were adjusted to P values < 0.017 (0.05/3) and < 0.005 (0.05/10) for comparisons between 3 and 5 treatment groups, respectively. Analysis of longitudinal echocardiographic data was performed in R statistical software using the Laird-Ware mixed effects model.33 The model was fitted with indicator variables for the 5 treatment groups and 4 time points as well as 12 two-way interaction terms between these variables, and included the random effect of animal identification number to estimate the variability caused by individuals. Two-way ANOVAs were employed to evaluate four outcome variables with Bonferroni multiple testing corrections, using P < 0.0125 (0.05/4) to determine significance.
Results
Characterization of Injectates prior to Implantation
Cardiomyocytes (cTnI+) comprised 64 ± 4% of cells across all aliquots (Supplementary Figure 1A). 27 ± 6% of cells stained positively for the cardiac-specific transcription factor, Nkx2.5, indicating some cardiomyocytes still in an early phase of differentiation; 15 ± 8% of cells were Ki67+, denoting a mitotic population (Supplementary Figure 1B, C). Human endothelial-like cells (hCD31+) were rare (< 0.1%, data not shown). No difference in cell composition was seen between CoPP-pretreated vs. PBS-pretreated injectates.
Injectate Retention within the Heart after Intra-infarct Injection
Recovery of cell-sized microspheres was used to estimate the extent of immediate cell loss following intra-infarct injection. Fifteen minutes after delivery, 68 ± 16% of injected microspheres were retained within the heart, the majority situated along the needle injection track (Figure 1). Ten ± 5% had dispersed to the lungs (P < 0.0001 vs. heart specimens), and no signal was detected in the spleen (0.001 ± 0.001, P < 0.0001 vs. heart specimens). Immediate microsphere loss (and, hence, expected cell loss) through leakage and intramyocardial collateral flow thus totaled about 30% of the injectate with the redistribution primarily into the pulmonary, not the systemic, circulation.
Figure 1.
Microsphere biodistribution after intra-infarct injection. Representative example of an infarcted heart 15 minutes after intramyocardial injection of yellow fluorescent microspheres. A-C, Gross specimen showing the anterior LV with infarct (circled). Black triangles mark ligation sites on the LAD and diagonal arteries. White arrows indicate the direction of trans-infarct needle injection. C, Blue arrows point to the majority of microspheres which remain within the infarct along the injection tract, effectively spanning the infarct. D-F, Consecutive cross-sections through the same heart illustrating the microspheres within the mid-level sections. A and D, Darkfield epi- illumination images; B and E, fluorescence imaging to visualize microspheres; C and F, merged images. Scale bar = 5 mm. G, Relative microsphere retention in heart, lung, and spleen as quantified by flow cytometry (n = 5). *** P < 0.0001 vs. lung or spleen.
Graft Histology at 8 Weeks Post-implant
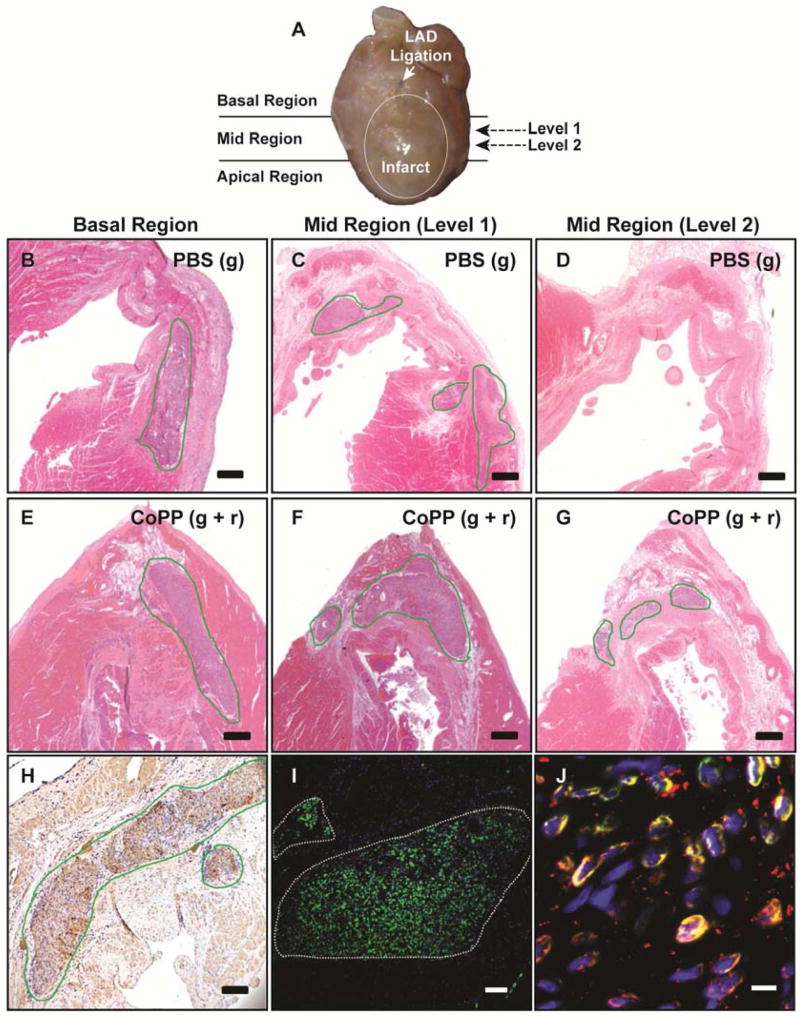
At 8 weeks post-implant, surviving hESC-CM grafts were found in all hearts receiving hESC-CM injections (Figure 2B-G), corroborated by βMHC immunostaining (Figure 2H). Immunostaining with human-specific lamin A/C further confirmed these grafts to be of human origin (Figure 2I). By this time, gap junction proteins were well expressed within the grafts (Figure 2J).
Figure 2.
Histology of hESC-CM grafts at 8 weeks after trans-infarct implantation. A, Sectioning diagram for fixed hearts to survey the infarct for the presence of human grafts along the diagonally-oriented injection tract. Further serial sectioning was performed within each region to determine the extent of human cell engraftment in the infarct center (e.g., Levels 1 and 2). B-G, H&E staining of representative hearts from the group receiving PBS-pretreated hESC-CMs [PBS(g): B-D] and from the group that received CoPP-pretreated hESC-CMs plus recipient treatment with systemic CoPP [CoPP(g+r): E-G]. B and E, In basal regions, hESC-CM grafts in both treatment groups are seen extending into non-infarcted host myocardium. Note the lack of fibrotic encapsulation around the grafts. C and F, In the mid-infarct region, grafts are found at the lateral infarct margins, near the infarct border zone, under both experimental conditions (Level 1). However, grafts are present in the infarct center only in the CoPP-treated group (F) where the potential for infarct replacement by graft and the creation of infarct-spanning bridges is evident. D and G, In deeper levels of the infarct (Level 2), grafts are seen only in the CoPP-treated heart, with no graft present in the heart that received PBS-treated cells. H and I, Grafts are confirmed to be of human origin by immunostaining with β-MHC (dark brown, H) and human lamin A/C (green, I). J, Dual immunostaining demonstrates gap junction proteins (connexin 43, red) on graft cells (human lamin A/C, green) with yellow indicating color convergence. Scale bars: B-G, 500 μm; H, 50μm; I, 25 pm; J, 5 pm.
Grafts of CoPP-pretreated hESC-CMs are Primarily Located within the Infarct Center
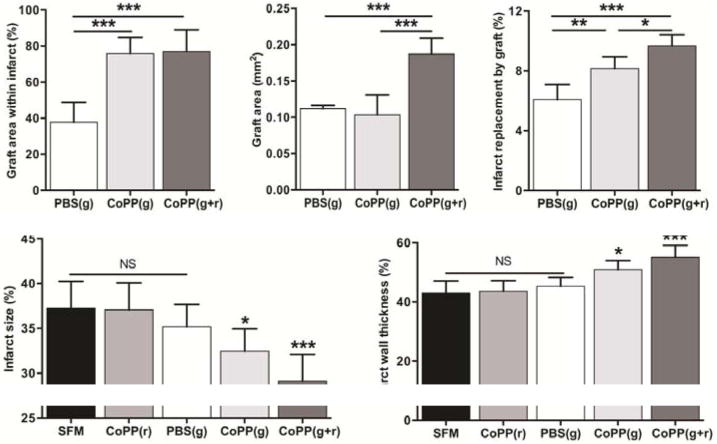
What most distinguished CoPP-pretreated hESC-CM grafts from PBS-pretreated controls was the extent to which cells engrafted within the infarct center (Figure 3A). By 8 weeks post-implant, three-quarters of the total graft area in CoPP-pretreated grafts was located deep within the infarct (76 ± 9% for CoPP-pretreated grafts; 77 ± 12% for CoPP-pretreated grafts plus systemic CoPP). In contrast, surviving PBS-pretreated hESC-CMs were found primarily at the infarct margins with just 38 ± 11% of the graft area within the infarct center (P < 0.0001 vs. each CoPP-treated group).
Figure 3.
Left ventricular morphometry at 8 weeks after acute infarction and cell therapy. A, Percentage of the total graft area located within the infarct. B, Mean graft areas cumulative over all surveyed sections/heart. C, Extent of total infarct area replaced by cellular graft. A-C, * P < 0.01; ** P < 0.001; ***P < 0.0001 between indicated groups. D, Infarct size quantified as a percentage of the total left ventricular circumference. * P < 0.02 vs. SFM and CoPP (r); *** P ≤ 0.0001 vs. SFM, CoPP (r), and PBS (g). E, Mean wall thickness in the infarct area as a percentage of the wall thickness in the adjacent, non-infarcted left ventricle. * P < 0.002 vs. SFM and CoPP (r); ***P < 0.0001 vs. SFM, CoPP (r), and PBS (g). Data represent mean values ± standard deviations; n = 8/group.
Treatment groups. Post-infarct cell therapies: PBS(g), graft hESC-CMs pretreated with PBS; CoPP(g), graft hESC-CMs pretreated with CoPP; CoPP(g+r), graft hESC-CMs pretreated with CoPP plus recipient treatment with systemic CoPP. Control groups without cell therapy: SFM, infarct injections with serum-free media (vehicle-only control); CoPP(r), infarct recipients treated with systemic CoPP.
Adding Systemic CoPP Increases Graft Size at 8 Weeks
At 8 weeks, rats receiving CoPP-pretreated hESC-CMs plus a 4-week course of systemic CoPP had larger cumulative graft areas (0.19 ± 0.02 mm2), effectively double those in the two other cell therapy groups (P < 0.0001 vs. each group, Figure 3B). Without systemic CoPP, graft sizes in hearts receiving CoPP-pretreated hESC-CMs were similar to those receiving PBS-pretreated cells (0.10 ± 0.03 mm2 vs. 0.11 ± 0.00 mm2, respectively, P = 0.67).
CoPP Treatment Increases Infarct Replacement by Engrafted Cells
Planimetry revealed that a larger percentage of the infarct area was comprised of graft cells in hearts receiving CoPP-pretreated vs. PBS-pretreated hESC-CMs (8.2 ± 0.8% vs. 6.1 ± 1.0%, P < 0.001, Figure 3C). Infarct replacement by graft cells was further increased when CoPP-pretreatment of the graft was combined with a 4-week course of systemic CoPP (9.7 ± 0.8%, P < 0.01 vs. CoPP-pretreated and P < 0.0001 vs. PBS-pretreated grafts, Figure 3C).
Infarct Size is Reduced in Hearts Receiving CoPP-pretreated hESC-CMs
Histomorphometry at 8 weeks revealed that infarct sizes ranged from 29 ± 3% to 37 ± 3% of the ventricular circumference across all groups, confirming that all hearts had sustained substantial infarction. Infarct size was not altered by cell therapy alone, as infarct sizes in hearts with PBS-pretreated hESC-CMs (35.2 ± 2.5%) were not smaller than in hearts receiving vehicle-only (SFM) injections (37.2 ± 3.0%, P > 0.9999). Also, a transient, 4-week course of systemic CoPP, by itself, did not affect infarct size at 8 weeks (37.1 ± 3.0%, P > 0.9999 vs. vehicle-only controls). In contrast, however, infarct sizes were markedly reduced in both groups receiving CoPP-pretreated hESC-CMs. In hearts receiving CoPP-pretreated hESC-CMs alone, infarct size was limited to 32.4 ± 2.5% of the ventricular circumference (P ≤ 0.02 vs. each of the two groups without cell therapy). Adding a 4-week course of systemic CoPP to CoPP-pretreated hESC-CM injections produced a further decrease in the infarct area to 29.1 ± 3.0% of the ventricle (P < 0.0001 vs. the two groups without cell therapy and P = 0.001 vs. hearts with PBS-pretreated hESC-CMs; Figure 3D).
Cell Therapy with CoPP-pretreated hESC-CMs Preserves Infarct Wall Thickness
Marked wall thinning was seen in infarcted hearts that received vehicle-only injections, reducing ventricular wall thickness to 43.1 ± 4.0% of that in adjacent, uninfarcted heart wall (Figure 3E). Cell therapy with PBS-pretreated hESC-CMs did not improve wall thickness at 8 weeks (45.3 ± 3.0%, P > 0.9999 vs. each of the two control groups without cell therapy). Also, a 4-week course of systemic CoPP alone (without cell therapy) did not improve wall thickness (43.7 ± 3.5%, P > 0.9999 vs. vehicle-only controls).
In contrast, wall thickness was much better preserved in hearts receiving CoPP-pretreated hESC-CM grafts, with or without systemic CoPP supplementation. Infarct wall thickness was 51.0 ± 3.0% of normal in hearts given CoPP-pretreated hESC-CMs (P ≤ 0.002 vs. the two groups without cell therapy and P = 0.03 vs. hearts with PBS-pretreated cells). In the group that received CoPP-pretreated hESC-CMs plus systemic CoPP, wall thickness was maintained at 55.1 ± 4.0% of normal (P < 0.0001 vs. the same three groups). Infarct areas in hearts that received CoPP-pretreated hESC-CMs plus systemic CoPP appeared well cellularized, with resident cells consisting not only of graft cells, but also surviving native cardiomyocytes (Figure 2F).
Cell Therapy Improves Post-infarct Global Heart Function
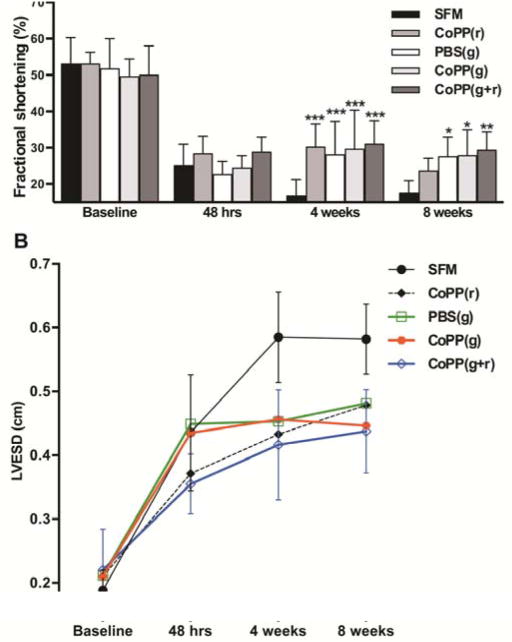
Global heart function, gauged by mean FS, dropped precipitously in all hearts within 48 hours after infarct creation (from a mean of 51.6 ± 6.7% pre-MI to 25.5 ± 4.9% at 48 hours post-MI, Figure 4A). In hearts receiving vehicle-only injections, FS continued to decline by another 30-35% over the first month post-infarct, reaching 17.4 ± 3.5% at 8 weeks (P = 0.001 at 4 weeks and 0.015 at 8 weeks vs. 48 hours post-MI). In contrast, FS in all 3 groups of hearts receiving cell therapy at the time of infarction showed a 13-18% improvement by 4 and 8 weeks, with mean FS at 8 weeks rising to 28.2 ± 5.7%. FS in each cell therapy group was notably higher than the vehicle-only (SFM) group at both 4 (P ≤ 0.0001) and 8 weeks (P < 0.01 for groups with PBS- and CoPP-pretreated grafts, P < 0.001 for the group receiving CoPP-pretreated hESC-CMs plus systemic CoPP). The protocol using CoPP combination therapy resulted in the highest FS at both 4 and 8 weeks (31.0 ± 6.4% and 29.3 ± 5.0%, respectively), although this was not statistically different from the two other cell therapy groups (P > 0.0125).
Figure 4.
Echocardiographic assessment of functional parameters at baseline, 48 hours, 4 and 8 weeks post infarction. A, Fractional shortening (%); data represent mean values ± standard deviation. * P < 0.01; ** P ≤ 0.001; *** P ≤ 0.0001 vs. SFM. B, LVESD (cm); points indicate mean values. Error bars showing standard deviations are limited to the two outermost groups so that trends in means can be more easily visualized.
LVESD, left ventricular end-systolic diameter; cm, centimeter.
The primary contributor to the improvement in ejection fraction was a reduction in end- systolic dimensions in hearts receiving cell therapy (Figure 4B). LVESDs in all treatment groups bettered vehicle-only controls at 4 weeks (each P ≤ 0.0002), but by 8 weeks, only the 2 groups with] cells had lower LVESDs than vehicle-injected groups (each P ≤ 0.002). At 8 weeks, differences in LVEDDs between treatment groups were not statistically significant (P > 0.0125) but, nonetheless, hearts in the two groups with CoPP-pretreated cells also had the lowest LVEDDs (data not shown).
Effects of Systemic CoPP Alone on Functional Parameters
Weekly doses of CoPP, without cell therapy, also improved post-infarct ventricular function during ongoing treatment. FS was higher and LVESD lower at 4 weeks in the group treated with systemic CoPP alone compared to vehicle-only controls (both P ≤ 0.0001, Figure 4A, B). Notably, at 4 weeks, rats receiving systemic CoPP had a FS of 30.2 ± 6.3%, compared to only 16.8 ± 4.3% in controls given vehicle injections. However, the improvement in FS was a transient one, and lost at 8 weeks (P > 0.0125), one month after CoPP dosing had been discontinued.
Discussion
Producing infarct-spanning bridges of neomyocardium, directionally aligned with the inherent helical myocardial bands of contraction,1 has the potential to correct post-infarct ventricular dysfunction in ways that are not currently possible. However, development of such techniques has been hampered by the early demise of cells implanted into infarcts.5-8 To assess the cytoprotective effects of CoPP pharmacotherapy, hESC-CMs were implanted under the most adverse conditions--immediately after acute infarct creation, directly into the infarct center, and into an infarct with permanent coronary occlusion (without reperfusion), conditions that have previously precluded hESC-CM survival. Thus, the most noteworthy consequence of CoPP pretreatment of hESC-CMs was that 76% of the resulting graft area at 8 weeks was found situated deep within the infarct center, double that of PBS-treated cells, making the creation of infarct-spanning myocardial bridges feasible.
Some recipient groups were also given a course of systemic CoPP to extend the pharmacologic effects over the first month post-implant. Hearts receiving the combined therapy were found to have larger grafts and a greater extent of infarct replacement by grafted cells compared to the cell therapy groups without systemic CoPP, suggesting that continued expression of HO-1 (and perhaps nrf2-related antioxidants)14,15,21 may have further stabilized these nascent grafts during infarct maturation. Populations of surviving native myocytes were also seen within infarcts in hearts receiving combined therapy. This was not unexpected, given that induced HO-1 expression is known to improve native myocyte survival in the face of ischemia/infarction.16-18 In this protocol, the injected CoPP-pretreated cells may have acted as delivery vehicles for HO-1 within the unvascularized infarct center, producing early local cytoprotective effects (e.g., through released carbon monoxide) that would also have benefited resident host cardiomyocytes and endothelial cells.
Importantly, in addition to enhancing graft viability, injections of CoPP-pretreated hESC-CMs also positively affected ventricular remodeling in the native heart by reducing infarct size and LVESD, while preserving wall thickness in the infarct region. Notably, these effects on remodeling were seen only in groups receiving CoPP-pretreated hESC-CMs, and not in hearts receiving PBS-pretreated cells. Further, both improvements in infarct size and wall thickness were even more pronounced when cell therapy was accompanied by the concomitant course of systemic CoPP.
All hearts receiving cell therapy were found to have better ventricular function at 1 and 2 months, with or without CoPP treatments, compared to hearts with vehicle-only injections. Thus, the larger graft sizes and mitigated remodeling seen in hearts receiving CoPP-supplemented grafts did not translate into advantages in FS beyond those achieved by cell therapy without CoPP. However, direct mechanical contributions from these still-immature cardiomyocytes were not expected at this time. That said, trends revealed that, by 8 weeks, ventricular dimensions were stabilizing in the 2 groups receiving CoPP-pretreated cells while continuing to increase in hearts from other groups, suggesting that functional gains might occur over time as these larger intrainfarct grafts mature and proliferate, especially in models with graft-host conduction.
Given the known effects of HO-1 overexpression on post-infarct ventricular function and remodeling,16,19,34 systemic CoPP was given without cell therapy to an additional control group. In the absence of cell therapy, systemic CoPP did not alter infarct size. However, infarcted hearts exposed to weekly systemic CoPP alone did exhibit markedly improved FS by 1 month. But, once systemic CoPP was discontinued, these early gains in global function were lost by 2 months. In contrast, improvements in heart function were sustained at 2 months only when the peri-infarction course of systemic CoPP was combined with cell therapy.
Limitations of this study are the xenograft model (without graft-host conduction), and caveats regarding extrapolation of rodent data to humans. However, a new report on infarct repair in non-human primate hearts demonstrates hESC-CM graft survival at 3 months with electrical coupling to host cardiomyocytes,35 raising expectations that cellular transplants can persist and integrate into host ventricles. If so, this clinically-relevant model could be used to determine whether structural purposing of cellular grafts by the proposed strategies would offer long-term functional advantages. As CoPP alternatives, other less specific, but FDA-approved, HO-1 inducers (e.g., statins, phosphodiesterase-5 inhibitors) might be explored. In future clinical application, such infarct-spanning “myobridges” could be surgically implanted during CABG procedures or minimally-invasive interventions for heart failure, using pre-procedural imaging and predictive simulation modeling to optimize orientation for individual hearts.
Supplementary Material
Video 1. Contracting hESC-CMs in the injectate just prior to implantation. Imaged cells have undergone ex vivo CoPP pretreatment, followed by cryopreservation, and thawing.
Video 2. Cell delivery technique into an acute infarct in a rat heart. The needle is inserted at a 30° angle to the epicardial surface from each of the two infarct margins until reaching the infarct center. The cells are then injected during needle withdrawal with a goal of producing a track that spans the infarct from one uninjured edge to the other.
Supplemental Figure 1. Characterization of cell injectates prior to implantation. Aliquots of differentiated hESC-CM injectates were stained with antibodies to (A) cTnI, denoting cardiomyocytes, green fluorescence; (B) Nkx2.5, an early cardiomyocyte-specific transcription factor, in red, or (C) Ki67, indicating mitotic cells, in red. Nuclei in all panels were counterstained with Hoechst dye (blue). Scale bars = 50 μm.
Acknowledgments
This study was supported by National Institutes of Health grants R01 HL064387 (M.D.A. and M.A.L.) and R01 HL086709 (M.D.A).
The authors thank Benjamin van Biber for stem cell preparation, Drs. Robert Welikson and Virginia M. Green for reviewing and editing the manuscript; Dr. Jason M. Kim for assistance with surgical procedures; and Dr. Pamela Johnson and Mary Beauchamp for preparation of tissue sections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Luo and Weaver contributed equally to this article.
Disclosures: The authors have no conflicts of interest or commercial support to declare.
References
- 1.Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, Carrio I, et al. Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J Thorac Cardiovasc Surg. 2001;122:389–392. doi: 10.1067/mtc.2001.113745. [DOI] [PubMed] [Google Scholar]
- 2.Buckberg G, Mahajan A, Saleh S, Hoffman JI, Coghlan C. Structure and function relationships of the helical ventricular myocardial band. J Thorac Cardiovasc Surg. 2008;136:578–589. 589 e571–511. doi: 10.1016/j.jtcvs.2007.10.088. [DOI] [PubMed] [Google Scholar]
- 3.Buckberg GD. Basic science review: the helix and the heart. J Thorac Cardiovasc Surg. 2002;124:863–883. doi: 10.1067/mtc.2002.122439. [DOI] [PubMed] [Google Scholar]
- 4.Spotnitz HM. Macro design, structure, and mechanics of the left ventricle. J Thorac Cardiovasc Surg. 2000;119:1053–1077. doi: 10.1016/S0022-5223(00)70106-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 6.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RK, Mickle DA, Weisel RD, Rao V, Jia ZQ. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001;72:1957–1963. doi: 10.1016/s0003-4975(01)03216-7. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 11.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure postmyocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Weaver MS, Cao B, Dennis JE, Van Biber B, Laflamme MA, et al. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Trans Med. 2014 doi: 10.5966/sctm.2013-0189. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamoto S, Flynn JP, Shi Q, Sakr SW, Luo J, Allen MD. Heme oxygenase-1 induction enhances cell survival and restores contractility to unvascularized three-dimensional adult cardiomyocyte grafts implanted in vivo. Tissue Eng Part A. 2011;17:1605–1614. doi: 10.1089/ten.tea.2010.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, et al. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 17.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 18.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, et al. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H48–59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 20.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 21.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 22.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 23.Chachques JC, Cattadori B, Herreros J, Prosper F, Trainini JC, Blanchard D, et al. Treatment of heart failure with autologous skeletal myoblasts. Herz. 2002;27:570–578. doi: 10.1007/s00059-002-2422-3. [DOI] [PubMed] [Google Scholar]
- 24.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 25.Lakkisto P, Kyto V, Forsten H, Siren JM, Segersvard H, Voipio-Pulkki LM, et al. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 26.Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, et al. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 27.L'Abbate A, Neglia D, Vecoli C, Novelli M, Ottaviano V, Baldi S, et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- 28.Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, et al. In vivo myogenic potential of human CD133(+) muscle-derived stem cells: a quantitative study. Mol Ther. 2009;17:1771–1778. doi: 10.1038/mt.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 30.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, et al. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length- based approaches. J Appl Physiol (1985) 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishbein MC, Maclean D, Maroko PR. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978;90:57–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 34.Lakkisto P, Siren JM, Kyto V, Forsten H, Laine M, Pulkki K, et al. Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med (Maywood) 2011;236:1437–1448. doi: 10.1258/ebm.2011.011148. [DOI] [PubMed] [Google Scholar]
- 35.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stemcell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014 doi: 10.1038/nature13233. In press. Epub ahead of print April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Contracting hESC-CMs in the injectate just prior to implantation. Imaged cells have undergone ex vivo CoPP pretreatment, followed by cryopreservation, and thawing.
Video 2. Cell delivery technique into an acute infarct in a rat heart. The needle is inserted at a 30° angle to the epicardial surface from each of the two infarct margins until reaching the infarct center. The cells are then injected during needle withdrawal with a goal of producing a track that spans the infarct from one uninjured edge to the other.
Supplemental Figure 1. Characterization of cell injectates prior to implantation. Aliquots of differentiated hESC-CM injectates were stained with antibodies to (A) cTnI, denoting cardiomyocytes, green fluorescence; (B) Nkx2.5, an early cardiomyocyte-specific transcription factor, in red, or (C) Ki67, indicating mitotic cells, in red. Nuclei in all panels were counterstained with Hoechst dye (blue). Scale bars = 50 μm.