Abstract
The aim of our present study is to explore the anti-arthritic potential effect of total steroid saponins (TSSN) extracted from the rhizome of Dioscorea zingiberensis C.H.Wright (DZW) and to investigate the underlying mechanisms. This work was performed using adjuvant-induced arthritis (AIA) rats in vivo and lipopolysaccharide (LPS) simulated 264.7 macrophage cells in vitro. In AIA-induced arthritic rats, TSSN significantly alleviated the arthritic progression through evaluating arthritic score, immune organ indexes, paw swelling, and body weight. This phenomenon was well correlated with significant suppression of the overproduction of inflammation cytokines (IL-1, IL-1β, IL-6, and TNF-α), oxidant stress makers (MDA and NO), eicosanoids (LTB4 and PGE2), and inflammatory enzymes (5-LOX and COX-2) versus the AIA rats without treatment. On the contrary, the release of SOD and IL-10 was profoundly increased. What’s more, TSSN could obviously ameliorate the translocation of NF-κB to the nucleus through phosphorylation of the p65 and IκBα in vivo and vitro. The current findings demonstrated that TSSN could protect the injured ankle joint from further deterioration and exert its satisfactory anti-arthritis properties through anti-inflammatory and anti-oxidant effects via inactivating NF-κB signal pathway. This research implies that DZW may be a useful therapeutic agent for the treatment of human arthritis.
Keywords: Dioscorea zingiberensis C.H.Wright, total steroid saponins, adjuvant-induced arthritis, lipopolysaccharide, NF-κB
1. Introduction
Rheumatoid arthritis (RA) is a common chronic and relapsing systemic autoimmune disease characterized by synovial hyperplasia, vasculogenesis, cartilage destruction, bone deformity and functional disability of the joint [1,2]. This systemic disorder is caused by progressive inflammation of the joint lining tissue, which can cause pain, stiffness, swelling, as well as many other symptoms [3]. RA is prevalent throughout the world and affects some of the human population causing long-term disability and premature mortality. Therefore, it is important to continue pathophysiological and pharmacological studies on this disease to discover the new therapeutical drugs.
Currently, RA is clinically treated mainly by synthetic medicines belonging to non-steroidal anti-inflammatory drugs (NSAIDs) including ibuprofen, aceclofenac, and naproxen combined with the steroid hormones like cortisone and prednisone [4]. However, these drugs only transiently suppress inflammation and ameliorate symptoms, but they do not significantly improve the long-term disease outcome [5]. Moreover, during these therapeutic treatment, many patients eventually lose response to the drugs or they are forced to interrupt drug administration due to severe adverse side effects such as gastrointestinal ulcergenicity [6], cardiovascular complication, hematologic toxicity and renal morbidity [7,8], hence utility of these medicines are limited for the treatment of RA. Owing to these shortcomings, the exploration of new anti-RA drugs with high efficacy and less toxicity are eagerly needed.
Traditional Chinese medicine (TCM), a unique medical system characterized by the use of multi-component drugs, can hit multiple targets with its components, improve therapeutic efficacy, reduce drug-related side effects, and may also be an effective way of decreasing drug resistance [9,10]. Recently the study of TCM has aroused much interest due to its superiority in the treatment of complex multi-factor diseases [11]. Thus, herbal medicines maybe constitute a potentially important avenue leading to novel therapeutic agents for RA that may not only prevent structural damage of arthritic joints caused by tissue and bone breakdown, but also be safe, relatively inexpensive, highly tolerated and convenient for many patients. Therefore, naturally originated drugs with minimum side effects are highly desired to substitute chemical therapeutics.
In recent years, steroid saponins isolated from herbs have attracted scientific attention because of their structural diversity and significant biological activities. DZW, one of the most commonly used raw material from a unique plant growing in China, contains a high level of steroid saponins which have been applied as a folk treatment for cough, anthrax, rheumarthritis, tumefaction, sprain as well as cardiac disease in the TCM for a long time [12]. Bioactivities of these steroid saponins, including antitumor, antifungal, antivirus, coronary heart disease, etc., have been reported [13]. However, to our knowledge no research has been reported on its anti-arthritic effect. Therefore, our current study was designed to confirm its anti-arthritic effect and explore its potential mechanism of the total steroid saponin extracted from the rhizome of DZW on AIA-treated rats in vivo and macrophage cells in vitro.
2. Materials and methods
2.1. Regents
FCA was purchased from Difco laboratories (Detroit, MI, USA). Methotrexate was obtained from Shanghai Sine Pharmaceutical Co., Ltd (Shanghai, China). ELISA test kits were purchased from R&D systems (Minneapolis, USA). All other chemicals and reagents used for study were of analytical grade procured from approved organizations.
2.2. Plant material and preparation of total steroid saponin extracts
The rhizomes of DZW were provided by Yangtze River Pharmaceutical Industry Co., Ltd. (Jiangsu, China), and authenticated by Prof. Y.Z. Wang (Northwest University, Xi’an of Shaanxi, China). A voucher specimen (HJ20100925-10) has been deposited in School of Pharmacy, Fourth Military Medical University, Shaanxi, China.
Dried raw material of DZW was powdered and extracted with 70% ethanol for three times. The ethanol extracts were combined and evaporated to dryness under reduced pressure with a rotary evaporator. The residue was redissolved in water and subjected to centrifugation. The supernatant was separated on a D-101 macroporous resin column by eluting with 60% ethanol. The eluate was concentrated under reduced pressure. The syrup thus obtained was dissolved in water again, and extracted with an equal volume of n-butanol six times successively. The pooled n-butanol extract was concentrated to obtain residues for the subsequent experimental use.
2.3. Phytochemical investigation of TSSN by HPLC-ELSD and HPLC-ESI-MS
The compounds in the TSSN has been analyzed by HPLC-ELSD and HPLC-ESI-MS in our laboratory [14]. The HPLC analysis was performed on a Waters Alliance 2695 equipment (Waters, Milford, MA, USA) including Alltech 2000ES (Alltech, USA), and the mass spectrometer equipped with a Q-TOF Premier, a quadrupole and orthogonal acceleration time-of-flight tandem mass spectrometer with an electrospray ionization interface.
2.4. Cell culture and NF-κB expression
The RAW 264.7 macrophage cell line acquired from American Type Culture Collection (Rockville, MD, USA) was used in the current study. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and antibiotic namely penicillin (100 units/mL) and streptomycin sulfate (100 μg/mL) in a humidified atmosphere of 5% CO2 [15]. They were incubated with TSSN (25, 50, and 100 μg/mL) and corresponding positive control Methotrexate (35 μg/mL), followed by adding with LPS for another 12 h (LPS, 1μg/mL). Non-stimulated normal control cells were also simultaneously cultured as the control. After stimulating with LPS for 24 h, the culture supernatants were collected and the total protein was extracted according to the previous description [16]. When finishing this procedure, the total p65 of NF-κB and IκBα were determined by western blotting.
2.5. Animals preparation
Healthy adult male Sprague-Dawley rats aged 8-10 weeks (weighing 250-280 g) were purchased from the Experimental Animal Center of The Fourth Military Medical University (Shaanxi, China). One week before the experiment, the animals were acclimatized in an environment at 24°C ± 1°C, with relative humidity of 45-55% and 12:12 h dark/light cycle under specific pathogen-free (SPF) conditions. Enough rat food rich in various necessary nutritional ingredients was supplied, and water was changed every day. What’s more, their cages were cleaned every two days to make their home comfortable. All experimental procedures were in strict accordance with the National Institutes of Health Guide to the Care and Use of Laboratory Animals. Animal experiments were approved by the local institutional review board at the authors’ affiliated institutions.
2.6. Induction of adjuvant arthritis and drug administration
Before the onset of arthritis, sixty Sprague-Dawley rats were randomly divided into six groups, namely the normal group, the FIA (Freund’s adjuvant induced arthritis) control group, the positive control group of Methotrexate, the TSSN low-dose group, TSSN middle-dose group and TSSN high-dose group, with 10 rats in each group. Except for the normal group, the arthritis was induced by a single injection of 0.1 ml of FCA, which contained 10 mg/ml of heat-killed Mycobacterium tuberculosis in liquid paraffin, into the palmar surface of the right hind paw [17]. This operation was conducted under gentle anesthesia with diethyl ether. After this primary immunization, the TSSN-treated groups were orally administered with TSSN extracts at three levels, which are high (200 mg/kg), middle (100 mg/kg), and low dose (50 mg/kg). Methotrexate (MTX, 3 mg/kg) was used as a reference drug of the positive control group and given by intragastric (ig) administration twice a week, while the normal control and FIA control groups were given an equal volume of normal saline at the same time. All groups were orally administered those items daily after arthritis induction until the end of the experiment (day 28).
After establishing the arthritis model, some related measures were taken to ameliorate this suffering during the subsequent experiment. Soft sawdust was placed in cage to avoid hard touching with the swelling leg, and this packing was changed to keep dry and soft every three days. The touch times with arthritic hinds were reduced as much as possible, when the drugs were administrated to rats. What’s more, the arthritic rats were raised in a quiet environment to prevent them from activating pain by noise.
During the period from onset of arthritis to the end, some clinical signs such as body weight, fur colour, diet, and changes in faeces were monitored and recorded according to day-by-day observations. if any abnormal physical signs besides arthritis are appear, the cause was analyzed to improve the condition.
2.7. Measurement of arthritis progression
2.7.1. Assessment of arthritis scores
The rats were assessed every three days for signs of arthritis between day 1 and 28 post-FCA using a well-established, widely used scoring system developed to evaluate the severity of AIA. Arthritis were examined and graded for severity and loci of erythema and swelling using a 4-point scale in which 0=normal, 1=mild swelling and erythema of digits, 2=swelling and erythema of the digits, 3=severe swelling and erythema, 4=gross deformity and inability to use the limb. The total score of each animal was calculated as the arthritic index, with a maximum possible score of 8 (4 points x 2 hind paws) [18]. Assessment of the arthritis score was carried out by a double-blind test.
2.7.2. Evaluation of paw swelling
In order to evaluate the progression of AIA, the right hind paw volumes of all animals were measured [19]. This work was started just before FCA injection on day 0 and thereafter continued at every three days till day 28 with a vernier calliper (GBT1214-1986, Shanghai) to measure ankle (tibiotarsal) joint perimeters by an independent observer without prior knowledge of the experimental groups. Each joint was measured three times and the average data were noted as a final record.
2.7.3. Recording the body weight
During the course of the treatment, the body weight of each rat was measured with a 0.1 g precision balance (METTLLER TOLDEO, PB1501) every 3 days, and the change of body weight was expressed as weight growth (g) [20].
2.8. Measurement the index of thymus and spleen
At the end of the experiment, the animals were sacrificed via anesthesia with intraperitoneal injection of chloral hydrate (0.35 g/kg) after sampling blood. The thymus and spleen were immediately removed from each animal and weighed. The index of thymus and spleen was expressed as the ratio (mg/g) of thymus and spleen wet weight versus body weight, respectively [20]. After the tissues were gently collected from the rats, their bodies were kept intact and clean as far as possible and buried under a specific tomb.
2.9. Estimation of inflammatory cytokines, oxidant stress makers, and eicosanoids concentrations in rat serum
On the 28th day of FCA inoculation, rats were anaesthetized with chloral hydrate (0.35 g/kg) intraperitoneally (i.p) and sacrificed by withdrawing the blood through abdominal vein. The blood sample was centrifuged at 3000 rpm for 20 min to obtain serum and stored at −20 °C prior to analysis. The levels of pro-inflammatory cytokines (IL-1, IL-1β, IL-6, IL-10 and TNF-α), oxidant stress makers (SOD, MDA and NO), and eicosanoids (LTB4 & PGE2) in the serum of FIA rat were analyzed by commercially available assay kits. All the procedures of the used kits were performed according to the manufacturer’s instructions. The results were expressed in picograms per milliliter. The assay was performed by an investigator blinded to the treatment group assignments.
2.10. Histopathological assessment of the arthritic knee joints
The animals were sacrificed on day 28 via anesthesia after blood sampling. Ankle joints were gently separated from the hind paw, and immersed in 4% paraformaldehyde (PF) in 0.1 mol/L phosphate buffer (pH=7.4) for overnight at 4°C followed by decalcification in decalcifier solution for 2-3 days [20]. Then, the joints were trimmed and embedded in paraffin, and 4 μm sections of whole ankle joints were cut with a microtome for subsequent use. For western blotting, the ankle joints were immediately removed and frozen in liquid nitrogen and stored at −80°C until use. After deparaffinization and hydration with a graded alcohol series, histological sections were stained with hematoxylin and eosin (H&E) for light microscopic examination (Olympus, BH-2, Japan) by an experienced technician/observer blinded to the identity of the sample being examined to avoid any bias.
2.11. Western blotting analysis
The prepared ankle joint tissue described in section 2.8. was homogenized by adding 1:5 tissue weight of protein extraction buffer in a glass homogenizer. The protein concentrations of ankle joint tissue and obtained in section 2.4. were determined using a BCA protein assay kit. Homogenate samples (50 μg), mixed with an equal volume of a sample buffer and heated at 95°C for 5 min, were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes [21]. The membrane was blocked with PBST containing 5% non-fat dry milk for 1 h, and then incubated in corresponding primary antibodies (5-LOX and COX-2 for ankle joint tissue. p-p65 and IκBα for ankle joint tissue and RAW 264.7 macrophage cells) for 1 h at room temperature. After washing three times with TBST, the membrane was incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies, diluted in the blocking buffer (dilution: 1:1000) for 1 h, and washed again three times in PBST buffer. β-Actin of which protein expression levels were normalized was used as a loading control.The protein band of interest was visualized using an ECL chemiluminescence system (ECL plus; Amersham Biosciences, NJ, USA) and the density of each band was quantified using an image analysis software.
2.12. Statistical analysis
All the results were expressed as mean ± standard deviation (SD) and carried out by SPSS version 19.0 program. Statistical comparisons were made between drug-treated groups and arthritic control groups. The data of disease activity index were statistically analyzed by two-way ANOVA followed by Bonferroni test. The data of biochemical estimation were analyzed by one-way ANOVA followed by Dunnett’s multiple range tests. The minimal level of statistical significance was considered at P values less than 0.05 (p < 0.05).
3. Results
3.1. The quantification and qualitation of chemical constituents in TSSN
We have isolated and purified 9 related phytoconstituent compounds from the TSSN, and their structures (1-9) were presented in Fig. 1.
Figure 1.
The chemical structures and their names of 9 kinds of steroid saponins from the rhizomes of Dioscorea zingiberensis C.H.Wright.
According to the ion pattern of peaks based on the previously reported HPLC-ESI-MS analysis conditions, TSSN contains many kinds of steroid saponins with various aglycones each having different sugar moieties as shown in Fig. 1. After optimizing the HPLC-ELSD parameters, quantitative analytical results of 9 compounds in TSSN as shown in Fig. 2 were 1.31 (1), 2.82 (2), 2.94 (3), 2.23 (4), 2.53 (5), 2.75 (6), 1.48 (7), 1.55 (8), and 1.43 (9) mg/g. Since dioscin (7) can suppress the NF-κB signaling pathways, it may play an important role in regulating the inflammatory response [22]. What’s more, diosgenin, the skeleton of 6-9, exhibits significant potential of anti-inflammatory effects through down-regulating mitogen-activated protein kinase (MAPK) and inhibiting the corresponding pro-inflammatory cytokines [23]. According to these reported results, it was implied that the steroid saponins with diosgenin aglycone seemed to be the candidate compounds responsible for the anti-arthritis effect of TSSN.
Figure 2.
The represent HPLC-ELSD chromatograms of (a) 9 standards and (b) the TSSN from the rhizomes of Dioscorea zingiberensis C.H.Wright. The peaks labeled 1-9 were the same as Fig. 1. The binary mobile phase composed with Acetonitrile (A)-Water (B) was used as the solvent system and the gradient elution mode was as follows: 0-5 min, 25% to 30% A; 5-20 min, 30% to 30% A; 20-35 min, 30% to 35% A; 35-45 min, 35% to 47% A; 45-47 min, 47% A to 70% A; 47-60 min, 70% to 70%.
3.2. Effect of TSSN on arthritis scores, paw swelling and body weight
The preventive effect of TSSN on FCA induced arthritis (FIA) was studied by evaluating the arthritis scores and ankle perimeter along with the change in body weights of normal and drug-treated AIA rats. As presented in Fig. 3d, clinical signs were displayed after injection of FCA into the subplantar of hind paws. A significant increase (p<0.01) in arthritis scores and ankle perimeter began to appear after 4 days following intraplantar administration of FCA (Fig. 3a and b), as compared to the normal ones. As time went by, the symptoms became much severer reaching the maximum on day 16. There was a significant difference (p<0.01) in these symptoms of arthritis in the control group when compared with the normal group. Administration of TSSN (200 mg/kg, 100 mg/kg) and MTX produced substantial dose suppressive effect in the treated groups which was considerably different from the control group (p<0.01, p<0.05, and p<0.01, respectively), whereas no evident inhibition was observed in the treated group with low dose (50 mg/kg).
Figure 3.

Effect of TSSN on the development of adjuvant arthritis in rats. Rats were immunized by a single injection of Freund’s complete adjuvant into the subplantar surface of the right hind paw on day 0. TSSN and Methotrexate were orally administered once daily and every 3 days, respectively, from day 0 to day 28. (a) Arthritis scores were evaluated every 4 days. (b) Hind ankle joint diameter of each rat was measured every 4 days. (c) Rat body weight changes from day 0 to day 22. (d): Morphological typical chromatograms of hind paw on day 16 after subplantar injection of FCA. All data were expressed as mean ± SD (n=10 per group). #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (3 mg/kg); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
Statistically significant body weight loss (p<0.05), which steadily continued up to the 28th day, was observed in the control group compared to the normal group, starting after 4 days of FCA injection apparently due to the generation of immune response (Fig. 3c). After treatment with TSSN (200 mg/kg, 100 mg/kg), the AIA rats exhibited significant dose-dependent body weight gain (p<0.01, and p<0.05, respectively) when compared to the control group in the subsequent days. However, no remarkable body weight increase was observed in the MTX and 50 mg/kg groups. MTX, used in low doses, is a therapeutic drug for the treatment of RA. Although various chronic toxicities preclude the clinical use of MTX at high anti-inflammatory and immunomodulatory doses, the difference in body weight between MTX group and TSSN group in the present study could not be attributed to its chronic toxicities.
3.3. Effect of TSSN on the indexes of thymus and spleen
Thymus and spleen are vital organs involved in immune responses. The indexes of these organs were determined at day 28 after FCA injection. The control group showed high indexes (p<0.01, Fig. 4) compared with the normal group.. There was a marked reduction of indexes of thymus and spleen in the TSSN treatment group at the doses of 200 mg/kg and 100 mg/kg (p<0.01, and p<0.05, respectively), while this phenomenon was absent at the dose of 50 mg/kg. These indexes were both decreased in the MTX treatment group (p<0.01).
Figure 4.
Effects of TSSN on the indexes (mg/g) of thymus and spleen of AIA rats in different groups at day 28 after immunization. There was markedly reduction of index of thymus and spleen in Methotrexate and TSSN (at the dose of 200 mg/kg and 100 mg/kg). All data were expressed as mean ± SD (n=10 per group). #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (3 mg/kg); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
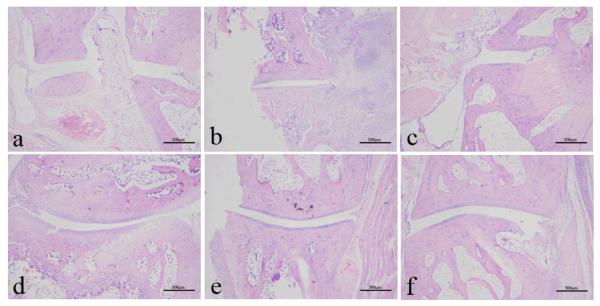
3.4. Effect of TSSN on histopathological changes
RA is well characterized with synovial membrane hyperplasia, pannus formation, and cartilage and bone destruction in the joint. In histopathological evaluation by H&E staining, the ankle joints of the control group rats displayed prominent destructive inflammation of articular bone and extra-articular tissues, joint swelling with cellular infiltration, synovial hyperplasia, and narrowing joint space with severe pannus formation along with the thinning of the cartilage plate (Fig. 5b). In contrast, the normal group showed normal articular cartilage, absence of synovial membrane hyperplasia and open articular cartilage (Fig. 5a). These pathological changes were evidently reduced by the administration of TSSN (Fig. 5e-5f) as compared to the control group. Particularly, there was only mild synovial infiltation with few inflammatory cells and no obvious damage in cartilage bone erosion in the high dose of TSSN. Similar data were also obtained in the MTX group (Fig. 5c).
Figure 5.
Histopathological changes of hind ankle joints were evaluated by H&E staining at the end of the experiment. The representative examples of each group: (a): Normal group; (b): Control group; (c): Methotrexate group; (d): Low dose of TSSN (50 mg/kg); (e): Middle dose of TSSN (100 mg/kg); (f): High dose of TSSN (200 mg/kg). (H&E staining, magnification 40 X; scale bar, 500 μm).
3.5. Effect of TSSN on inflammatory cytokines, oxidant stress makers, and eicosanoids levels in rat serum
To gain an insight into the release of inflammatory cytokines (IL-1, IL-1β, IL-6, TNF-α, and IL-10), oxidative status (SOD, MDA, and NO), and eicosanoids production (LTB4 and PGE2), we measured their levels in the serum. As shown in Fig. 6, the total inflammatory cytokines of the control group displayed significant 1.52, 1.26, 1.97, and 1.34 fold increases in the content of IL-1 (p<0.01) , IL-1β (p<0.01), IL-6 (p<0.01), and TNF-α (p<0.01), respectively, as compared to normal rats, whereas the IL-10 exhibited 1.27 decrease (p<0.01) suggesting that IL-10 possessed an anti-inflammatory effect during the FIA process. However, TSSN alleviated the change of these parameters as compared with control group. Interestingly, the effective regulation on the inflammatory cytokines by TSSN, especially in the 200 dose, was almost equivalent to that by the reference drug MTX.
Figure 6.

Effects of TSSN on inflammatory cytokines production in rats the serum. Levels (ng/L) of IL-1, IL-6, IL-10, IL-1β and TNF-α were determined by ELISA on day 28. All data were expressed as mean ± SD (n=10 per group). #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (3 mg/kg); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
Regarding the oxidant stress makers, there was a remarkable increase in the level of MDA (1.03, p<0.05) as well as NO (1.24, p<0.01), and a significant decrease in SOD activity (1.36, p<0.01) in the TSSN group compared with the normal group as seen from Fig. 7. Noticeably, administration of TSSN resulted in attenuating the production of MDA and NO, while elevating the SOD activity compared with the control group. Although, the 50 and 100 mg/kg doses of TSSN had only slightly reversed these parameters.
Figure 7.

Effects of TSSN on oxidative stress markers in the serum of rats on day 28. (a) Levels of SOD (U/mL) and NO (μmol/L). (b): Levels of MDA (nmol/L). All data were expressed as mean ± SD (n=10 per group). #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (3 mg/kg); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
For the eicosanoids, there were 1.38-fold (p<0.01) and 1.20-fold (p<0.01) rises of LTB4 and PGE2 in the serum, respectively, in the control group compared to the normal group (Fig. 8). Pre-treatment of TSSN inhibited these changes compared with the control group. What’s more, the mitigation of TSSN on the PGE2, especially in the 200 mg/kg dose, was almost equivalent to that of MTX. However, the 50 and 100 mg/kg doses suppressed the release of two eicosanoids similarly without any significant difference.
Figure 8.

Effects of TSSN on eicosanoids in the serum of rats on day 28. (a) Levels of LTB4 (ng/L). (b): Levels of PGE2 (ng/L). All data were expressed as mean ± SD (n=10 per group). #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (3 mg/kg); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
3.6. Effect of TSSN on NF-κB expression and inflammatory mediators
Since NF-κB signaling pathway is a key regulator in RA joints, we further evaluated the effect of TSSN on LPS-induced activation in RAW 264.7 macrophage cells. LPS is found in the outer membrane of gram-negative bacteria, where it maintains bacterial structure and protects from external effects. On the other hand, LPS also acts as an endotoxin and trigger a variety of the immune response in mammalian cells. The inflammatory reaction is stimulated through release of inflammatory mediators by RAW 264.7 macrophages cells when it is exposed to LPS [24]. Upon LPS recognition, LPS is transferred to CD14 by LPS binding protein and recognized by TLR4-MD-2 complex on the cellular surface leading to the rapid and coordinated activation of various intracellular signaling pathways including translocation of NF-κB [25]. The activated NF-κB could mediate in turn the inflammation by regulating the inflammatory mediators expression. Therefore, we chose the LPS as the ideal stimulant to initiate the inflammatory reaction in RAW 264.7 macrophage cells.
In normal physiological conditions, the NF-κB is in an inactivited state and located in cytoplasm of cells. Activation of NF-κB requires phosphorylation of p65 (a subunit of NF-κB) and IκBα at certain parts of their structure. Therefore, both p65 and IκBα protein expressions were measured in the present study. They were noticeably increased (p<0.01) after induction of LPS as compared with the control without LPS stimulation in Fig. 9 a-b, suggesting that the activated NF-κB may be translocated from the cytoplasm to the nucleus. However, increase of these two indicators was markedly alleviated by TSSN-administration in a dose dependent manner, and the translocation of NF-κB was inhibited to a great extent. Consistent with these findings in vitro, the p65 and IκBα protein expressions in arthritic ankle joint extracts revealed the same variation trend in the TSSN pre-treatment rat group versus the AIA group. The underlying mechanisms of TSSN on NF-κB were similar in vivo and in vitro suggesting that TSSN would suppress NF-κB activation by inhibiting the phosphorylation p65 and IκBα.
Figure 9.

Effects of TSSN on the expression of NF-κB induced by LPS on RAW 264.7 cells and the expression of inflammatory enzyme proteins and NF-κB in ankle joint tissue of the rats on day 28. (a): The RAW 264.7 cells were initially pre-treatmented with indicated concentration of TSSN and Methotrexate (positive control), then stimulated by LPS (1 μg/mL). (c): Representative results of Western blotting. (b-d): Quantitative analysis. The protein levels were normalized against the β-actin, which served as loading control. Values are expressed in relative optical density and represented as means ± SD at least of three independent tests. #p versus the Normal group and *p versus the Control group, respectively. NG, normal group; CG, control group; MTX, Methotrexate group (35 μg/mL in vitro and 3 mg/kg in vivo); LDG, low dose of TSSN group (50 mg/kg); MDG, middle dose of TSSN group (100 mg/kg); HDG, high dose of TSSN group (200 mg/kg).
Seen from Fig. 9c-d, there were significant increases in COX-2 (p<0.01) and 5-LOX (p<0.01) protein expression of ankle joint tissue in the FCA-induced rats compared with the normal group. Administration of TSSN (200 mg/kg, and 100 mg/kg) markedly inhibited these two protein expressions compared with the control group. At the same time, the decreasing trend of these two proteins was also observed in MTX-treated rats.
4. Discussion
When the normal biological system are exposed to harmful stimulants, some reactions produce abundant adverse substances which could further influnce the physical activity in a cascading manner. So is the same in Rheumatoid arthritis (RA). The inflammatory reaction, an important pathophysiologic process, is firstly initiated after the injection of Freund’s complete adjuvant (FCA). Evidences have demonstrated that many types of cells such as macrophages, lymphocytes, synovial cells and neutrophils are infiltrated during the pathophysiologic process of RA [26]. These cells are aggregated into the articular cavity and release mediators and inflammatory cytokines. These released materials establish a complex network to aggravate joint damage through pannus formation, narrow joint space, and cartilage and bone destruction, finally leading to disability [27]. Due to these abovementioned reasons, we investigated whether the TSSN has potential anti-inflammatory effects on a Freund’s Complete Adjuvant-induced arthritis model in vivo and on RAW 264.7 macrophages cell system in vitro.
Oral administration of TSSN for 28 days markedly attenuated the progress of arthritis by reduction in arthritic scores (Fig. 3a), paw swelling (Fig. 3b), body weight growth (Fig. 3c), indexes of the organ (Fig. 4), and amelioration in ankle histopathological changes (Fig. 5). These effects may be produced through decreasing pro-inflammatory (TNF-α, IL-1, IL-1β, along with IL-6 in Fig. 6) and inflammatory enzymes (5-LOX and COX-2 in Fig. 9c-d) as well as their products of eicosanoids (LTB4 and PGE2 in Fig. 8) while increasing anti-inflammatory cytokines (IL-10 in Fig. 6). The transcriptional factor NF-κB released by macrophages is extensively involved in the inflammatory response [28]. In normal cells, the inactivated NF-κB is trapped in the cytoplasm by IκB proteins [29]. Once stimulated and activated by harmful stimuli, the p65 and p50 (two subunits of NF-κB) are immediately translocated to the nucleus where it activates many genes to transcribe various kinds of cytokines and inflammatory mediators [30]. We propose that TSSN may inhibit the activity of NF-κB. In an effort to test this hypothesis, the RAW 264.7 macrophages, a widely used system to observe the inflammatory reactions in vitro, were applied in vitro. As expected, the western blot results (Fig. 9a-b) revealed that TSSN suppressed the phosphorylation of IκBα, and then inhibited nuclear p65 levels induced by LPS, and finally led to the inactivation of NF-κB after exposed to LPS, a potent activator of NF-κB pathways corresponding to the IκBα/p65/NF-κB line. In order to further verify the TSSN effect on NF-κB, its activity was also examined in vivo, and the similar results were observed (Fig. 9c-d). The inhibitory activity of TSSN on NF-κB might trigger various signal transduction cascades as follows: it lowers encoding of the genes of inflammatory cytokines such as 5-LOX and COX-2, and subsequently decreases the secretion of relative cytokines, LTB4 and PGE2. Consequently, it alleviates the deterioration of ankle injury.
Since our previous phytochemical studies, the TSSN extracts that contain a large amount of steroid saponins have drawn scientists’ tremendous attraction, because of their various bioactivities. Steroid saponins consist of two main parts, i.e. C27-carbon skeleton of aglycones and various kinds of sugar moieties as shown in Fig. 1. Because of the linkage to the sugar moiety, they reveal high polarity. Some steroid saponins, especially ones with diosgenin aglycone, might lose some sugar fragments by the breakage of glycosidic bond under various enzymes in vivo, reduce their polarity, pass through the cell membrane barrier, and finally produce pharmaceutical effect. As previously reported [23], diosgenin in TSSN could inhibit the activation of NF-κB. TSSN has exhibited satisfactory anti-arthritis efficacy through suppressing the inflammatory response, and the inhibition of NF-κB signalling pathway might be a possible explanation.
Oxidative stress has also been implicated in the pathogenesis of RA. FCA-induced arthritis may be caused by generation of free radical which results in destruction of the joint structure via down regulation of endogenous antioxidant defense system [31]. In the present study, the enhanced oxidative stress was clearly evidenced by marked increases in MDA and NO with decrease in SOD activity in the adjuvant-arthritic rats. Treatment with TSSN provoked protection against oxidative stress through restoration of three altered indicators towards their normal level. As aforementioned, diosgenin, the promising compound of TSSN and the secondary metabolite of spirostane steroid saponins, inhibited not only the iNOS expression but also the production of ROS [23]. Then, the reduction in iNOS and SOD suppresses NO and MDA release, respectively. Steroid saponins preserve the endogenous antioxidant effects [32,33] where the hydroxyls in their structures might be responsible for this efficacy. From this aspect, other aglycones metabolized from the TSSN may also be the effective constituents. Based on these results, the anti-arthritis effect of TSSN may be explained on the basis of the anti-oxidant mechanism.
Due to these aforementioned satisfactory underlying mechanisms, the TSSN displays tremendous capacity as a new anti-arthritis agent in future. Because it is the source of diosgenin which is used as the precussor substances of producing steroid hormone, Dioscorea zingiberensis C.H.Wright, a unique Traditional Chinese Medicine, has been successfully cultivated in several Chinese provinces on a large scale, so that the raw material could be easily obtained. In order to acquire high quality TSSN, the technological process was finally determined and shown in section 2.2 after numerous attempts and optimization. From this preparative procedure, there was no need of special instrument or reagents. TSSN could be easily prepared according to the step-by-step operation. After establishing the preparative condition in laboratory, the TSSN was obtained on a large scale for industrial production. A satisfactory result was reached after analyzing the quality of obtained TSSN on this preparative mode. Therefore, it was feasible to produce TSSN in the industrial scale which will advance the research on TSSN toward the development of a new medicine.
5. Conclusion
In summary, ample evidences indicated that TSSN possessed potential therapeutic anti-arthritic effects. As in the aforementioned discussions, the underlying mechanisms for the beneficial effects of TSSN could be mediated via several factors such as regulating the pro-inflammatory cytokines along with anti-inflammatory cytokines, alleviating of oxidative stress, suppressing eicosanoids (PGE2 & LTB4), and ameliorating over-expression of COX-2 and 5-LOX enzymes. TSSN might influence the NF-κB signalling pathway and the endogenous anti-oxidant system. Although these results seem to be promising the use of TSSN for RA treatment, further research should be carried out to elucidate the more detailed molecular mechanisms involved in these anti-arthritic properties of TSSN.
Reference
- [1].Zuo J, Xia Y, Li X, Chen JW. Therapeutic effects of dichloromethane fraction of Securidaca inappendiculata on adjuvant-induced arthritis in rat. J. Ethnopharmacol. 2014;153:352–8. doi: 10.1016/j.jep.2014.02.015. [DOI] [PubMed] [Google Scholar]
- [2].Zheng CJ, Zhao XX, Ai HW, Lin B, Han T, Jiang YP, Xing X, Qin LP. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine. 2014;21:838–46. doi: 10.1016/j.phymed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- [3].Lee LT, Tsai YF, Hu NY, Wang CW, Huang KK, Hsiao JK, Shih YC, Munekazu L. Anti-arthritis effect of mangostins from G. Mangostana. Biomedicine & Preventive Nutrition. 2013;3:227–32. [Google Scholar]
- [4].Patil MVK, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomedicine & Aging Pathology. 2012;2:6–15. [Google Scholar]
- [5].Sinduhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- [6].Zhang YQ, Xu W, Li H, Zhang X, Xia YF, Chu KD, Chen LD. Therapeutic effects of total alkaloids of Tripterygium wilfordii Hook f. on collagen-induced arthritis in rats. J. Ethnopharmacol. 2013;145:699–705. doi: 10.1016/j.jep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- [7].Raveendhara R, Bannuru MDFAGE, Elizaveta E, Vaysbrot MDMS, Matthew C, Sullivan BA, Thimothy E, McAlindon MDMPH. Relative ef ficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: A systematic review and meta-analysis Semin. Arthritis. Rheu. 2014;43:593–9. doi: 10.1016/j.semarthrit.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [8].Wood RC, III, Wyatt JE, Bullins KW, Hanley AV, Hanley GA, Denham JW, Panus PC, Harirforoosh S. Effects of rebamipide on nephrotoxicity associated with selected NSAIDs in rats. Eur. J. Pharmacol. 2013;54:138–46. doi: 10.1016/j.ejphar.2013.10.035. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, Chen SJ, Chen Z. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–31. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang X, Chen X, Liang Q, Zhang H, Hu P, Wang Y, Luo G. Metabonomic study of Chinese medicine Shuanglong formula as an effective treatment for myocardial infarction in rats. J Proteome Res. 2011;10:790–9. doi: 10.1021/pr1009299. [DOI] [PubMed] [Google Scholar]
- [11].Yue R, Zhao L, Hu YH, Jiang P, Wang SP, Xiang L, Liu WC, Zhang WD, Liu RH. Rapid-resolution liquid chromatography TOF-MS for urine metabolomic analysis of collagen-induced arthritis in rats and its applications. J. Ethnopharmacol. 2013;145:465–75. doi: 10.1016/j.jep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- [12].Li H, Huang W, Wen YQ, Gong GH, Zhao QB, Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H.Wright. Fitoterapia. 2010;81:1147–56. doi: 10.1016/j.fitote.2010.07.016. [DOI] [PubMed] [Google Scholar]
- [13].Wei YL, Xu YS, Han X, Qi Y, Xu LN, Xu YW, Yin LH, Sun HJ, Liu KX, Peng JY. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol. 2013;59:118–28. doi: 10.1016/j.fct.2013.05.054. [DOI] [PubMed] [Google Scholar]
- [14].Zhang XX, Liang JR, Liu JL, Zhao Y, Gao J, Sun WJ, Ito Y. Quality control and identification of steroid saponins from Dioscorea zingiberensis C.H. Wright by fingerprint with HPLC-ELSD and HPLC-ESI-Quadrupole/Time-of-fight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;91:46–59. doi: 10.1016/j.jpba.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jia N, Li YW, Wu Y, Zhang XX, Hur GM, Xi MM, Cui J, Sun WJ, Wen AD. Comparison of the anti-inflammatory and analgesic effects of Gentiana macrophylla Pall. and Gentiana straminea Maxim., and identification of their active constituents. J. Ethnopharmacol. 2012;144:638–45. doi: 10.1016/j.jep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- [16].Shin JS, Noh YS, Lee YS, Cho YW, Baek NI, Choi MS, Jeong TS, Kang E, Chung HG, Lee KT. Arvelexin from Brassica rapa suppresses NF-kappaB-regulated pro-inflammatory gene expression by inhibiting activation of IkappaB kinase. Br. J. Pharmacol. 2011a;164:145–58. doi: 10.1111/j.1476-5381.2011.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xue M, Jiang ZZ, Wu T, Li J, Zhang L, Zhao Y, Li XJ, Zhang LY, Yang SY. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine. 2012;19:998–1006. doi: 10.1016/j.phymed.2012.06.006. [DOI] [PubMed] [Google Scholar]
- [18].Vysakh A, Ratheesh M, Rajmohanan TP, Pramod C, Premlal S, Girishkumar B, Sibi PI. Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action. Int. Immunopharmacol. 2014;20:124–30. doi: 10.1016/j.intimp.2014.02.026. [DOI] [PubMed] [Google Scholar]
- [19].Refaat R, Salama M, Meguid EA, Sarha AE, Gowayed M. Evaluation of the effect of losartan and methotrexate combined therapy in adjuvant-induced arthritis in rats. Eur J Pharmacol. 2013;698:421–8. doi: 10.1016/j.ejphar.2012.10.024. [DOI] [PubMed] [Google Scholar]
- [20].Lin B, Zhang H, Zhao XX, Rahman K, Wang Y, Ma XQ, Zheng CJ, Zhang QY, Han T, Qin LP. Inhibitory effects of the root extract of Litsea cubeba (lour.) pers. on adjuvant arthritis in rats. J. Ethnopharmacol. 2013;147:327–34. doi: 10.1016/j.jep.2013.03.011. [DOI] [PubMed] [Google Scholar]
- [21].Huang XY, Zhang XM, Chen FH, Zhou LL, Deng XF, Liu YJ, Li XJ. Anti-proliferative effect of recombinant human endostatin on synovial fibroblasts in rats with adjuvant arthritis. Eur J Pharmacol. 2014;723:7–14. doi: 10.1016/j.ejphar.2013.10.068. [DOI] [PubMed] [Google Scholar]
- [22].Qu XH, Zhai ZJ, Liu XQ, Li HW, Ouyang ZX, Wu CL, Liu GW, Fan QM, Tang TT, Qin A, Dai KR. Dioscin inhibits osteoclast differentiation and bone resorption though down-regulating the Akt signaling cascades. Biochem. Bioph. Res. Co. 2014;443:658–65. doi: 10.1016/j.bbrc.2013.12.029. [DOI] [PubMed] [Google Scholar]
- [23].Jung DH, Park HJ, Byun HE, Park YM, Kim TW, Kim BO, Um SH, Pyo S. Diosgenin inhibits macrophage-derived infl ammatory mediators through downregulation of CK2, JNK, NF-κB and AP-1 activation. Int. Immunopharmacol. 2010;10:1047–54. doi: 10.1016/j.intimp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- [24].Heo SJ, Jang JY, Ye BR, Kim MS, Yoon WJ, Oh CH, Kang DH, Lee JH, Kang MC, Jeon YJ, Kang SM, Kim D. Chromene suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 cells. Food Chem Toxicol. 2014;67:169–75. doi: 10.1016/j.fct.2014.02.023. [DOI] [PubMed] [Google Scholar]
- [25].Hsu CC, Lien JC, Chang CW, Chang CH, Kuo SC, Huang TF. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem Pharmacol. 2013;85:385–95. doi: 10.1016/j.bcp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- [26].Chen XM, Xia J, Zhou T, Yuan Q, Zhang WF, Hu CP, Li YJ, Jiang JL. Involvement of DDAH/ADMA pathway in the pathogenesis of rheumatoid arthritis 2 in rats. Int Immunopharmacol. 2013;16:322–31. doi: 10.1016/j.intimp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [27].Abdin AA, Ei-Halim MSA, Hedeya SE, EI-Saadany AAE. Effect of atorvastatin with or without prednisolone on Freund’s adjuvant. Eur J Pharmacol. 2012;676:34–40. doi: 10.1016/j.ejphar.2011.11.052. [DOI] [PubMed] [Google Scholar]
- [28].Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- [29].Naidu VGM, Babu KRD, Thwin MM, Satish RL, Kumar PV, Gopalakrishnakone P. RANKL targeted peptides inhibit osteoclastogenesis and attenuate adjuvant induced arthritis by inhibiting NF-κB activation and down regulating inflammatory cytokines. Chem-biol Interact. 2013;203:467–79. doi: 10.1016/j.cbi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- [30].Kong XY, Liu CF, Zhang C, Zhao J, Wang JZ, Wan HY, Zhu HW, Zhang P, Chen WH, Xiao YQ, Lin N. The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model. J. Ethnopharmacol. 2013;148:842–50. doi: 10.1016/j.jep.2013.05.023. [DOI] [PubMed] [Google Scholar]
- [31].Arab HH, EI-Sawalhi MM. arvedilol alleviates adjuvant-induced arthritis and subcutaneous air pouch edema: Modulation of oxidative stress and inflammatory mediators. Toxicol Appl Pharm. 2013;268:241–8. doi: 10.1016/j.taap.2013.01.019. [DOI] [PubMed] [Google Scholar]
- [32].Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J. Agric. Food Chem. 2003;51:2555–8. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- [33].Tang W, Eisenbrand G. Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine. Springer-Verlag; Berlin/New York: 1992. [Google Scholar]