Abstract
Rationale
Periodontal disease (PD) strongly correlates with increased mortality post-myocardial infarction (MI); however, the underlying mechanisms are unknown. Matrix metalloproteinase (MMP)-9 levels directly correlate with dysfunction and remodeling of the left ventricle (LV) post-MI. Post-MI, MMP-9 is produced by leukocytes and modulates inflammation. We have shown that exposure to Porphyromonas gingivalis lipopolysaccharide (PgLPS), an immunomodulatory molecule identified in PD patients, increases LV MMP-9 levels in mice and leads to cardiac inflammation and dysfunction.
Aims
To determine if circulating PgLPS exacerbates the LV inflammatory response post-MI through MMP-9 dependent mechanisms.
Methods and Results
We exposed wild type C57BL/6J and MMP-9−/− mice to PgLPS (ATCC 33277) for a period of 28 days before performing MI, and continued to deliver PgLPS for up to 7 days post-MI. We found systemic levels of PgLPS 1) increased MMP-9 levels in both plasma and infarcted LV resulting in reduced wall thickness and increased incidence of LV rupture post-MI and 2) increased systemic and local macrophage chemotaxis leading to accelerated M1 macrophage infiltration post-MI and decreased LV function. MMP-9 deletion played a protective role by attenuating the inflammation induced by systemic delivery of PgLPS.
Conclusion
In conclusion, MMP-9 deletion has a cardioprotective role against PgLPS exposure, by attenuating macrophage mediated inflammation.
Keywords: Matrix metalloproteinase-9, myocardial infarction, periodontal disease, Porphyromonas gingivalis;, proteomics, cardiac function
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States. Multiple epidemiological studies correlate periodontal disease (PD) with CVD, as PD patients have increased mortality and heart failure (HF) incidence post-myocardial infarction (MI) [1-3]. An estimated 75% of the US adult population has PD. Meta-analysis of over 200,000 individuals revealed that PD increases CVD risk by 35%, implicating PD as a significant public health concern [4]. While the link between oral health and CVD is strong, the cellular and molecular mechanisms that associate oral health and CVD are still unknown.
PD is a complex disease, where multiple causal factors simultaneously and interactively play a role in progression and severity of the disease. There are four main causal risk factors: 1) the subgingival microbiota, 2) individual genetic variations, 3) life style, and 4) systemic factors [5]. The link between periodontitis and CVD are thought to be caused by inflammatory mechanisms initiated by bacteria, associated with periodontal lesions [6]. Porphyromonas gingivalis (Pg) is the most frequent PD pathogen detected in the gums/circulation of patients with PD [7-9]. As the infection progresses deeper into the tissue and enters the blood stream, an innate inflammatory response is activated to combat the infection.
In order to elucidate effects of a specific periodontal pathogen in cardiac function, we previously infused mice with sub-septic concentrations (0.8 μg/g body weight/day) of PgLPS ATCC 33277, a strain identified in over 25% of PD patients, and found increased systemic inflammation, evidenced by elevated plasma levels of interleukin (IL-1)α and matrix metalloproteinase (MMP)-9 [10-12]. These results were similar to those seen in PD patients [7]. We concluded that systemic inflammation due to a periodontal pathogen induced a subtle yet significant decrease in cardiac function that was MMP-9 dependent.
MMP-9 is a critical player post-MI, as it modulates the inflammatory and scar formation responses [13, 14]. In animal models, deletion of MMP-9 attenuates LV dysfunction post-MI [15]. Accordingly, we hypothesized that systemic sub-septic levels of PgLPS would amplify the inflammatory response in the left ventricle (LV) post-MI, leading to a decrease in cardiac function that would be attenuated by MMP-9 deletion.
2. Materials and Methods
2.1 Mice
C57BL/6J wild type (WT) and MMP-9−/− male and female mice, 4-7 months of age, were used in this study (n≥6/sex/group). The MMP-9−/− mice are on a C57BL/6J background. The MMP-9−/− mice were generated by Zena Werb’s laboratory and backcrossed by Lynn Matrisian’s laboratory [16, 17]. WT and MMP-9−/− mice were each separated into 3 groups: day 0 (D0) no MI controls (n≥6/sex/group), saline D1, D3, D5, and D7 post-MI (n≥6/sex/day), and PgLPS infused D1, D3, D5, and D7 post-MI (n≥6/sex/day), for a combined total of 6 groups. Mice were kept in a light-controlled environment with a 12:12 hour light-dark cycle and given free access to standard mice chow and water. WT mice were ordered from Jackson laboratories. MMP-9−/− colonies were bred in-house and maintained in the same room with WT mice. Groups were examined simultaneously, with the evaluator blinded to genotype. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and the University of Mississippi Medical Center in accordance with the “Guide for the Care and Use of Laboratory Animals”.
2.2 Experimental Design
To elucidate the effects of the periodontal pathogen P. gingivalis (Pg) on LV remodeling; mice were exposed to PgLPS ATCC 33277 (0.8 μg/g body weight/day; Invivo Gen) by osmotic mini-pumps (model 2004, 1003D, and 1007D; Durect). PgLPS ATCC 33277 is a strain commonly identified in over 25% of PD patients [10, 11]. Previously, we showed this concentration induces low-grade systemic inflammation similar to what is seen in plasma of PD patients following dental cleanings [12]. After 28 days of PgLPS exposure mice did not have elevated body temperature (p=0.6873) confirming PgLPS concentration did not induce sepsis. Except for D0 animals, mice were infused with either saline or PgLPS for 28 days before MI. After 28 days, coronary artery ligation as well as removal and replacement of pump were performed. No mortality was observed from PgLPS exposure alone, and the saline treated group served as the negative technical control for this procedure. MI was induced through permanent ligation of the left anterior descending coronary artery using a well-established method [18]. In summary, mice were anesthetized with 1-2% isoflurane in oxygen, intubated, and put on a standard rodent ventilator. An incision was made between the 3rd and 4th rib and a rib retractor was used to allow visualization of the heart. An 8-0 suture was used to ligate the left coronary artery at a location approximately 1-2 mm distal to the left atrium, and MI was confirmed by LV blanching and ECG changes showing ST segment elevation. Prior to the surgery, buprenorphine was administered to ease pain. Animals were sacrificed at 1, 3, 5, or 7 days post-MI. Day 0 controls did not undergo surgery prior to sacrifice.
2.3 Echocardiography
Transthoracic echocardiography was performed using the Visual Sonics Vevo 770 system (VisualSonics) with a 30-MHz image transducer at baseline (before MI) and at termination. Mice were anesthetized with 1-2% isoflurane in an oxygen mix, and electrocardiograms and heart rates were monitored throughout the imaging procedure. All images were acquired at heart rates >400 bpm to ensure physiological relevance. Measurements were taken from the parasternal long axis B- and M-mode views. For each parameter, 3 images from consecutive cardiac cycles were measured and averaged [16, 19]. Echocardiography data were analyzed, and no sex or age differences were found between groups (for example, p=0.60 for the fractional shortening comparison).
2.4 Survival Analysis and Tissue Harvest
The mice were checked daily for the survival analysis. At necropsy, cardiac rupture was confirmed by the presence of coagulated blood in the thoracic cavity or observation of ruptured site on the LV. For tissue collection, mice were anesthetized with 1-2% isoflurane in an oxygen mix. Five minutes after heparin administration (4 U/g body weight, i.p.), blood was collected from the common carotid artery and immediately centrifuged for collection of plasma. A 1x proteinase inhibitor cocktail (Roche, 50-720-4060) was added to the plasma, which was stored at −80°C. Plasma samples (100 μL) were sent to Rules Based Medicine (Austin, TX) for multi-analyte proteomic profiling [20]. The coronary vasculature was flushed with cardioplegic solution (69 mM NaCl; 12 mM NaHCO3; 11 mM glucose; 30 mM 2,3-butanedione monoxime; 10 mM EGTA; 0.001 mM Nifedipine; 50 mM KCl; and 100 U Heparin in 0.9% saline, pH 7.4). Hearts were removed, and the LV and right ventricle (RV) separated and weighed individually. The LV was sliced transversely into apex, middle, and base sections. Infarct areas were calculated as described previously [21]. The infarct region (LVI) was dissected from the apex and base sections, frozen in liquid nitrogen, and stored at −80°C for RT2-PCR, immunoblotting, or bioplex analyses. The middle section was fixed in 10% zinc formalin for histological examination.
2.5 Leukocyte Isolation
Neutrophils and macrophages were isolated from infarcted hearts as previously described [19]. The LV tissue was dissociated into single-cell suspension using 600 U/mL collagenase type 2 (Worthington Biochemicals, CLS-2) and 60 U/mL DNase1 (AppliChem, A3778.0500). Cells were washed and resuspended in cold PBS supplemented with 0.5% BSA and 2 mM EDTA. Cells were sequentially incubated with Ly-6G microbeads (Miltenyi Biotec, 130-092-332) to remove neutrophils, and with CD11b microbeads (Miltenyi Biotec, 130-049-601) to isolate macrophages. Positive cells were isolated using magnetic MS columns (Miltenyi Biotec, 130-042-201). RNA extraction was performed on the isolated neutrophils and macrophages using PureLink RNA Mini Kit (Invitrogen, 12183-018A). RNA levels were quantified using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific). Reverse transcription of equal RNA content (400 ng) was performed using the High Capacity RNA-to-cDNA Kit (Life Technologies 4837406). Quantitative RT-PCR was performed using taqman gene expression assays (Applied Biosystems) to evaluate mRNA expression of pro-inflammatory M1 and anti-inflammatory M2 macrophage markers as shown in Table 1. Isolated cells were also analyzed for MMP-9 expression (Mm00442991_m1). MMP-9 mRNA levels were normalized against cell number to determine MMP-9 levels/cell. Macrophages were analyzed for the inflammatory analytes listed in Table 1. All values were normalized to hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) (Mm01545399_m1) housekeeping gene. Analysis of PCR data was done using the MIQE guidelines.
Table 1.
RT2-PCR primers (Applied Biosystems) used for M1 and M2 macrophage phenotyping.
| M1 | M2 |
|---|---|
| Ccl3 | arginase-1 |
| (Mm00441259_g1) | (Mm00443258_m1) |
|
| |
| IL-1β | Fizz |
| (Mm01336189_m1) | (Mm00445109_m1) |
|
| |
| IL-6 | mannose receptor 1 |
| (Mm00446190_m1) | (Mm00485148_m1) |
|
| |
| TNF-α | Ym1 |
| (Mm00443258_m1) | (Mm00474091_m1) |
2.6 Real Time RT2-PCR
RNA was extracted from the infarcted LV (LVI) using TRIzol® Reagent and Purelink® RNA (Invitrogen) according to the manufacturer’s instructions. RNA levels were quantified using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific). Reverse transcription of equal RNA content (400 ng) was performed using the RT2 First Strand Kit (Qiagen 330401). Quantitative Real Time RT2-PCR gene array for inflammatory cytokines and receptors (Qiagen PAMM-011A) and MMPs and tissue inhibitors of metalloproteinase (TIMPs) (Qiagen PAMM-013A) were used to quantify mRNA levels in the LVI. All values were normalized to the Hprt1 housekeeping gene, since the expression of this gene does not change following injury. Analysis of PCR data was done using the MIQE guidelines.
2.7 Protein Extraction and Analysis
Protein was extracted by homogenizing the samples sequentially in phosphate buffered saline (PBS) with 1× protease inhibitor cocktail (16 μL per mg tissue, soluble protein fraction), and in protein extraction reagent type 4 (Sigma; 7 M urea, 2 M thiourea, 40 mM Trizma® base and the detergent 1% C7BzO, 15 μL per mg tissue) with 1x protease inhibitor cocktail (insoluble protein fraction). Protein concentrations were determined by the Quick Start™ Bradford Protein Assay (Bio-Rad). Total protein (10 μg) was separated on 4-12% Criterion™ XT Bis-Tris gels (Bio-Rad), transferred to a nitrocellulose membrane (Bio-Rad), and stained with MemCode™ Reversible Protein Stain Kit (Thermo Scientific) to verify protein concentration and loading accuracy. After blocking with 5% nonfat milk (Bio-Rad), the membrane was incubated with an antibody against galectin-3 (R&D, AF1197, 1:500), secondary antibody (Santa Cruz, SC2020, 1:5000), and detected with ECL Prime Western Blotting Detection Substrate (Amersham). Galectin-3 levels were quantified by densitometry using the IQ-TL image analysis software (GE Healthcare, Waukesha, WI). The relative expression for each immunoblot was calculated as the densitometry of the protein of interest divided by the densitometry of the entire lane of the total protein stained membrane. Measurement of cytokines in the LVI were determined by fluorescent bead-based Luminex technology (BioRad; Bio-plex Pro Mouse Cytokine 23-plex panel MD0-009RDPD and 9-plex panel MD0-00000EL), in accordance with the manufacturer instructions. Samples (10 μg of homogenized lysates) were incubated for 30 min in a 96-well plate. Detection antibodies were added, and the plate was incubated for 30 min with shaking. After incubation, Streptavidin was added to each well, and the plate was incubated for 10 min. Samples were resuspended in assay buffer, and the plate was measured for fluorescence emission on the Bio-plex system. Concentrations were calculated from standard curves for each of the analytes.
2.8 Histology
The middle section of the LV (mid-papillary region) was embedded in paraffin and sectioned at 5 μm. Immunohistochemistry was conducted using the Vectastain ABC Kit (Vector Laboratories). Antibodies specific for neutrophils (Cedarlane; CL8993AP; 1:100) and macrophages (Mac-3, Cedarlane CL8943AP; 1:100) were used. HistoMark Black (KPL 54-75-00) was used to visualize positive staining, with eosin as a counterstain. Negative controls were incubated with no primary antibody. For each LV section, five 60× magnification images were captured. The percentage of positive staining per field of view (macrophage levels) was measured by Image-Pro Plus version 6.2. Results are shown as percent stained area.
2.9 Statistical Analyses
Data are presented as mean±SEM. Multiple group comparisons were analyzed by one-way ANOVA, followed by the Student Newman-Keuls when the Bartlett’s variation test passed, or by the nonparametric Kruskal-Wallis ANOVA test, followed by Dunn post-hoc test when the Bartlett’s variation test did not pass. The survival rate was analyzed by Kaplan-Meier survival analysis and compared by the log-rank test. Rupture rates were analyzed by Fisher’s exact test. A value of p<0.05 was considered statistically significant.
3. Results
3.1 PgLPS exposure increased rupture rates and kinetics post-MI in WT mice but not in MMP-9−/−
Survival numbers post-MI were not affected by PgLPS (Figure 1A). As reported previously, MMP-9 deletion reduced mortality post-MI in the unexposed mice (p<0.05) [22]. MMP-9 deletion also reduced mortality post-MI in the PgLPS exposed mice (p<0.05). PgLPS exposure increased deaths due to rupture, compared to non-exposed MI controls, as 8/8 (100%) had rupture in WT+LPS vs. 11/23 (47%) in non-exposed MI mice (p<0.05). This increase in rupture was attenuated by MMP-9 deletion, where only 2/3 (66%) deaths were due to rupture in MMP-9−/−+LPS (Figure 1B; p<0.05). In the murine permanent occlusion model, cardiac rupture normally occurs between days 3 to 7 post-MI. Interestingly, PgLPS exposure promoted earlier ruptures at D1 post-MI in WT mice but not in MMP-9−/−. These results indicate that systemic PgLPS infusion accelerates the occurrence and timing of post-MI rupture. Based on this result, we determined what mechanisms occurred at D1 post-MI to cause this increased rupture and what cell populations modulated this effect.
Figure 1.
PgLPS increased rupture rates and accelerated time of death in WT mice. (A) PgLPS exposure did not affect final day 7 survival rate post-MI; however, MMP-9 deletion attenuated mortality post-MI. (B) Rupture rate was accelerated by PgLPS in WT mice which was attenuated by MMP-9 deletion. Kaplan-Meier survival analysis and log-rank test; #p<0.05 vs respective MI; †p<0.05 vs WT MI+LPS.
3.2 MMP-9 levels increased in plasma and LVI after PgLPS exposure in WT mice
MMP-9 in the early phase of MI has been shown to impair infarct healing and aggravate early remodeling, which in turn causes cardiac rupture [15, 23]. We reported that chronic PgLPS exposure increased MMP-9 in plasma and LV [12]. In order to determine if PgLPS presence can modulate MMP-9 production post-MI, proteomic profiling and gene array analysis were performed in plasma and LVI up to 7 days post-MI. MMP-9 levels increased significantly post-MI in both plasma and LVI gene levels in WT animals (both p<0.05). This increase was intensified by PgLPS exposure (Figure 2). By D7 post-MI, plasma MMP-9 levels were returning to levels comparable to unexposed controls. This suggests that the intensified inflammatory response has subsided by D7 post-MI in the PgLPS exposed WT mice. No sex differences were identified from MMP-9 levels (p=0.23). These results indicate that MMP-9 increases post-MI, and this effect was amplified with PgLPS exposure.
Figure 2.
Exposure to PgLPS exacerbated MMP-9 levels in both plasma and LVI. MMP-9 levels increased post-MI in (A) plasma protein levels, as determined by proteomic profiling (n=12/group) and (B) infarcted LV mRNA levels, as determined by qRT-PCR in WT mice (n = 6/group); ANOVA with Student Newman-Keuls post-test; *p<0.05 vs respective D0 (Day 0); #p<0.05 vs respective MI.
3.3 PgLPS accelerated M1 macrophage infiltration in WT mice and MMP-9 deletion attenuated this effect
To determine if PgLPS exposure amplified leukocyte infiltration into the infarcted tissue, LV sections were stained for neutrophils and macrophages. Neutrophil levels were elevated similarly in both unexposed and PgLPS exposed mice at D1 post-MI (Figure 3A-B). Unexpectedly, macrophages were present at D1 post-MI in WT+LPS (Figure 3C-E). Significant numbers of macrophages do not normally infiltrate the LV post-MI until D3, and peak infiltration occurs at D5 [16].
Figure 3.
PgLPS induced accelerated macrophage infiltration at D1 post-MI. (A, B) Staining for neutrophils showed PgLPS had no effect on cell number at D1 post-MI. (C, D) Staining for Mac3 showed higher macrophage numbers in the LV post-MI at D1 in the PgLPS exposed groups. (C) Immunoblot of galectin-3, a marker for macrophages, confirmed increased macrophages at D1 post-MI in WT mice. This effect was attenuated by MMP-9 deletion; n=6/group; ANOVA with Student Newman-Keuls post-test; *p<0.05 vs respective d0; #p<0.05 vs respective MI; †p<0.05 vs WT MI+LPS. Scale bars are 100 μm.
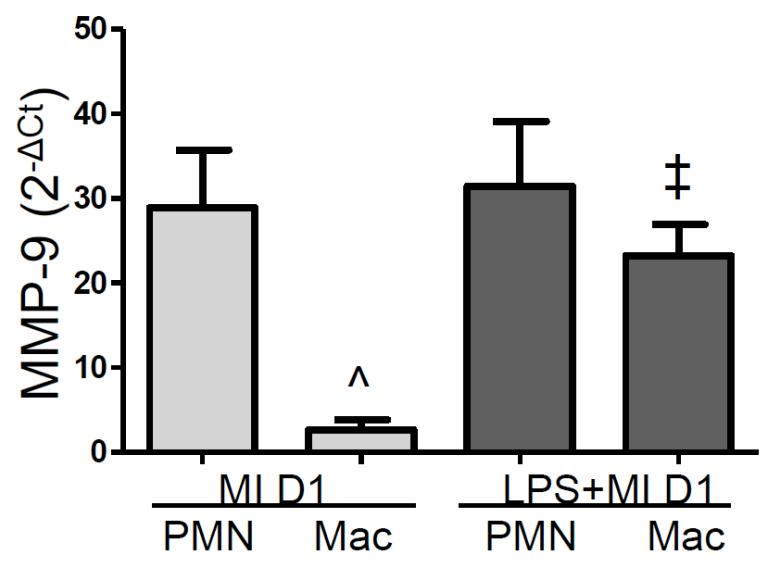
In the post-MI setting, MMP-9 is expressed predominantly by the neutrophils and macrophages. At D1 post-MI, MMP-9 is expressed 2600-fold higher in neutrophils compared to the rest of the cells combined (p<0.05). To determine if the acceleration in macrophage recruitment is responsible for the increase in MMP-9, RT2-PCR was performed on neutrophils and macrophages isolated from the LVI at D1 post-MI. As expected, neutrophils expressed higher levels of MMP-9 per cell compared to macrophages in the control mice (Figure 4). Of note, after PgLPS exposure neutrophils and macrophages were equally responsible for the increase in MMP-9, suggesting the acceleration in macrophage recruitment increased overall MMP-9 levels. This result also suggested that the macrophage phenotype might be altered.
Figure 4.
PgLPS stimulated macrophages to increase expression of MMP-9. Isolated neutrophils and macrophages were analyzed for MMP-9 expression; n=6/group; ANOVA with Student Newman-Keuls post-test; ^p<0.05 vs MI D1 neutrophils; ‡p<0.05 vs MI D1 macrophages.
To determine the macrophage phenotype, macrophages isolated from the LVI at D1 were analyzed for M1 (pro-inflammatory) and M2 (anti-inflammatory) markers. M1 markers, Ccl3, IL1β, and TNFα, mRNA levels were increased in WT+LPS compared to unexposed and MMP-9−/−+LPS MI groups (Figure 5). Il6 was significantly higher in the MMP-9−/−+LPS MI group, and this cytokine has been reported to be produced by both M1 and M2b macrophages [24-26]. The M2 marker arginase (Arg)1 was significantly higher in the WT+LPS MI group, whereas Fizz1 was significantly higher in the MMP-9−/−+LPS MI group.
Figure 5.
PgLPS induces M1 macrophage polarization in WT but not MMP-9−/−. Isolated macrophages were analyzed for M1 and M2 macrophage markers; n=6/group; ANOVA with Student Newman-Keuls post-test; *p<0.05 vs respective d0; #p<0.05 vs respective MI; †p<0.05 vs WT MI+LPS.
Combined, these results indicated that PgLPS induced an earlier and more pro-inflammatory influx of macrophages into the ischemic myocardium. However in the absence of MMP-9, PgLPS induced both M1 and M2 polarization, suggesting that PgLPS modulation of macrophage-driven inflammation occurs in an MMP-9 dependent manner through several mechanisms. Macrophage polarization is dynamic and flexible, with classically activated pro-inflammatory M1 cells initiating and sustaining inflammation and anti-inflammatory M2 cells resolving chronic inflammation [27, 28]. The accelerated influx of M1 macrophages in the WT after PgLPS exposure may explain the increased rupture rates [29, 30].
3.4 PgLPS increased macrophage mediated inflammation in WT mice at D1 post-MI is attenuated by MMP-9 deletion
MMP-9 processes cytokines affecting the inflammatory response post-MI [31]. To determine which signaling molecules were responsible for the earlier recruitment of macrophages, we performed multi-analyte proteomic profiling of plasma and LVI, as well as RT2-PCR cytokine and ECM gene arrays on the LVI tissue (Figures 6-7 and Table 2). Compared to D0 samples, D1 post-MI plasma showed elevations in 12 of the 40 analytes examined, including IL-6 that served as a positive control [32]. Eotaxin, granulocyte macrophage colony-stimulating factor (GM-CSF), Cxcl1, MCP-1, and tumor necrosis factor (TNF)α increased in the LVI of all groups post-MI, indicating responses that were independent of PgLPS or MMP-9.
Figure 6.
Plasma proteomic profiling revealed increased macrophage associated chemokines, (A) macrophage inhibitory protein (MIP)-1β, (B) monocyte chemotactic protein (MCP)-1, and (C) MCP-3 in PgLPS infused WT mice post-MI compared to unexposed controls. MMP-9 deletion attenuated this effect; n=12/group; ANOVA with Student Newman-Keuls post-test; *p<0.05 vs respective D0; #p<0.05 vs respective MI; †p<0.05 vs WT MI+LPS.
Figure 7.
Proteomic profiling of LV tissue revealed PgLPS increased the inflammatory response post-MI in WT mice and this effect was attenuated by MMP-9 deletion. Majority of the increased analytes are associated with macrophages demonstrating PgLPS induced macrophage immunity post-MI; n=6/group; ANOVA with Student Newman-Keuls post-test; *p<0.05 vs respective d0; #p<0.05 vs respective MI; †p<0.05 vs WT MI+LPS.
Table 2.
Summary of changes in inflammatory mRNA levels. Arrows indicate differences in D1 post-MI samples compared to D0 controls (↑- increased and ↓- decreased). Bolded analytes are intensified by PgLPS and underlined analytes are attenuated by MMP-9 deletion.
| WT MI D1 |
MMP-9−/− MI D1 |
WT PgLPS+MI D1 |
MMP-9−/− PgLPS+MI D1 |
|
|---|---|---|---|---|
| ↑ | Ccl2, Ccl6, Ccl9, Ccl17, Ccr1, Ccr2, Cxcl1, Cxcl4, Cxcl5, IL1r1,IL1r2, IL8rb, Itgam, Itgb2, MMP- 3, MMP-8, MMP-14, MIPiβ, Tnfrsfla |
Ccl9, Ccl17, Ccr1, Ccr2, Ccr3, IL1r1, Itgam, Itgb2, MMP-3, MMP-8, MMP-14, Spp1, Tgfb1 |
Ccl6, Ccl7, Ccl9, Ccl12, Ccl17, Ccr1, Ccr2, Ccr3, Ccr5, Cxcl1, Cxcl4, Cxcl5, Cxcl10, ILip, IL1r1, IL1r2, IL8rb, IL11, Itga3, Itgal, Itgam, tgav, Itgb2, MIP1β, MMP-3, MMP-8, MMP-14, Spp1, Tgfb1, Tnfrsf1a |
Ccl6, Ccl7, Ccl8, Ccl9, Ccl12, Ccl17, Ccl22, Ccr1, Ccr2, Ccr3, Ccr5, Ccr7, Cxcl1, Cxcl4, Cxcl5, Cxcl10, IL1a, IL1r1, IL1r2,IL6ra, IL8rb, IL11, Itgam, Itgb2, Ltb, Mif, MIP1β, MMP-3, MMP-8, MMP-14, Spp1, Tgfb1, Tnf, Tnfrsf1a |
| ↓ | Ccr10, Cxcl11, IL10rb, IL15, MMP- 15, TIMP-2 |
IL10rb, IL15, MMP- 15, TIMP-2, |
Ccr10, IL10rb, IL15, MMP-15, TIMP-2 |
Ccr10, IL10rb, IL15, MMP-15, TIMP-2 |
n=6/group; All p<0.05 by Kruskal-Wallis ANOVA with Dunn post-hoc test
Compared to D1 post-MI WT samples, plasma and LVI tissue levels of the signaling molecules interferon (IFN)γ, interleukins (IL)-1β, −6 and −18, macrophage inflammatory proteins (MIPs), monocyte chemotactic proteins (MCPs) and integrins were intensified by PgLPS (Figures 6-7 and Table 2). This effect was attenuated by MMP-9 deletion. MIP-1β, a macrophage chemotactic protein, was the only inflammatory molecule exacerbated in both plasma and LVI by PgLPS exposure. Of note, IFNγ, IL-1β, IL-6, and IL-18 are known to promote macrophage M1 polarization [33]. This increase in macrophage chemoattractants explains the unexpected presence of macrophages at D1 in the LVI of WT+LPS animals [34, 35].
MMP-9 also processes extracellular matrix proteins and proangiogenic factors such as collagen, vascular endothelial growth factor (VGEF), and fibroblast growth factor (bFGF) to influence post-MI remodeling [31]. Despite the accelerated rupture rate seen in the WT MI mice exposed to PgLPS, the ECM gene array showed no changes in collagen types I and III at D1 post-MI, compared to the D1 saline WT MI control. This was not unexpected, since infarct scar formation does not begin until around day 3 post-MI [36]. Vascular endothelial growth factor (VEGF) was increased in the WT groups only. VEGF stimulates angiogenesis and is associated with increased MMP activity [37, 38]. PgLPS also increased bFGF and platelet derived growth factor (PDGF) in the LVI at D1 post-MI (Figure 7). This effect was attenuated by MMP-9 deletion.
The expression of 11 MMPs and 3 TIMPs were evaluated by gene array. MMP-1, MMP-2, MMP-7, MMP-10, MMP-11, MMP-13, TIMP-1, and TIMP-3 were not changed at D1 post-MI over respective day 0 values. Without MI, MMP-9 deletion resulted in a compensatory increase in MMP-13 that has been reported previously and was not further changed with MI [39, 40]. At D1 post-MI, MMP-8, and MMP-14 increased and MMP-15 and TIMP-2 decreased in all groups compared to the D0 control, indicating an MI effect that was not altered by PgLPS or MMP-9 deletion (Table 2). MMP-3 expression was intensified by PgLPS exposure in WT mice, and MMP-9 deletion attenuated this effect. MMP-12 increased in PgLPS exposed MMP-9−/− mice at D1 post-MI.
PgLPS exposure stimulated genes and protein analytes associated with leukocyte chemotaxis. The majority of these genes and analytes are linked to macrophage recruitment and infiltration. MMP-9 deletion attenuated the increase of these analytes, demonstrating that MMP-9 deletion has a cardioprotective role against PgLPS exposure, by attenuating macrophage mediated inflammation.
3.5 PgLPS intensified LV dysfunction post-MI and this was attenuated by MMP-9 deletion
Our data showed that PgLPS accelerated early M1 macrophage infiltration post-MI in the WT, which would increase damage to the myocardium eventually affecting LV function [29, 30]. In order to determine how these early changes affected cardiac function after 1 week of MI, we measured LV function at D7 using 2-dimensional echocardiography (Table 3). Interestingly, both infarct wall thinning and reduced FS were amplified in WT+LPS mice, and this effect was attenuated by MMP-9 deletion (p<0.05 for all). These data demonstrate that PgLPS exposure intensified LV dysfunction in the WT mice post-MI but not in MMP-9−/−, indicating a role for MMP-9 in PgLPS-induced cardiac dysfunction.
Table 3.
PgLPS exacerbates LV dysfunction post-MI in WT, but not MMP-9−/− mice.
| D0 | MI D7 | LPS+MI D7 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| WT | MMP-9−/− | WT | MMP-9−/− | WT | MMP-9−/− | |
|
|
||||||
| Heart Rate (bpm) | 469±9 | 448±12 | 475±15 | 485±10 | 465±8 | 479±14 |
| Infarct area (%) | N/A | N/A | 58±3 | 56±2 | 55±5 | 50±5 |
| Wall Thickness (mm) | 1.23±0.04 | 1.19±0.03 | 0.59±0.05* | 0.57±0.05* | 0.39±0.01*# | 0.49±0.04*† |
| EDD (mm) | 3.50±0.11 | 3.22±0.16 | 5.60±0.22* | 5.36±0.34* | 5.53±0.17* | 5.28±0.20* |
| ESD (mm) | 2.28±0.08 | 2.05±0.15 | 5.21±0.26* | 4.98±0.36* | 5.27±0.17* | 4.94±0.20* |
| FS % | 35±1 | 37±1 | 7±1* | 7±1* | 5±1*# | 7±1*† |
Values are mean ± SEM; EDD-End diastolic dimension; ESD-End systolic dimension; FS-Fractional shortening; n=12/group (6M/6F); ANOVA with Student Newman-Keuls post-hoc test
p<0.05 vs respective D0
p<0.05 vs WT MI
p<0.05 vs WT MI+LPS
4. Discussion
The goal of this study was to investigate the mechanisms that underlie the connection between periodontal disease (PD) and post-MI remodeling of the left ventricle (LV). Our results show that PgLPS exposure: 1) increased MMP-9 levels in both plasma and LV infarct, resulting in augmented infarct wall thinning and decreased LV function; 2) increased systemic and local macrophage chemotaxis, resulting in accelerated M1 macrophage infiltration post-MI and increased deaths due to rupture post-MI; and 3) MMP-9 deletion played a protective role by attenuating the exacerbated inflammation caused by systemic delivery of PgLPS. These data reveal that PD plays key roles in MI by inducing a strong macrophage mediated inflammatory response regulated by MMP-9.
The occurrence of rupture post-MI is an unpredictable event [39]. Excessive inflammation post-MI has been shown to impair infarct healing, which in turn causes cardiac rupture [23, 40]. Our data revealed that PgLPS increased and accelerated ruptures post-MI in WT mice and this was due to an increase in the amount of post-MI LV wall thinning. This was accompanied by an increase in MMP-9 and M1 pro-inflammatory macrophages, illustrating that PgLPS exposure can accelerate rupture through an acceleration of MMP-9 mediated macrophage infiltration. In addition to increased levels of MMP-9, PgLPS also increased MMP-3 levels in WT mice at D1 post-MI. Studies in a rabbit permanent occlusion model showed increased MMP-3 levels post-MI led to LV dysfunction by causing myocyte disruption and misalignment [41].
Circulating periodontal pathogens stimulates inflammation and is believed to be the source of CVD risk in PD patients [12, 42, 43]. Our results showed that PgLPS exposure enhanced systemic and local inflammation post-MI in WT mice, through increased levels of inflammatory markers associated with macrophage chemotaxis. This effect was attenuated by MMP-9 deletion. Immunohistochemistry showed PgLPS accelerated macrophage infiltration post-MI in the LV of WT mice, and these macrophages were pro-inflammatory (M1 phenotype). This finding indicates that PgLPS induced increased myocardial damage through MMP-9 regulation of macrophage mediated inflammation to decrease LV function.
In conclusion, PD induces increased risk for heart failure post-MI by altering the inflammatory response in a MMP-9 dependent manner. The data suggest that an increase in peripheral inflammation by PgLPS exposure accelerated macrophage infiltration into the LV. This in turn increased the amount of tissue damage as demonstrated by the increase in LV rupture post-MI. The early changes observed at D1 led to a decrease in D7 LV function, which was attenuated by MMP-9 deletion. These results highlight the importance of future studies evaluating the use of MMP-9 inhibitors in high risk patients with periodontal disease to prevent cardiovascular events. Periodontal disease is associated with increased risk for heart failure post-MI. Previously, we reported that chronic PgLPS exposure induced a subtle yet significant decrease in LV function [12]. It is expected that PgLPS would decrease LV function further at later points post-MI. Future studies focused on longer time points post-MI would be necessary to identify changes that lead to heart failure. These findings give us insight into the molecular events that increase the risk of heart failure in periodontal disease patients who have MI.
Highlights.
Mice were given PgLPS for 28 days before and through 7 days of myocardial infarction (MI).
The post-MI increase in MMP-9 in both the plasma and infarct was amplified by PgLPS.
PgLPS increased wall thinning post-MI in WT mice, resulting in increased LV rupture.
M1 macrophage infiltration was accelerated in WT mice exposed to PgLPS post-MI.
MMP-9 deletion attenuated PgLPS effects post-MI.
Acknowledgments
Funding
This work was supported by American Heart Association [13POST14350034 to KYD-P, 14SDG18860050 to LEdCB, and 14POST18770012 to RPI], and by the National Institute of Health/National Heart, Lung, and Blood Institute [HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center and R01HL075360 to MLL] and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award [5I01BX000505 to MLL], and by HL051971 and GM104357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no conflict of interest.
References
- [1].Kaisare S, Rao J, Dubashi N. Periodontal disease as a risk factor for acute myocardial infarction. A case-control study in Goans highlighting a review of the literature. Br Dent J. 2007;203:E5. doi: 10.1038/bdj.2007.582. discussion 144-5. [DOI] [PubMed] [Google Scholar]
- [2].Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- [3].Holmlund A, Holm G, Lind L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J Periodontol. 2010;81:870–6. doi: 10.1902/jop.2010.090680. [DOI] [PubMed] [Google Scholar]
- [4].Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Papantonopoulos G, Takahashi K, Bountis T, Loos BG. Artificial neural networks for the diagnosis of aggressive periodontitis trained by immunologic parameters. PLoS One. 2014;9:e89757. doi: 10.1371/journal.pone.0089757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84:S51–69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- [7].Shaddox LM, Wiedey J, Calderon NL, Magnusson I, Bimstein E, Bidwell JA, et al. Local inflammatory markers and systemic endotoxin in aggressive periodontitis. J Dent Res. 2011;90:1140–4. doi: 10.1177/0022034511413928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oliveira FJ, Vieira RW, Coelho OR, Petrucci O, Oliveira PP, Antunes N, et al. Systemic inflammation caused by chronic periodontite in patients victims of acute ischemic heart attack. Rev Bras Cir Cardiovasc. 2010;25:51–8. doi: 10.1590/s0102-76382010000100013. [DOI] [PubMed] [Google Scholar]
- [9].Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:2174–80. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- [10].Kojima T, Yasui S, Ishikawa I. Distribution of Porphyromonas gingivalis in adult periodontitis patients. J Periodontol. 1993;64:1231–7. doi: 10.1902/jop.1993.64.12.1231. [DOI] [PubMed] [Google Scholar]
- [11].Missailidis CG, Umeda JE, Ota-Tsuzuki C, Anzai D, Mayer MP. Distribution of fimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol Immunol. 2004;19:224–9. doi: 10.1111/j.1399-302X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- [12].Deleon-Pennell KY, Bras LE, Lindsey ML. Circulating lipopolysaccharide resets cardiac homeostasis in mice through a matrix metalloproteinase-9 dependent mechanism. Physiol Rep. 2013;1:e00079. doi: 10.1002/phy2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, et al. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–14. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- [14].Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–H9. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- [17].Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Salto-Tellez M, Yung Lim S, El Oakley RM, Tang TPL, Almsherqi ZAM, Lim S-K. Myocardial infarction in the C57BL/6J mouse: A quantifiable and highly reproducible experimental model. Cardiovascular Pathology. 2004;13:91–7. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- [19].Zamilpa R, Kanakia R, Cigarroa Jt, Dai Q, Escobar GP, Martinez H, et al. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H1418–H26. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chiao YA, Dai Q, Zhang J, Lin J, Lopez EF, Ahuja SS, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet. 2011;4:455–62. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circulation research. 2013;112:675–88. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ramirez TA, Iyer RP, Ghasemi O, Lopez EF, Levin DB, Zhang J, et al. Aliskiren and valsartan mediate left ventricular remodeling post-myocardial infarction in mice through MMP-9 effects. J Mol Cell Cardiol. 2014;72:326–35. doi: 10.1016/j.yjmcc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tao ZY, Cavasin MA, Yang F, Liu YH, Yang XP. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life Sci. 2004;74:1561–72. doi: 10.1016/j.lfs.2003.09.042. [DOI] [PubMed] [Google Scholar]
- [24].Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- [25].Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–30. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fujiu K, Wang J, Nagai R. Cardioprotective function of cardiac macrophages. Cardiovasc Res. 2014;102:232–9. doi: 10.1093/cvr/cvu059. [DOI] [PubMed] [Google Scholar]
- [30].Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- [31].Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–70. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Puhakka M, Magga J, Hietakorpi S, Penttila I, Uusimaa P, Risteli J, et al. Interleukin-6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. J Card Fail. 2003;9:325–32. doi: 10.1054/jcaf.2003.38. [DOI] [PubMed] [Google Scholar]
- [33].Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008:130, 147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–11. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [37].Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:4360–7. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- [38].Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, et al. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–84. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- [39].Mishra PK, Pathi V, Murday A. Post myocardial infarction left ventricular free wall rupture. Interact Cardiovasc Thorac Surg. 2007;6:39–42. doi: 10.1510/icvts.2006.138511. [DOI] [PubMed] [Google Scholar]
- [40].Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–42. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- [41].Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68:799–814. doi: 10.1016/s0024-3205(00)00982-6. [DOI] [PubMed] [Google Scholar]
- [42].Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- [43].Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler Thromb Vasc Biol. 2007;27:1433–9. doi: 10.1161/ATVBAHA.106.138743. [DOI] [PubMed] [Google Scholar]