Abstract
The role of endogenous analgesic mechanisms has largely been viewed in the context of gain modulation during nociceptive processing. However, these analgesic mechanisms may play critical roles in the extraction and subsequent utilization of information related to spatial and temporal features of nociceptive input. To date, it remains unknown if spatial and temporal filtering of nociceptive information is supported by similar analgesic mechanisms. To address this question, human volunteers were recruited to assess brain activation with functional MRI during conditioned pain modulation (CPM) and offset analgesia (OA). CPM provides one paradigm for assessing spatial filtering of nociceptive information while OA provides a paradigm for assessing temporal filtering of nociceptive information. CPM and OA both produced statistically significant reductions in pain intensity. However, the magnitude of pain reduction elicited by CPM was not correlated with that elicited by OA across different individuals. Different patterns of brain activation were consistent with the psychophysical findings. CPM elicited widespread reductions in regions engaged in nociceptive processing such as the thalamus, insula and SII. OA produced reduced activity in SI, but was associated with greater activation in the anterior insula, dorso-lateral prefrontal cortex, intra-parietal sulcus, and inferior parietal lobule relative to CPM. In the brainstem, CPM consistently produced reductions in activity while OA produced increases in activity. Conjunction analysis confirmed that CPM related activity did not overlap with that of OA. Thus, dissociable mechanisms support inhibitory processes engaged during spatial vs. temporal filtering of nociceptive information.
Introduction
Sensory information undergoes substantial transformation during afferent processing by differential recruitment of inhibitory vs. excitatory processes. For example, afferent input can be transformed in the spatial domain by processes such as lateral inhibition and spatial summation. Similarly, afferent input can be transformed in the temporal domain by processes such as adaptation and temporal summation. In the nociceptive system, inhibitory processes contribute substantially to the processing of afferent information in both the spatial and temporal domains.
One mechanism involved in the spatial transformation of nociceptive information is the “diffuse noxious inhibitory controls” (DNIC), which is mediated via the spino-bulbo-spinal loop [17]. The DNIC phenomenon is manifested as a decrease in pain sensation to a noxious stimulus during or following application of another spatially remote noxious stimulus. This ‘pain inhibits pain’ phenomenon is suggested to involve a spatial filtering of pain that helps to extract nociceptive signals from the background noise [18]. Similar spatial regulation of nociceptive processing can also be accomplished solely at the spinal level without recruitment of descending inhibition [9]. Both forms of heterotopic inhibition are measured psychophysically in the laboratory by the conditioned pain modulation (CPM) paradigm [39].
Another inhibitory processing mechanism is offset analgesia (OA), which reflects temporal filtering of sensory information [11,43]. OA describes a phenomenon in which a small decrease in noxious stimulus intensity produces a robust change in perceived pain intensity that is disproportionally large relative to the actual decrease in temperature. The OA effect is time locked and lasts for approximately 10 s before pain ratings begin to increase toward values that would be predicted from a constant temperature stimulus of the same duration [11,43].
Although both CPM and OA evoke pain inhibition it remains unclear if they engage similar brain mechanisms. Functional imaging studies of CPM have identified reduced activity in several pain-processing areas including the thalamus, SI and SII as well as the anterior and middle cingulate cortex (ACC and MCC) and insula (INS) [28,30]. In contrast, OA reduces activity in SI, but produces greater activity in the periaqueductal gray (PAG), anterior INS, dorsalateral prefrontal cortex (DLPFC) and the MCC [7,42]. Since different noxious stimuli were applied across these different studies, it remains unclear if CPM and OA engage similar brain mechanisms of pain modulation. Moreover, if both CPM and OA rely on similar inhibitory mechanisms we would predict that the magnitude of inhibition produced by CPM would be strongly correlated with the magnitude inhibition produced by OA. Thus, the aim of the current study was to determine if spatial filtering of nociceptive information is accomplished by mechanisms that are similar to those engaged by temporal filtering of noxious information.
Materials & Methods
Subjects
Sixteen healthy subjects were enrolled in the study. Three subjects were excluded from the study due to either not tolerating the stimuli or having an unusual response to the offset analgesia paradigm (3 SD above the mean). Thus, our final sample included 13 right handed subjects (5 men, 8 women), mean age of 25.6± 2.8 (range 21-33 years), with race distribution of ten whites, one African American, one Hispanic and one Asian. Subjects had no history of chronic pain or neurological disorders and no magnetic resonance imaging (MRI) contraindications. All female subjects reported using a reliable method of birth control and were not pregnant while participating in this study. The Institutional Review Board (IRB) at Wake Forest University School of Medicine approved all procedures used in this experiment. Before participating in the study every subject gave written informed consent acknowledging that they understood all methods and procedures used in the experiment, they would experience painful stimuli, and they were free to withdrawal from the study at any time.
Study sessions
Subjects first participated in a familiarization session in the psychophysical assessment laboratory. During the familiarization session, subjects first received a standard set of heat stimuli to give them experience rating pain. They then experienced the CPM and OA stimulus paradigms to be used during the imaging session in order to ensure that these stimuli were tolerable. These familiarization data are not presented further. After successful completion of this session, subjects participated in an MRI scanning session on a separate day. In both sessions, the CPM and OA paradigms were delivered in a random order.
Heat stimulus delivery
Noxious heat stimuli were delivered using a MRI compatible thermode with a contact area of 16X16 mm (TSA II, Medoc, Israel). The temperature increase and decrease rate was 5°C/s from a baseline temperature of 35°C.
Pain assessments
In the familiarization session, subjects first received 32 heat stimuli (35-49°C, 5 s) on the posterior aspect of the lower leg in order to provide them experience rating pain intensity and unpleasantness. These stimuli were rated with a mechanical visual analog scale (VAS) [29]. The endpoints of the intensity scale were ‘no pain sensation’ and ‘most intense pain imaginable’ and the endpoints of the unpleasantness scale were ‘not unpleasant at all’ and ‘most unpleasant imaginable’. For each dimension these endpoints corresponded to numbers of 0 and 10, but these numbers were only visible to the experimenter.
After presentation of these training stimuli, all stimuli to be used during the imaging session were then presented. These stimuli were rated with a computerized VAS. Subjects manipulated the scale by moving a track ball. The scale was visually identical to the plastic VAS and was projected onto a computer monitor during the familiarization session or onto a MRI compatible goggles during the imaging session. Depending upon the stimulus to be rated, these ratings were acquired either in a continuous fashion over the duration of the noxious stimulus or at a single time point during noxious stimulation. These ratings were only obtained for pain intensity and were sampled at 100 Hz.
Stimulus conditions
CPM paradigm
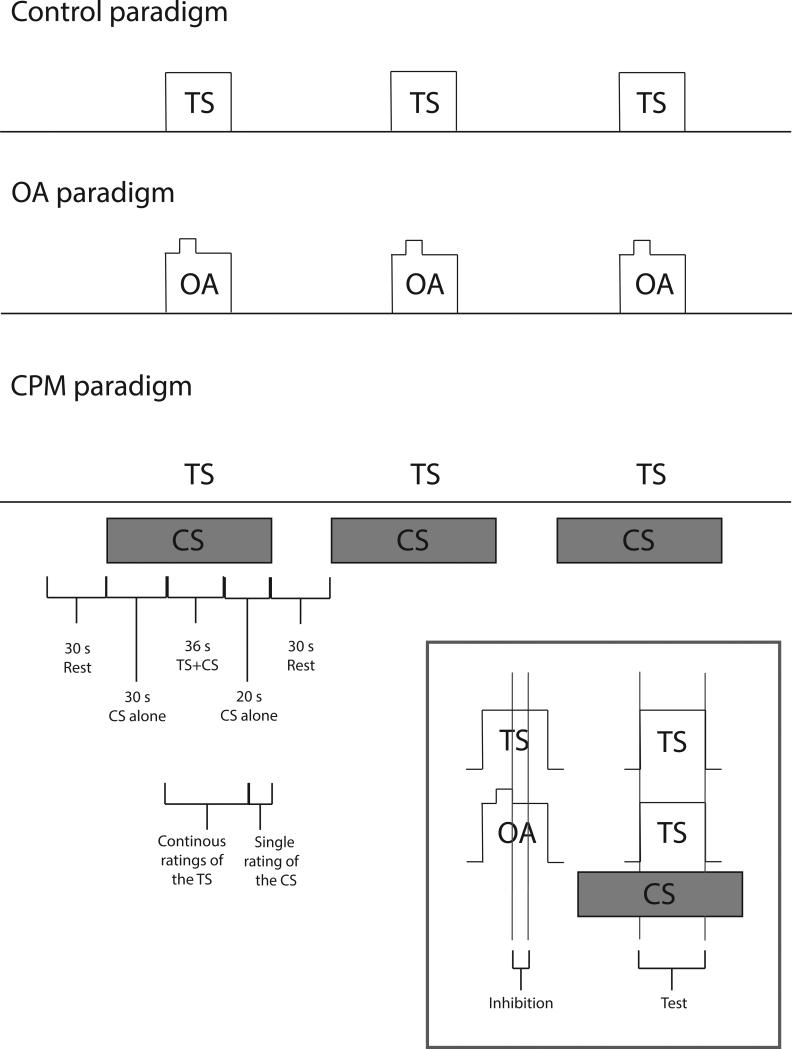
In order to evoke a CPM response, the effect of the conditioning stimulus (CS) on the test stimulus (TS) was examined. A parallel CPM design was employed such that the TS was delivered during the CS. Each pair of stimuli was repeated 3 times with an inter-stimulus interval of 30 s (Fig. 1).
Fig. 1. Study design.
The time course of the test-alone, OA and CPM paradigms. CPM was produced by immersion of the right foot into a cold water bath (10-12°C) and its effect on a noxious heat stimulus (49°C) to the lower left leg was assessed. OA was assessed using a 3 temperature stimulus paradigm (49-50-49°C) in which the 1°C decrease following the second temperature (T2) was used to evoke OA. The magnitude of both CPM and OA was assessed by comparisons with a control stimulus (49°C). Continuous ratings of pain intensity of the TS or OA stimulus were acquired in all paradigms while a single rating of the CS was additionally acquired during the CPM paradigm. Regressors were constructed to analyze brain activation during the 36 s of the TS during both the CPM and control paradigms (test phase). Since the duration of OA is shorter than that of CPM, brain activation was analyzed during a 10 s window following the T2-T3 temperature decrease (inhibition phase) during the OA and the control paradigms. TS- test stimulus, CS-conditioning stimulus, OA- offset analgesia
Test stimulus - the TS was a tonic heat pain stimulus that lasted for 30 s plateau duration at an intensity of 49°C and was delivered to the back of the lower left leg. An inter-stimulus interval of 87 s was maintained between successive TSs. Subjects provided continuous ratings of pain intensity of the TS.
Conditioning stimulus- the CS was delivered by immersion of the right foot into a cold (10-12°C) water bath for 87 s. Thirty seconds after the initiation of the CS the TS was delivered. After 75 s of foot immersion subjects were prompted to provide a single VAS rating of cold pain intensity.
OA paradigm
A three-temperature stimulus train was used to evoke OA. This train consisted of T1=49°C (5 s), T2=50°C (5 s) and T3=49°C (20 s) for a total plateau duration of 30 s. The decrease from T2 to T3 was used to evoke OA. This three-temperature stimulus train was repeated 3 times with a 87 s inter stimulus interval of rest, and was applied to the back of the lower left leg. Subjects provided continuous ratings of pain intensity of this stimulus.
Control paradigm
The same control paradigm was used to assess analgesia during both CPM and OA paradigms. This stimulus was identical to the TS, lasted for 30 s (plateau duration) at an intensity of 49°C and was applied to the back of the lower left leg. This stimulus was repeated 3 times with 87 s inter-stimulus interval of rest. Subjects provided continuous ratings of pain intensity of this stimulus. In order to optimize comparisons with both the CPM and OA paradigms, this paradigm did not involve immersion of the right foot in a water bath.
MRI acquisition
BOLD (blood oxygenation level dependent) signal was used to assess regional brain activation. Functional data were acquired on a 1.5-T General Electric echo-speed Horizon LX scanner with a birdcage head coil (GE Medical Systems, Milwaukee, WI). An anatomical scan was obtained using 170 x 1 mm thick slices, 0.93 × 0.93 mm in-plane resolution, echo time=4.75, repetition time=11.52 s, flip angle 12°. T2*-weighted fMRI scans were acquired with an echo-planar pulse imaging sequence using 28 x 5 mm thick axial slices, 240 x 180 field of view, 64 x 48 matrix size, 3.75 × 3.75 mm in-plane resolution, echo time = 40 ms, repetition time = 2 s, flip angle 80°. After a 20 s equilibration period, a total of 203 volumes were acquired within a single series. Each series lasted for 6 minutes and 46 seconds.
During the imaging session, each stimulus paradigm was presented to each subject at least twice in a random order. Between each series there was 1 min break except for the CPM paradigm in which an 8 min break was applied.
Image processing
Both structural and functional MRI data were analyzed using the FSL software package (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Data from each functional series were normalized for global signal intensity on a volume-by-volume basis and were corrected for both motion and slice timing. These data were then spatially smoothed (5 mm) and underwent high pass temporal filtering to remove slow drifts in signal intensity. The functional data were registered to their structural data using a 6-parameter linear 3-D transformation.
Structural data were transformed into standard stereotaxic space (Montreal Neurological Institute 152 T1 template) using a 12-parameter linear 3-D transformation (FLIRT [13]) followed by a nonlinear transformation using a 10 mm warp resolution. Functional data were transformed into standard space using the nonlinear transform derived from the structural data.
Statistical analysis
Findings from a previous experiment indicated that the CPM response diminished across repetitions within a series of stimuli [23]. Accordingly, preliminary repeated measures analyses of variance (RM-ANOVA) were used to determine if the magnitude of analgesia elicited by CPM and OA differed over time within series. This analysis confirmed that both OA and CPM responses diminished significantly over time. Thus, all analyses are focused on data derived from the first stimulus in every series. We have previously demonstrated that this single epoch design is sufficiently sensitive to detect pain related brain activation as well as activation associated with pain modulation [15,16].
fMRI analyses
In order to determine which brain areas exhibited increased or decreased activation during OA and CPM responses, all voxels within the brain were examined with conventional general linear model-based multiple regression analyses using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl).
For analysis of brain activity during the CPM paradigm, 3 regressors were constructed. The regressor test was set to 0 during rest periods and +1 during the 36 s of TS (combination of 3 s of increase from baseline, 30 s of stimulus and 3 s of decrease to baseline); the regressor cond was set to 0 during rest periods and +1 during the 87 seconds in which the CS was delivered; the regressor rating was set to 0 during rest periods and +1 during the 10 s rating of the CS. All regressors were orthogonalized against each other to identify variability unique to each condition. This procedure also ensures that activation related to the CS is incorporated into the statistical model.
For analysis of brain activity during the OA paradigm, 3 regressors were constructed. The regressor pain was set to 0 during rest periods and +1 during the 13 s of T1+T2; the regressor inhibition was set to 0 during rest periods and +1 during the 10 seconds following the decrease in stimulus intensity from T2 to T3; the regressor recovery was set to 0 during rest periods and +1 during the last 13 s of T3, in which the inhibition effect of the OA disappeared and the painful sensation increased. The inhibition regressor examined a 10 s period of time during the maximal effect of OA [42]. All regressors were orthogonalized against each other to identify variability unique to each condition.
For analysis of brain activity during the control paradigm three regressors were generated and were time matched to the regressors from the OA paradigm (see Fig. 1). The 10 s corresponding to the inhibition period of OA were used to assess analgesia during the OA paradigm (control_10). All time periods of stimulation (36 s) were used to assess analgesia during the CPM paradigm (control_30).
The hemodynamic response function for each regressor described above was modeled by convolving the regressor with a gamma variate (delay 6 s, SD 3 s). The temporal derivative of this hemodynamic response function was added to the statistical model as a regressor of no interest. These regressors were then temporally filtered using the same parameters that were applied to the functional images.
Fixed effects general linear modeling analyses were conducted within each series to identify brain activation associated with the modeled hemodynamic response functions. Second level fixed effects analyses were used to assess these effects across different series within subjects. Finally, third level random effects analyses were used to identify stimulus-related brain activation across individuals. Regional changes in whole-brain activation were identified in Z (Gaussianised T/F) statistic images that were thresholded using clusters determined by Z > 2.3 and a cluster significance threshold of p < 0.05 [37]. This procedure ensures that the probability of false positives is corrected for multiple comparisons across all brain voxels. Moreover, all analyses were executed using a hypothesis-independent whole brain search in which both positive and negative signal changes were examined. This conservative approach minimizes confirmation biases associated with ROI-based analyses.
Three contrasts were conducted to fully examine CPM related activation: TS related activation during the CS in the CPM paradigm (CPMtest > rest), TS related activation in the absence of the CS in the control paradigm (control_30 > rest), and CPM response related activation (CPMtest-rest > control_30-rest). Similarly, 3 contrasts were conducted to fully examine OA related activation: OA-related activation (OAinhibition > rest), control stimulus-related activation (control_10 > rest), differences between OA and control (OAinhibition –rest > control_10-rest).
In order to examine the differences in activation evoked by CPM and OA the following contrast was conducted [(CPMtest-rest > control_30-rest) vs. (OAinhibition –rest > control_10-rest)]. In order to determine if CPM evoked activation similar to that of OA, a conjunction analysis of the two third-level contrasts of the CPM and OA responses was conducted [25].
Statistical analysis of psychophysical data
Mean VAS ratings from different windows of time were analyzed for the CPM and OA paradigms. For the CPM response, we calculated the mean pain ratings during the 30 s plateau of the TS stimulus during the CPM paradigm and the control paradigm (control_30). For the OA response we calculated the mean pain ratings of the 10 s period starting 2.25 s after the T2 period ended in the OA paradigm and the matching time period of the control paradigm (control_10). We chose to analyze the mean VAS ratings 2.25 s after the end of the T2 period to account for the latency between the T2 temperature decrease and the decrease in psychophysical ratings [43].
Paired t-tests were used to determine if CPM and OA elicited significant analgesia. Comparisons between mean pain ratings during CPMtest and control_30 were used to determine if the CS produced a significant reduction in pain intensity ratings of the TS. Comparisons between mean pain ratings during OAinhibition and control_10 were performed to determine if the T2-T3 temperature decrease during the OA paradigm elicited a significant reduction in pain intensity ratings.
In order to directly compare pain reduction during the CPM vs. the OA paradigm, we first calculated a) the difference between the mean pain ratings during the 30 s of the TS obtained in the CPM paradigm and in the control_30 paradigm for the CPM response, b) the difference between the mean pain ratings during the 10 s of OAinhibition and those obtained in the control_10 paradigm for the OA response. Efficient CPM and OA are represented by negative values. These differences were then compared using a paired t-test in order to determine if the magnitude of analgesia differed between paradigms.
A regression analysis was used to determine if individual differences in the magnitude of CPM were related to the magnitude of OA. Additional multiple regression analyses were used to evaluate 1) the effect of sex, age, and perceived pain intensity of the CS on the magnitude of the CPM response and 2) the effect of sex and age on the magnitude of the OA response. All analyses were conducted using JMP (SAS Institute, Cary, NC, USA).
Results
Psychophysical evidence for analgesia during CPM and OA
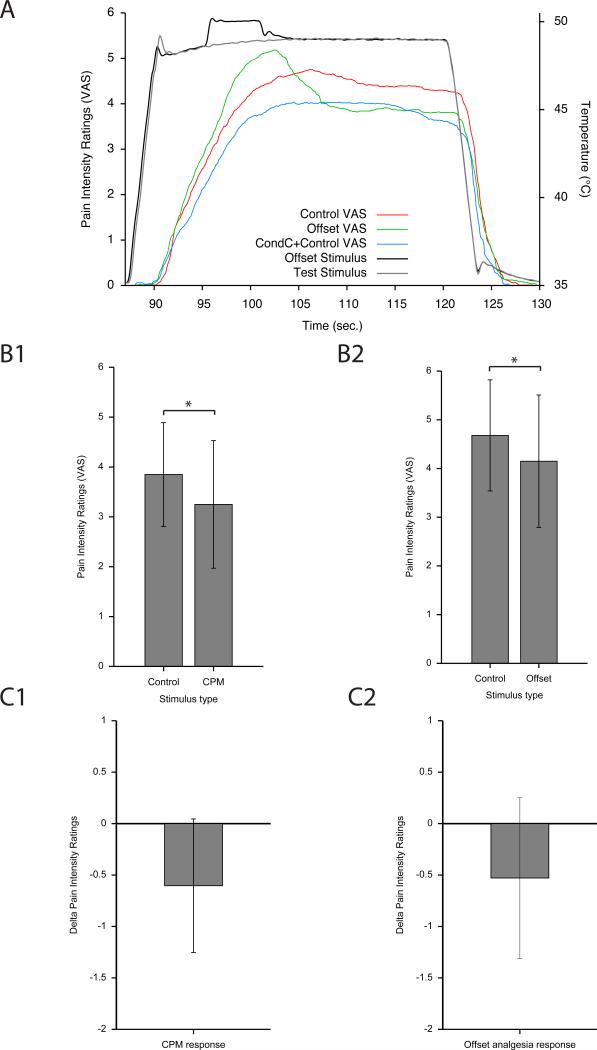
During MRI scanning, a robust CPM response was evoked during the CPM paradigm. There was a significant difference between the mean pain intensity ratings during the 30 s of the TS in the control and CPM paradigms [F=11.3, (df = 1, 12), p<0.006, Table 1 and Fig 2]. Consistent with the modulatory effect of the CS, subjects perceived the CS as painful with a mean pain intensity rating of 2.19±1.58.
Table 1.
the mean ratings of pain intensity during control, OA, and CPM paradigms
| First repetition | Second repetition | Third repetition | |
|---|---|---|---|
| Constant (10 s) | 4.68±1.14 | 4.20±1.15 | 4.22±1.37 |
| OA | 4.15±1.36 | 4.62±1.38 | 4.62±1.51 |
| Constant (30 s) | 3.85± 1.04 | 3.04± 0.87 | 3.06± 1.04 |
| CPM | 3.25 ±1.28 | 2.78± 1.15 | 2.96± 1.16 |
| CPM response | −0.60 ±0.65 | −0.27 ±0.57 | −0.10 ±0.51 |
| OA response | −0.53 ±0.78 | 0.42 ±0.87 | 0.41 ±0.79 |
For CPM and constant (30 s) paradigms, the presented pain ratings are averaged across the entire 30 s of the test stimulus. For constant (10 s) and OA paradigms, the presented pain ratings are averaged across the 10 s following 2.25 s of the decrease from T2 to T3. Pain ratings are in a scale between 0 (not painful) to 10 (the most intense pain sensation imaginable).
Fig. 2. Psychophysical responses for the CPM and OA paradigms during MRI scanning.
Both CPM and OA produced significant reductions in perceived pain intensity. The VAS ranged from 0 (no pain sensation) to 10 (most intense pain sensation imaginable). A. Continuous VAS ratings of the TS for the control, OA and CPM paradigms (mean of all subjects). B1. During the 30 s of the TS the conditioning stimulus produced a significant reduction in pain intensity relative to 30 s of the TS during the control paradigm (mean±SEM). B2. During the 10 s following the T2 to T3 decrease during the OA paradigm, pain intensity ratings were significantly lower than of those of the TS during the corresponding period of the control paradigm (* p<0.05, mean±SEM). C1, C2. The magnitude of the CPM response was not significantly different from the magnitude of the OA response (mean±SEM). Negative values indicate an efficient inhibitory response in both paradigms. TS- test stimulus;] CPM- conditioned pain modulation; OA offset analgesia
During MRI scanning, OA elicited by the 1°C decrease from 50°C (T2) to 49°C (T3) elicited substantial analgesia. Pain ratings during the inhibition period of the OA paradigm were significantly reduced below those of the control paradigm [F=6.0, (df = 1, 12), p=0.031, Table 1 and Fig 2].
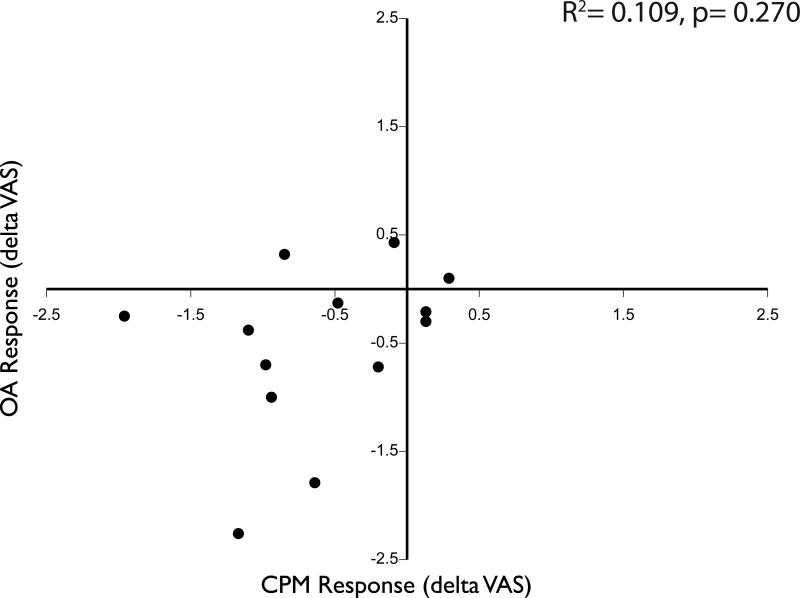
There was no significant difference between the magnitude of the CPM and OA responses (p=0.754), thus analyses of functional imaging data are not confounded by different psychophysical responses. However, despite this similarity, the magnitude of CPM was not correlated with the magnitude of OA within individuals (r2=0.109, p=0.270, Fig 3). This raises the possibility that CPM is supported by mechanisms that are distinct from those that are supported by OA.
Fig. 3. No correlation of psychophysical responses between CPM and OA during MRI scanning.
No significant correlation was found between the CPM and OA responses. Negative values in both the x and y dimensions indicate an efficient inhibitory response in both paradigms. CPM-conditioned pain modulation; OA offset analgesia
The magnitude of the CPM effect was not related to the subjects’ age, sex and their perceived intensity of the CS (p=0.914). Similarly, the magnitude of the OA effect was not related to subjects’ age and sex (p=0.675).
Brain activation and deactivation associated with CPM response
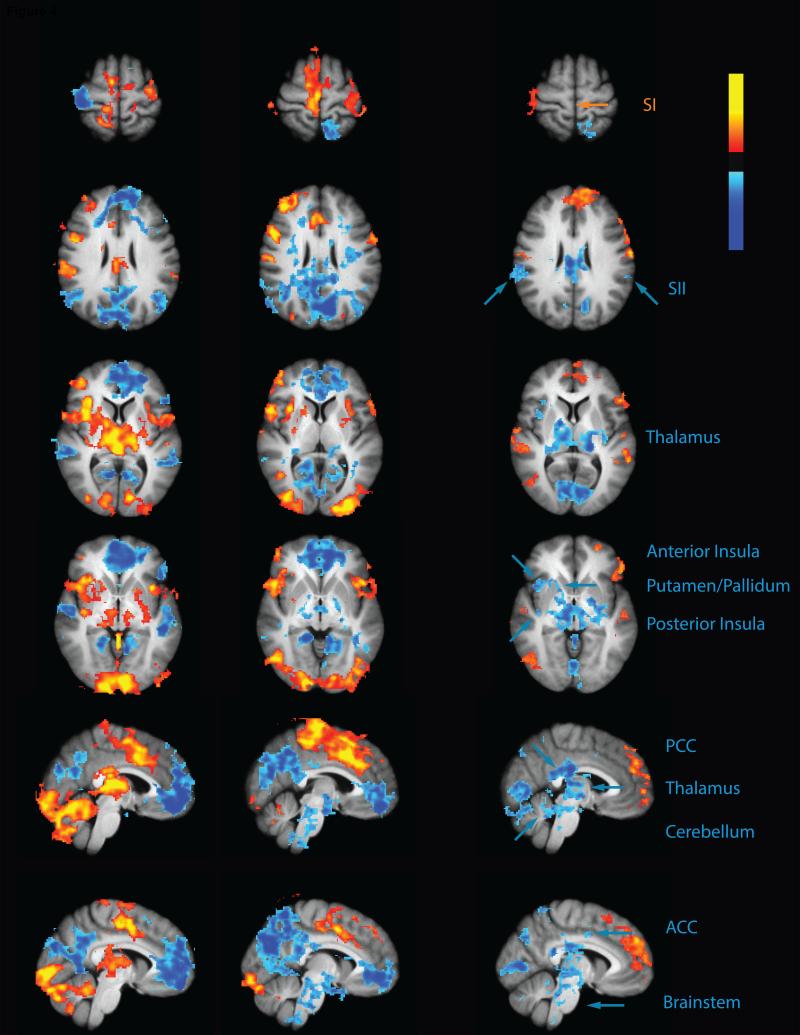
The thalamus, putamen, cerebellum, anterior INS, posterior INS, ACC and SII exhibited significantly reduced TS-related activity during CPM. These areas were all activated by the TS during the control paradigm, but exhibited significantly reduced TS activity when the CS was applied to the contralateral foot in the CPM paradigm (Fig 4 and Table 2). TS related activation of the leg region of SI was noted during both control and CPM paradigms however, no significant CPM effect was observed in this area.
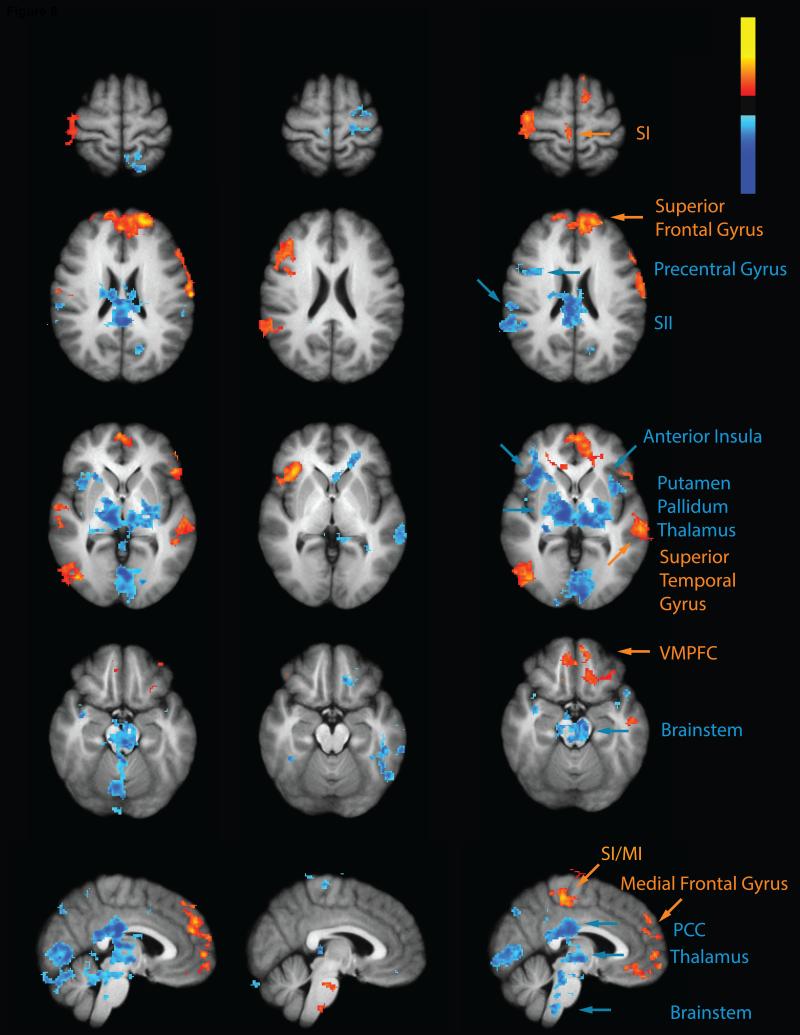
Fig. 4. CPM inhibits regions involved in nociceptive processing.
The test stimulus in the control paradigm (control>rest) produced activation (red-yellow) in the cerebellum, Putamen/Pallidum, thalamus, anterior INS, posterior INS, ACC, SI and SII together with deactivation in the PCC/precuneus, medial prefrontal cortex. During the CPM paradigm (CPM>rest) the test stimulus was associated with deactivation (blue) in the brainstem, thalamus and PCC/precuneus. However, test stimulus related activation was still evident in the cerebellum, ACC, anterior INS and SI. Results of the contrast between these two paradigms (CPM>control) revealed that CPM reduced test stimulus related activity (blue) in the brainstem, cerebellum, thalamus, posterior INS, SII, anterior INS, Putamen and ACC. CPM produced greater deactivation of the PCC/precuneus but produced activation in a region of the medial frontal cortex which was deactivated during the control paradigm (red-yellow). PCC-posterior cingulate cortex; ACC- anterior cingulate cortex; INS- insula; SI-primary somatosensory cortex; SII- secondary somatosensory cortex.
Table 2.
Significant differences in BOLD responses during CPM (CPM>control)
| a. Increased BOLD responses | |||
|---|---|---|---|
| Region | Coordinatesmax | BA | Zmax score |
| Frontal lobe | |||
| Superior Frontal Gyrus | 0, 52, 32 | 4.31 | |
| Frontal Pole | 0, 62, 10 | 3.94 | |
| Inferior Frontal Gyrus | −52, 24, 4 | 45 | 4.05 |
| Temporal lobe | |||
| Superior temporal gyrus | 62, −28, 2 | 22 | 3.25 |
| 60, −8, −10 | 21 | 3.22 | |
| Parietal lobe | |||
| SI | −64, −12, 30 | 1 | 4.06 |
| 38, −30, 46 | 2 | 3.98 | |
| 30, −34, 56 | 3b | 3.61 | |
| SII | −64, −8, 20 | 43 | 3.8 |
| Premotor cortex | −60, 2, 30 | 6 | 4.06 |
| Inferior Parietal Lobule | 42, −26, 42 | 40 | 3.57 |
| Occipital lobe | |||
| lateral occipital cortex | 44, −76, 0 | 3.71 | |
| b. Decreased BOLD responses | |||
|---|---|---|---|
| Region | Coordinatesmax | BA | Zmax score |
| Parietal lobe | |||
| Superior Parietal Lobule | −16, −42, 56 | 7 | 3.64 |
| Inferior Parietal Lobule | 64, −34, 36 | 40 | 3.57 |
| Precuneous cortex | −10, −72, 34 | 31 | 3.82 |
| Occipital lobe | |||
| Visual Cortex | 10, −76, 8 | 17 | 4.29 |
| Limbic lobe | |||
| PCC | −2, −42, 20 | 29 | 4.22 |
| Subcortical | |||
| Thalamus | 10, −20, 2 | 4.33 | |
| Brainstem | 2, −26, −20 | 2.93 | |
| Pons | 2, −34, −26 | 2.93 | |
ACC- anterior cingulate cortex, PCC- posterior cingulate cortex, SI- primary somatosensory cortex, MI- primary motor cortex.
During CPM, extensive deactivation was found in the midbrain, potentially encompassing the substantia nigra and PAG. Addition deactivations were noted in the brainstem at the superior portion of the pons and at the junction of the medulla and the pons. These deactivations are true deactivations since TS related deactivation was observed during the CPM paradigm, but no significant TS related changes were evident during the control paradigm.
The PCC exhibited a similar pattern of activity in that it was not activated during the control paradigm but deactivated during the CPM paradigm. Therefore, this area was mostly deactivated during CPM.
A number of other brain regions including medial prefrontal areas and regions at the temporo-parietal junction exhibited reduced deactivation during CPM. These areas exhibited TS related deactivation during the control paradigm but either exhibited no TS related signal changes or reduced deactivation during the CPM paradigm. Thus, the contrast detected greater activity in these areas during CPM.
Activation associated with OA response
SI and the cerebellum exhibited significantly reduced TS related activity during OA. The cerebellum was activated by the TS during the control paradigm but showed reduced TS related activation during the OA paradigm. In contrast, no significant TS related activation was detected in SI during either the control paradigm or the OA paradigm. However, in the OA vs. control contrast the leg representation exhibited decreased activity (Fig 5 and Table 3). This region of reduced activity corresponds exactly with SI activation detected during the 30 s window examined during the control paradigm for CPM (Fig 4). Thus, it is likely that there is sub-threshold activity during the 10 s window used in these comparisons.
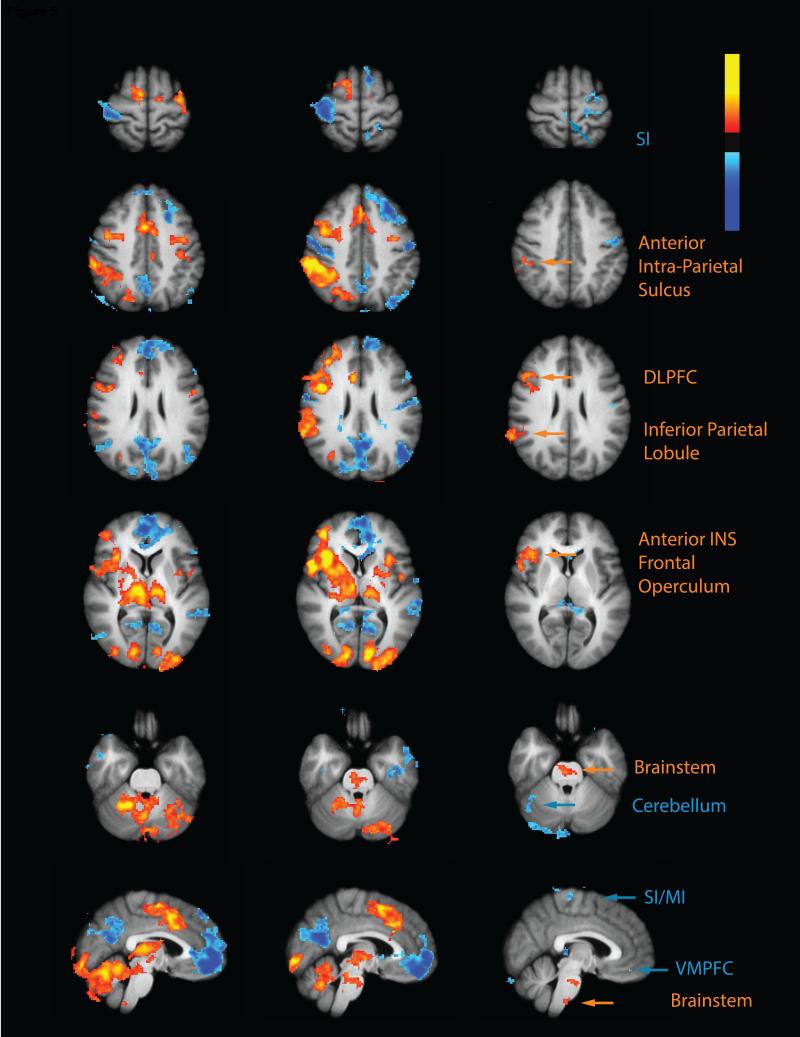
Fig. 5. OA activates regions important in pain modulation.
During the control paradigm (control>rest) the test stimulus produced activation (red-yellow) of the cerebellum, thalamus, putamen, ACC, anterior INS and SII together with deactivation (blue) of the PCC/precuneus and the medial frontal cortex. During the OA paradigm (OA>rest), the test stimulus produced activation of the brainstem, cerebellum, thalamus, putamen, posterior INS, SII, anterior INS, inferior parietal lobule, anterior intra parietal sulcus, and ACC. The contrast revealed greater test stimulus related activation (red-yellow) during OA (OA>control) in the DLPFC, intra parietal sulcus, inferior parietal lobule, anterior INS, frontal operculum cortex and brainstem and reduced activation (blue) in SI and the cerebellum. INS- insula; DLPFC-dorsa-lateral prefrontal cortex; PCC-posterior cingulate cortex; ACC- anterior cingulate cortex; SII- secondary somatosensory cortex.
Table 3.
Significant differences in BOLD responses during OA (OA>control)
| a. Increased BOLD responses | |||
|---|---|---|---|
| region | Coordinatesmax | BA | Zmax score |
| Frontal lobe | |||
| Frontal orbital cortex | 38, 28, −10 | 13 | 3.63 |
| Inferior frontal gyrus | 52, 24, 16 | 45 | 3.85 |
| Parietal lobe | |||
| Supramarginal gyrus | 62, −42, 26 | 40 | 3.58 |
| Temporal lobe | |||
| Angular gyrus | 64, −48, 26 | 3.48 | |
| Insular lobe | |||
| Insula | 36, 26, 0 | 13 | 3.72 |
| Subcortical | |||
| Brainstem | 0, −26, −46 | 3.21 | |
| Pons | 2, −24, −26 | 3.38 | |
| b. Decreased BOLD responses | |||
|---|---|---|---|
| region | Coordinatesmax | BA | Zmax score |
| Frontal lobe | |||
| Frontal orbital cortex | −34, 30, −22 | 47 | 3.38 |
| MI | 10, −30, 72 | 4a | 3.46 |
| Premotor cortex | −26, −16, 70 | 6 | 3.56 |
| Parietal lobe | |||
| Precentral gyrus | −52, −12, 42 | 4 | 3.5 |
| SI | −24, −30, 72 | 1 | 3.52 |
| −38, −16, 32 | 3a | 3.36 | |
| −50, −14, 32 | 3b | 3.52 | |
| Temporal lobe | |||
| inferior temporal gyrus | −48, −56, −18 | 3.68 | |
| Superior temporal gyrus | −66, −34, 2 | 22 | 3.68 |
| Occipital lobe | |||
| Lateral occipital cortex | −54, −66, −10 | 37 | 3.49 |
| Subcortical | |||
| Hippocampus | −10, −40, 2 | 30 | 3.11 |
| Posterior cerebellum | 34, −60, −42 | 3.62 | |
SI- primary somatosensory cortex MI- primary motor cortex.
The DLPFC, intra parietal sulcus, inferior parietal lobule, anterior INS and frontal operculum cortex exhibited significant activation during OA. These areas were all activated by the TS during the control paradigm, but exhibited significantly higher TS activity in the OA paradigm. Notably all these areas were in the right hemisphere (Fig 5).
In the brainstem, significant OA related activation was found in the superior part of the pons and in the border between the pons and the medulla. Activations in these regions may encompass the pontine components of the reticular formation such as the superior nucleus reticularis centralis and nucleus reticularis tegmenti pontis, as well as the parabrachial nucleus. No TS related activation was detected in these areas during the control paradigm while during the OA paradigm a significant activation was found. Thus, the contrast detected greater activity in these areas during OA (Fig 5). In contrast, PAG activity was detected in both the control and the OA paradigms, however, no statistically reliable differences were evident in the direct comparison of the two paradigms.
Differences between CPM and OA activation
In order to determine if CPM and OA engage different brain mechanisms during the production of analgesia we performed a contrast between brain activation during the CPM response and OA response [(CPM>control) > (OA>control), Fig 6 and Table 4].
Fig. 6. Differences in brain activation between CPM and OA.
CPM produced markedly different patterns of activity than OA. CPM reduced test stimulus activation (blue) in the brainstem, cerebellum, putamen, posterior INS and SII more than OA (CPM>OA). In contrast, OA reduced test stimulus related activation (red-yellow) more than CPM in SI (CPM>OA). OA was associated with greater activation of the anterior INS, dorso-lateral PFC, intra parietal sulcus, and inferior parietal lobule, while CPM was associated with less deactivation of the both the ventro-medial and dorso-medial PFC, superior temporal gyrus and lateral occipital cortex. INS- insula; PFC- dorsa-lateral prefrontal cortex; SII- secondary somatosensory cortex.
Table 4.
Significant differences between CPM and OA responses
| a. Increased BOLD responses in CPM compared to OA | |||
|---|---|---|---|
| Region | Coordinatesmax | BA | Zmax score |
| Frontal lobe | |||
| Frontal Pole | −14, 58, 28 | 4.01 | |
| Superior frontal gyrus | −8, 18, 54 | 3.92 | |
| Premotor cortex | 30, −24, 68 | 3.81 | |
| MI | 4, −30, 56 | 4 | 3.77 |
| Parietal lobe | |||
| SI | −62, −10, 30 | 1 | 4.01 |
| −46, −22, 44 | 2 | 3.67 | |
| 40, −24, 52 | 3b | 3.61 | |
| SII | −64, −6, 14 | 3.82 | |
| Occipital lobe | |||
| Inferior Lateral Occipital Cortex | 42, −72, −2 | 3.72 | |
| b. Increased BOLD responses in OA compared to CPM | |||
|---|---|---|---|
| Region | Coordinatesmax | BA | Zmax score |
| Parietal lobe | |||
| Superior Parietal Lobule | −6, −76, 44 | 7 | 3.6 |
| Inferior Parietal Lobule | 64, −40, 30 | 40 | 3.97 |
| Precuneous Cortex | 10, −70, 36 | 3.99 | |
| Occipital lobe | |||
| Visual cortex (V1) | −8, −92, 2 | 17 | 4.18 |
| Visual cortex (V2) | −6, −78, 4 | 18 | 3.81 |
| Insular lobe | |||
| Insula | 44, 14, −6 | 13 | 4.08 |
| Subcortical | |||
| Thalamus | 10, −14, 0 | 4.32 | |
| Brainstem | 4, −38, −48 | 3.6 | |
| Posterior Cerebellum | −28, −40, −42 | 3.36 | |
A number of brain regions associated with afferent nociceptive processing exhibited greater reduction in activity during the CPM vs. OA. These regions included the thalamus, putamen, posterior INS and SII. In contrast, SI exhibited greater deactivation during OA relative to CPM.
Brain regions associated with the modulation of afferent nociceptive processing and/or attention exhibited greater activity during OA than CPM. These regions include the anterior INS, DLPFC, intra parietal sulcus, and inferior parietal lobule.
Surprisingly, markedly different patterns of activity between the CPM and OA were seen in multiple levels of the brainstem. In the midbrain, pons and medulla CPM consistently produced reductions in activity while OA produced increases in activity.
Areas normally deactivated during pain were differently influenced by CPM and OA. CPM produced greater deactivation of the PCC and precuneus than OA. In contrast, OA produced greater deactivation of the VMPFC and more dorsal medial prefrontal regions and temporal parietal junction. In the temporal parietal junction, however, CPM reduced deactivation while OA increased deactivation.
Absence of similarities between CPM and OA activation
The conjunction analysis revealed no shared areas between the CPM and OA responses in either activations or deactivations. This finding further supports results from direct contrast of CPM and OA and indicates that CPM and OA do not engage the same brain areas to exert their analgesia.
Discussion
Spatial and temporal filtering of nociceptive information instantiated by CPM and OA represent dissociable analgesic phenomena that modulate afferent nociceptive information processing in different ways. The magnitude of pain reduction elicited by CPM was not correlated with that elicited by OA. Consistent with the lack of correlation in these subjective reports, brain activation during CPM was markedly distinct from that during OA. These data are the first to demonstrate that distinct mechanisms support inhibitory processes engaged during spatial vs. temporal filtering of nociceptive information.
CPM related activation
CPM elicited by heterotopic inhibition of noxious stimulus input represents a powerful mechanism of spatial filtering in the nociceptive system. Heterotopic inhibition of nociceptive processing may be mediated by both supraspinal as well as spinal components. The existence of such inhibitory mechanisms at multiple levels of the neuraxis underscores how these spatial filtering mechanisms represent a fundamental component of nociceptive information processing.
At the supraspinal level, heterotopic inhibition of spinal nociceptive processing has been shown to be mediated by activation of a spino-bulbo-spinal loop and has been termed DNIC[17]. However, determination of the exact brainstem sites supporting DNIC remains unclear. For example, focal lesions of the PAG, the rostral ventromedial medulla (RVM), parabrachial nucleus, or the locus coeruleus/subcoeruleus do not abolish DNIC [3,4,6]. Moreover, progressive sectioning of the brainstem reveals that DNIC is preserved despite complete disconnection of all structures in the midbrain and rostral medulla [5]. Lesions of subnucleus reticularis dorsalis (SRD) in the caudal medulla do reduce the DNIC response, but animals with medullary transections which spared this structure had reduced DNIC, indicating involvement of other structures in the caudal medulla [5,6,34].
At the spinal level, spatially remote noxious stimuli have long been known to inhibit the activity of neurons in the dorsal horn of the spinal cord [1,31,9,19]. The activity of spinothalamic tract neurons can be inhibited by noxious stimulation of remote body areas and this inhibition remains even after spinal cord transaction [9,19]. It is important to note that DNIC is distinct from heterotopic stimulation induced inhibition which is mediated by local circuits within the spinal cord because DNIC necessarily involves activation of descending inhibition from the brainstem [17]. The existence of multiple inhibitory mechanisms prompted the development of the more general term conditioned pain modulation (CPM) [39].
In the current study, CPM reduced test stimulus related activation of numerous brain regions engaged in nociceptive processing. Reduced test stimulus activity was detected in the cerebellum, thalamus, putamen, anterior and posterior INS and ACC. This reduced test stimulus brain activity is consistent with the CS induced reductions in pain intensity ratings of the test stimulus. Moreover, this pattern of reduced brain activation is similar to previous studies that examined brain activation during the CPM response [28,21,30].
Surprisingly, brain regions known to be involved in modulation of pain were not differentially activated during CPM. In fact, the brainstem exhibited reduced activation at medullary, pontine. and mesencephalic levels. Taken together with the widespread reductions in test stimulus related activity, these findings raise the possibility that the specific CPM paradigm utilized in the current study induced analgesia largely through a spinally mediated mechanism.
Previous studies of CPM have yielded conflicting results about the engagement of brainstem mediated descending inhibition. For example, Piche et al (2009) identified increased PAG activation during the CS. However, this PAG response was related to the reduced RIII reflex induced by the test stimulus, but not related to subjectively reported analgesia [28]. In contrast, Sprenger et al (2011) identified reduced test stimulus related activation of both the pons and medulla during the CS [30]. Taken together with the present results, these findings indicate that different CPM paradigms may differently engage spinal vs. supraspinal inhibitory mechanisms. This may explain the disassociation between subjectively reported analgesia and spinal RIII reflex modulation [32,28]. Differential engagement of spinal vs. supraspinal inhibition may also account for lack of associations between various CPM responses obtained using different CPM paradigms [24]. It remains an open question as to whether the location of the conditioning stimulus on the contralateral limb vs. the non-homologous limb would account for differential engagement of brainstem modulatory processes.
OA related activation
OA reflects a temporal filtering of sensory information [43]. OA is evoked by a small decrease in temperature that is followed by a disproportionally large reduction in perceived pain [11]. In the current study during the three-temperature paradigm, the T2 to T3 temperature decrease produced statistically significant OA. Pain intensity ratings in the 10 s window following this 1°C decrease were reduced below those elicited by a constant 49°C stimulus in the same window during the control paradigm. Consistent with the psychophysical findings, OA reduced pain related activity in SI and the cerebellum. These reductions in pain related activity are similar to findings of previous functional imaging studies of OA [42,7].
In sharp contrast with patterns of activity detected during CPM, numerous brain regions involved in pain modulation were activated during OA. OA produced increased activity in the DLPFC, anterior INS, frontal operculum cortex and the brainstem. This is similar to previous studies that examined brain activation during OA [42,7].
The increased activity in the anterior INS and DLPFC during the OA induction of analgesia is in line with previous studies that found involvement of these regions in analgesia using placebo, meditation and distraction [14,44,35,22,10]. Given that OA activation of the anterior INS and DLPFC mirrors the activity of brain regions engaged in the modulation of pain by cognitive processes, this finding raises the possibility that portion of OA is mediated or amplified by cognitive processes related to the prediction of the time course of pain.
The activation in the brainstem is also in line with previous studies that examined distraction and placebo analgesia [33,8]. Conclusive identification of specific nuclei within the brainstem is difficult due to their small size and movement of the brainstem itself. However, activation in the present investigation occurred within brainstem regions, which may include components of the reticular formation and the parabrachial nucleus. These regions are involved in descending modulation of pain [20,2,12].
OA may potentially be instantiated at multiple levels of the neuraxis, ranging from the primary afferent, to the spinal dorsal horn, to supraspinal sites. The present findings cannot directly address the potential contribution of peripheral or local spinal components to OA, but given the differential activation noted in both cortical and subcortical structures, suggest that supraspinal and/or descending control mechanisms are heavily involved. In contrast, the patterns of reduced activation of pain-related regions and smaller magnitude of deactivations seen with CPM are more consistent with a local spinal mechanism of action. Prospectively designed single-unit electrophysiological investigations of OA at the peripheral and spinal levels are needed to conclusively determine the involvement of these sites in OA.
Differences between CPM and OA
To date endogenous analgesia has been viewed predominantly as a mechanism by which the gain of nociceptive neurons is modulated. Here we show that spatial and temporal filtering of nociceptive information can be supported by clearly dissociable mechanisms involving endogenous analgesia. Spatial filtering evoked by CPM appears to be largely spinally mediated and results in widespread reductions in the activity of brain regions involved in nociceptive processing. In contrast, temporal filtering evoked by OA is associated with activation of numerous brain regions involved in pain modulation.
The lack of correlation between subjectively reported pain reductions evoked by CPM vs. OA found in the present investigation is in line with previous findings comparing CPM and OA [26]. The involvement of different mechanisms for each paradigm is further supported by the observation that ketamine blocked CPM but not OA [26].
The dissociation between spatial and temporal filtering mechanisms underscores the distinct functional role of each process. In the case of CPM, spatial filtering allows the most salient component of multiple, spatially distinct noxious inputs to drive processes supporting both conscious awareness of spatial location as well as processes optimizing reflex withdrawal responses to spatially complex noxious stimuli. In the case of OA, temporal filtering serves to inhibit after-sensations arising from slowly conducting nociceptive afferents, thereby producing a clear perceptual signal that noxious stimulus intensity is being reduced. Such information is critically important to inform the organism that measures taken to escape noxious stimulation are succeeding.
The independence of spatial and temporal filtering of nociceptive information may be of substantial clinical importance. Reduced efficacy of CPM is associated with some forms of chronic pain [38], and can even predict which individuals may develop chronic pain [40, 36] and which individuals will benefit from a pain medication [41]. Reduced OA is a feature of some forms of neuropathic pain [27] and may hold a potential to guide treatment decisions.
Conclusions
When taken together, the present findings provide strong evidence that endogenous analgesic mechanisms may differentially modulate multiple, distinct components of nociceptive input. This modulation is critically important for the transformation of nociceptive input to nociceptive information. Thus, endogenous analgesic mechanisms are intrinsically associated with the spatial and temporal filtering of afferent input and play a far greater role than simple gain modulation.
SUMMARY.
Endogenous analgesic mechanisms are intrinsically associated with the spatial and temporal filtering of afferent input and play a far greater role than simple gain modulation.
Acknowledgments
This study was supported by a United State-Israel Binational Science Foundation (BSF) grant number 2009097, NIH R01 NS039426, and NIH F31DA026278
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts of interest to report for any of the authors
References
- 1.Arendt-Nielsen L, Gotliebsen K. Segmental inhibition of laser-evoked brain potentials by ipsi- and contralaterally applied cold pressor pain. Eur J Appl Physiol Occup Physiol. 1992;64:56–61. doi: 10.1007/BF00376441. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008;71:217–21. doi: 10.1212/01.wnl.0000318225.51122.63. [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira D, Bing Z, Le Bars D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J Neurophysiol. 1990;64:1712–23. doi: 10.1152/jn.1990.64.6.1712. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Bing Z, Le Bars D. Effects of lesions of locus coeruleus/subcoeruleus on diffuse noxious inhibitory controls in the rat. Brain Res. 1992;571:140–4. doi: 10.1016/0006-8993(92)90520-j. [DOI] [PubMed] [Google Scholar]
- 5.Bouhassira D, Chitour D, Villaneuva L, Le Bars D. The spinal transmission of nociceptive information: modulation by the caudal medulla. Neuroscience. 1995;69:931–8. doi: 10.1016/0306-4522(95)00269-o. [DOI] [PubMed] [Google Scholar]
- 6.Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595:353–7. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire SW, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. Neuroimage. 2009;47:1002–6. doi: 10.1016/j.neuroimage.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–43. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Gerhart KD, Yezierski RP, Giesler GJ, Jr, Willis WD. Inhibitory receptive fields of primate spinothalamic tract cells. J Neurophysiol. 1981;46:1309–25. doi: 10.1152/jn.1981.46.6.1309. [DOI] [PubMed] [Google Scholar]
- 10.Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227–36. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol. 2002;87:2205–8. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- 12.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–25. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 14.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The single-epoch fMRI design: validation of a simplified paradigm for the collection of subjective ratings. Neuroimage. 2003 Jul;19(3):976–87. doi: 10.1016/s1053-8119(03)00119-8. [DOI] [PubMed] [Google Scholar]
- 16.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12950–5. doi: 10.1073/pnas.0408576102. Epub 2005 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 18.Le Bars D, Villanueva L, Bouhassira D, Willer JC. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Path Physiol Exp Ther. 1992;4:55–65. [PubMed] [Google Scholar]
- 19.McGaraughty S, Henry JL. Effects of noxious hindpaw immersion on evoked and spontaneous firing of contralateral convergent dorsal horn neurons in both intact and spinalized rats. Brain Res Bull. 1997;43:263–7. doi: 10.1016/s0361-9230(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 20.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 21.Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152:1469–77. doi: 10.1016/j.pain.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during distraction from pain: a comparative LORETA study with conditioned pain modulation. Brain Res. 2012;1435:105–17. doi: 10.1016/j.brainres.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 23.Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I. Waning of ‘conditioned pain modulation’: a novel expression of the subtle pronociception in migraine. Headache. 2013;53:1104–15. doi: 10.1111/head.12117. [DOI] [PubMed] [Google Scholar]
- 24.Nahman-Averbuch H, Yarnitsky D, Granovsky G, Gerber E, Dagul P, Granot M. The effect of stimulation parameters on ‘conditioned pain modulation’ response. Scandinavian Journal of Pain. 2013;4:10–14. doi: 10.1016/j.sjpain.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY. Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain. 2011;152:656–63. doi: 10.1016/j.pain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology. 2011;115:1063–71. doi: 10.1097/ALN.0b013e31822fd03a. [DOI] [PubMed] [Google Scholar]
- 28.Piché M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci. 2009;29:14236–46. doi: 10.1523/JNEUROSCI.2341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 30.Sprenger C, Bingel U, Büchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–39. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Taub A. Local, segmental, and supraspinal interaction with a dorsolateral spinal cutaneous afferent system. Exp Neurol. 1964;10:357–74. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- 32.Terkelsen AJ, Andersen OK, Hansen PO, Jensen TS. Effects of heterotopic- and segmental counter-stimulation on the nociceptive withdrawal reflex in humans. Acta Physiol Scand. 2001;172:211–7. doi: 10.1046/j.1365-201x.2001.00856.x. [DOI] [PubMed] [Google Scholar]
- 33.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res. 1995;28:113–25. [PubMed] [Google Scholar]
- 35.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–52. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother. 2010;24:119–28. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- 37.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 38.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–5. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 39.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Conditioned Pain Modulation (CPM): recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14(4):33. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–8. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–8. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29:10264–71. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134:174–86. doi: 10.1016/j.pain.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]