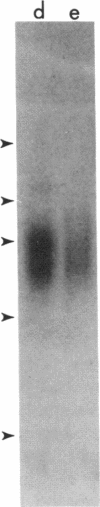
Abstract
Two different Fc receptors for IgG (Fc gamma R) have been identified on human leukocytes: a high avidity receptor (Fc gamma Rhi) present on monocytes but not on neutrophils, and a low avidity receptor (Fc gamma Rlo) present on neutrophils but not on monocytes. Fc gamma Rlo can be inhibited and the receptor precipitated by monoclonal antibody 3G8. We have used this monoclonal antibody to study the course of Fc gamma Rlo appearance on bone marrow cells, leukocytes of patients with chronic myelogenous leukemia (CML), and HL-60 and U937 cells induced to differentiate with agents such as dimethyl sulfoxide (DMSO), retinoic acid, phorbol myristate acetate, and lymphokine. We report that Fc gamma Rlo is a late differentiation antigen, first expressed at the metamyelocyte stage. Since precursors to metamyelocytes bear Fc gamma R, and the promyelocyte line HL-60 bears Fc gamma Rhi, there must be a progressive loss of Fc gamma Rhi during myeloid differentiation and the reciprocal expression of Fc gamma Rlo. Results of immunoprecipitation and polyacrylamide gel analysis of the proteins are consistent with these results. We have also studied the receptor for the C3bi complement component (CR3), which is blocked and immunoprecipitated by monoclonal antibody OKM10. During DMSO-driven differentiation of HL-60 cells, we find that CR3 is induced on all cells, whereas Fc gamma Rlo is induced on only 24% of cells, suggesting that CR3 appears earlier during differentiation than Fc gamma Rlo does.
Full text
PDF

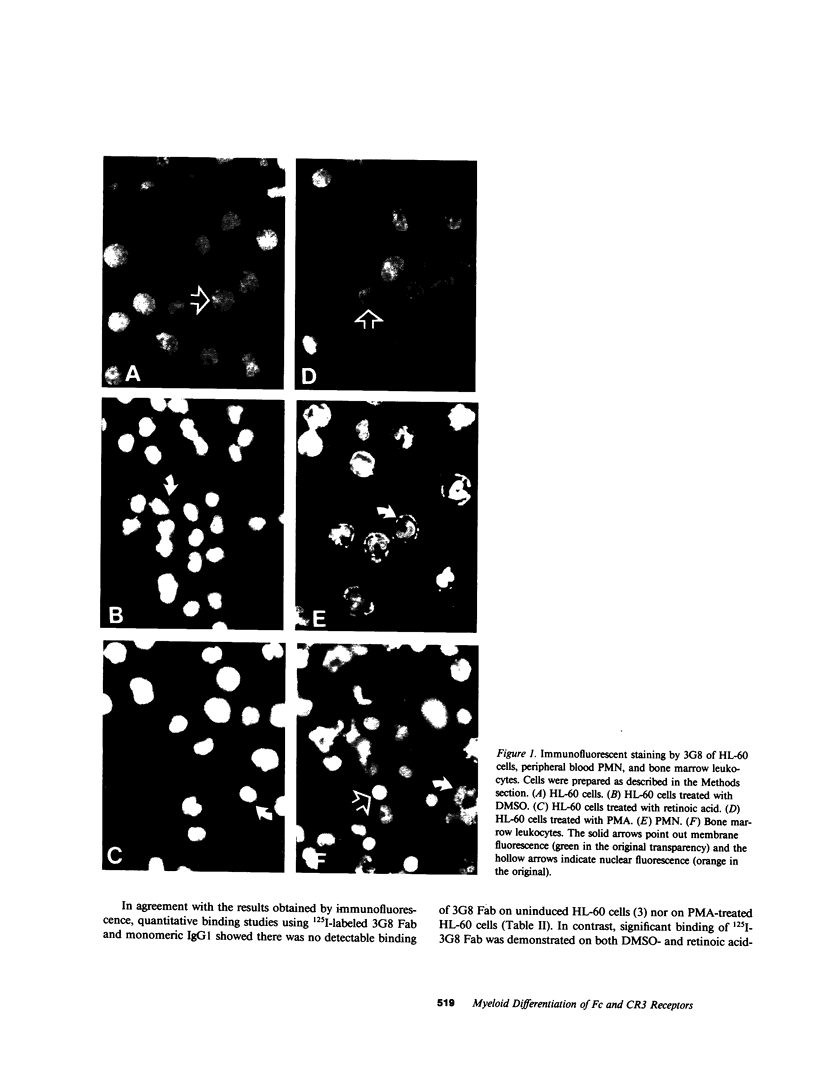
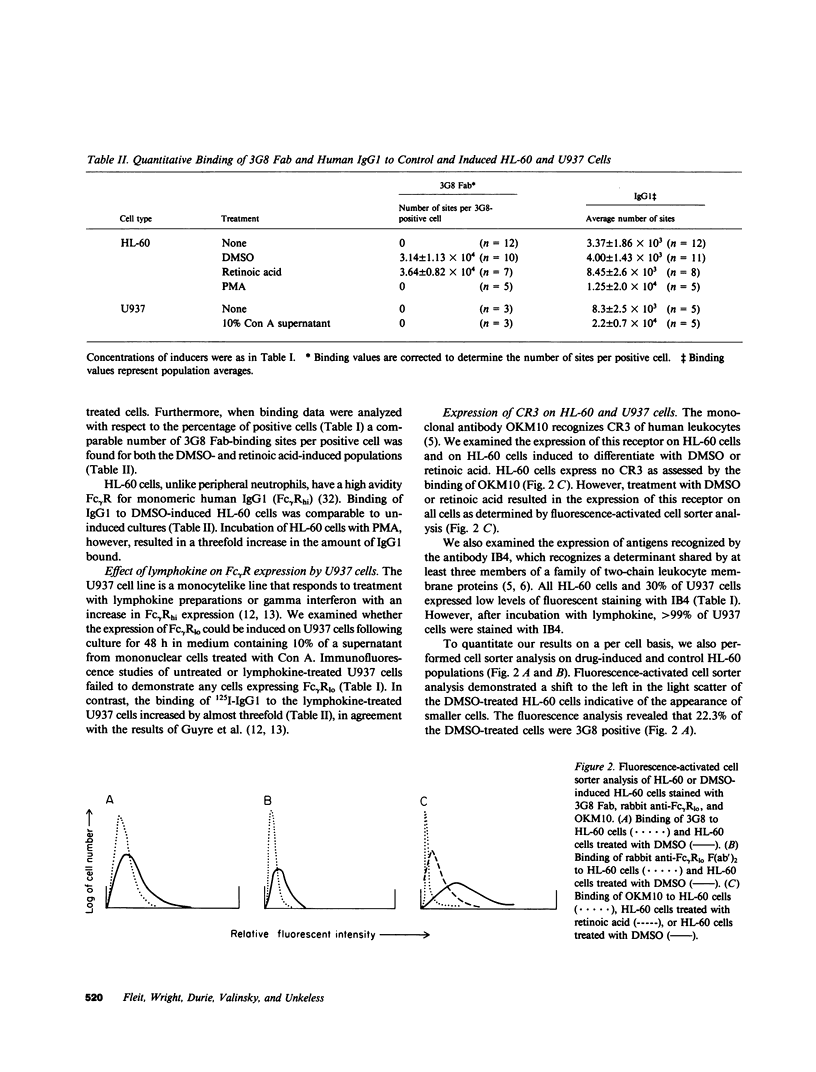
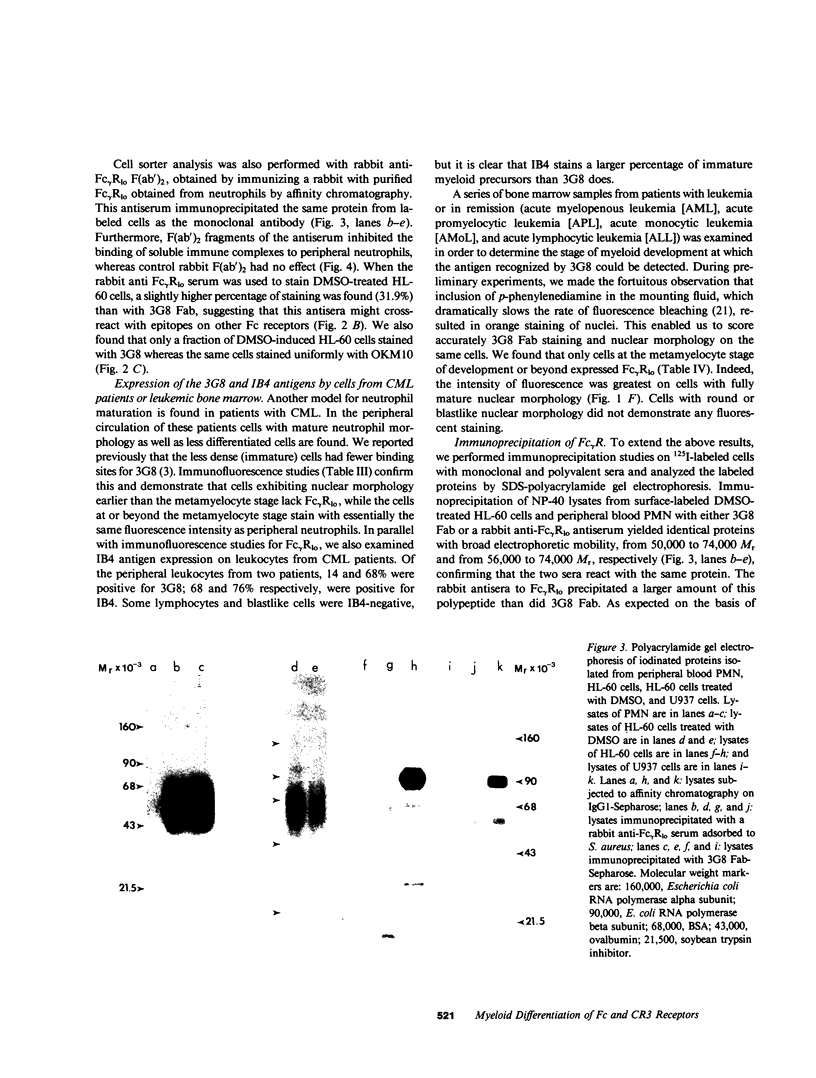
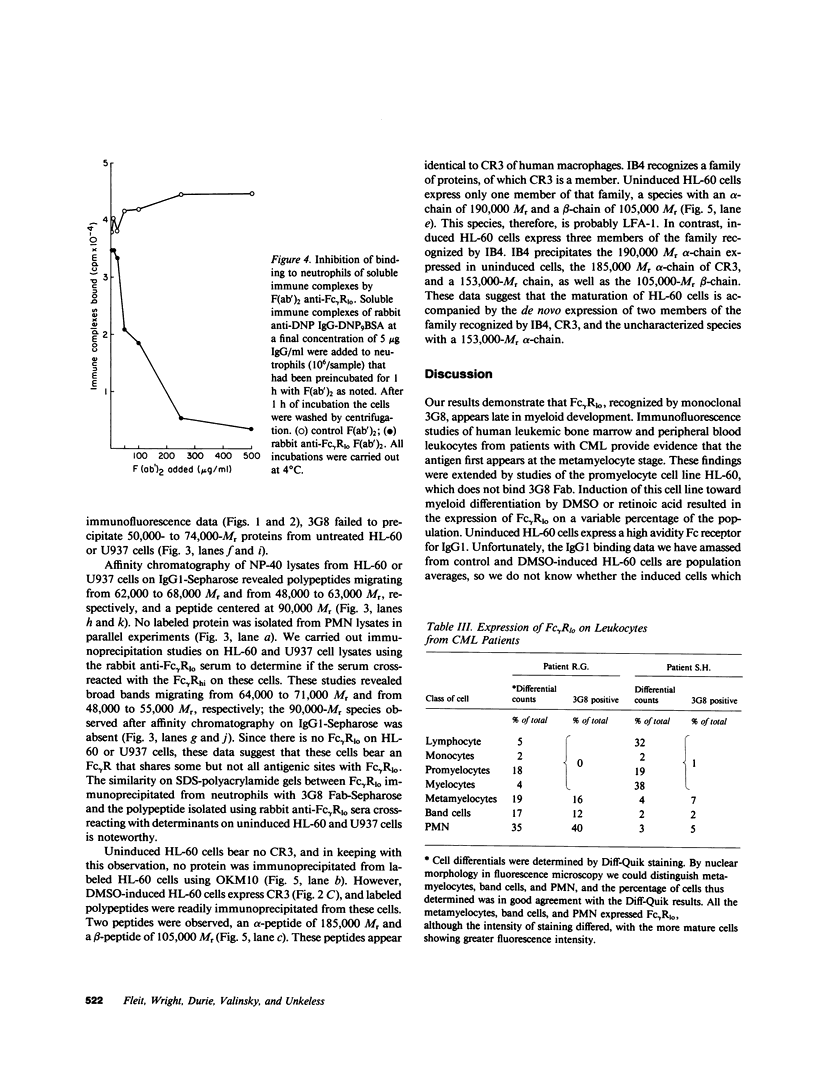
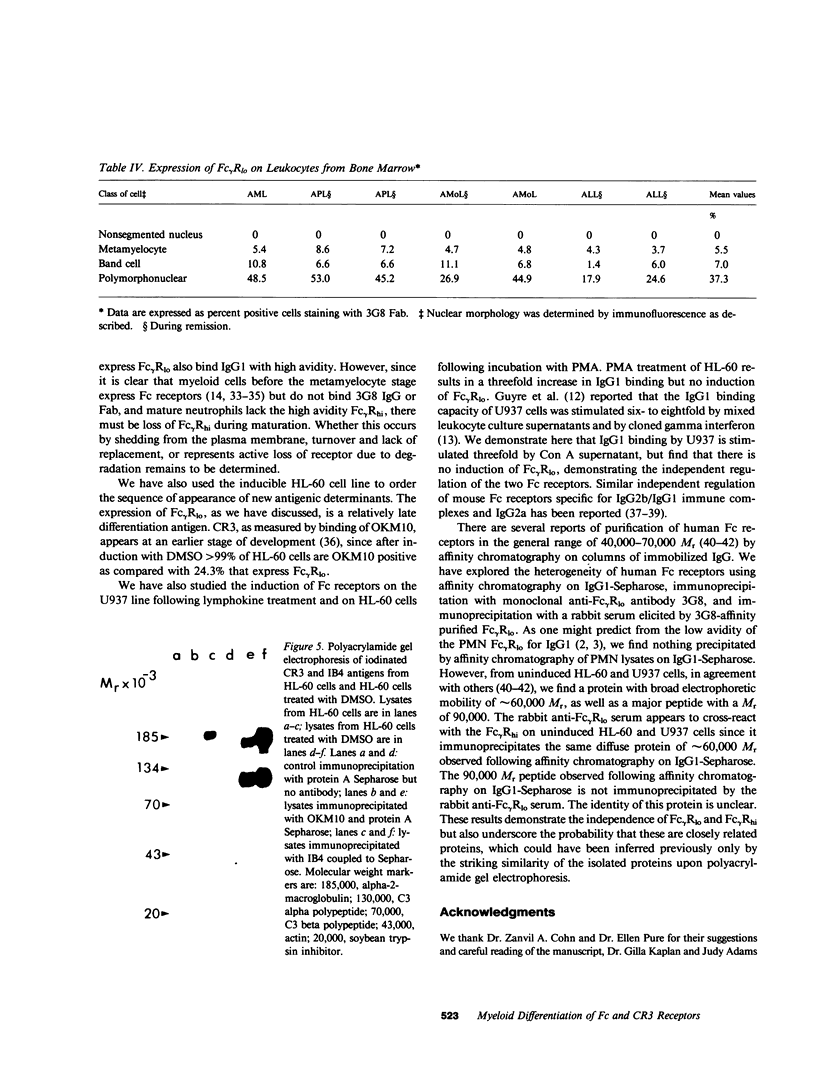


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L. Isolation of the receptor for IgG from a human monocyte cell line (U937) and from human peripheral blood monocytes. J Exp Med. 1982 Dec 1;156(6):1794–1806. doi: 10.1084/jem.156.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S. G., Hansen K. S., Bainton D. F. Differentiation of cell surface receptors on normal human bone marrow myeloid precursors. Br J Haematol. 1981 Jul;48(3):491–500. doi: 10.1111/j.1365-2141.1981.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Berman J. W., Basch R. S. Amplification of the biotin-avidin immunmofluorescence technique. J Immunol Methods. 1980;36(3-4):335–338. doi: 10.1016/0022-1759(80)90138-6. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns G. F., Cawley J. C. Membrane receptors of human leukaemic myeloid cells: sequential expression of the gamma Fc receptor. Br J Haematol. 1979 Aug;42(4):499–505. doi: 10.1111/j.1365-2141.1979.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Cohen L., Sharp S., Kulczycki A., Jr Human monocytes, B lymphocytes, and non-B lymphocytes each have structurally unique Fc gamma receptors. J Immunol. 1983 Jul;131(1):378–383. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Fc receptors of a human promyelocytic leukemic cell line: evidence for two types of receptors defined by binding of the staphylococcal protein A-IgG1 complex. J Immunol. 1980 Jul;125(1):448–453. [PubMed] [Google Scholar]
- Ezekowitz R. A., Bampton M., Gordon S. Macrophage activation selectively enhances expression of Fc receptors for IgG2a. J Exp Med. 1983 Feb 1;157(2):807–812. doi: 10.1084/jem.157.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Crabtree G. R., Bodwell J. E., Munck A. MLC-conditioned media stimulate an increase in Fc receptors on human macrophages. J Immunol. 1981 Feb;126(2):666–668. [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Herborn H. A., Valdimarsson H., Wickramasinghe S. N. Development of human granulocyte and monocyte Fc receptors. Scand J Haematol. 1979 Apr 4;22(4):364–368. doi: 10.1111/j.1600-0609.1979.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Honma Y., Takenaga K., Kasukabe T., Hozumi M. Induction of differentiation of cultured human promyelocytic leukemia cells by retinoids. Biochem Biophys Res Commun. 1980 Jul 31;95(2):507–512. doi: 10.1016/0006-291x(80)90813-x. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Wadell G., Jacobsson P. A., Svensson L. Preparation of specific antisera against adenoviruses by affinity bead immunization (ABI). J Immunol Methods. 1979;26(2):141–149. doi: 10.1016/0022-1759(79)90077-2. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Unkeless J. C., Cohn Z. A. Insertion and turnover of macrophage plasma membrane proteins. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3824–3828. doi: 10.1073/pnas.76.8.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Solanki L., Cohen L. Isolation and partial characterization of Fc gamma-binding proteins of human leukocytes. J Clin Invest. 1981 Dec;68(6):1558–1565. doi: 10.1172/JCI110410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S., Unkeless J. C. Purificaton of a functional mouse Fc receptor through the use of a monoclonal antibody. J Exp Med. 1980 Oct 1;152(4):1048–1069. doi: 10.1084/jem.152.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Rabellino E. M., Ross G. D., Trang H. T., Williams N., Metcalf D. Membrane receptors of mouse leukocytes. II. Sequential expression of membrane receptors and phagocytic capacity during leukocyte differentiation. J Exp Med. 1978 Feb 1;147(2):434–445. doi: 10.1084/jem.147.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. S. Maturation-linked expression of the Fc (IgG) receptor on developing human bone marrow and peripheral blood granulocytes. Clin Exp Immunol. 1979 Nov;38(2):300–305. [PMC free article] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Healey G. A. Quantitation of proteins and internal antigen pools by a monoclonal sandwich radioimmune assay. J Immunol Methods. 1983;56(1):1–11. doi: 10.1016/0022-1759(83)90043-1. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O., Mellman I. S., Broeze R. J., Garcia-Blanco M., Lengyel P. Interferon action: effects of mouse alpha and beta interferons on rosette formation, phagocytosis, and surface-antigen expression of cells of the macrophage-type line RAW 309Cr.1. Cell Immunol. 1982 Oct;73(1):128–140. doi: 10.1016/0008-8749(82)90441-5. [DOI] [PubMed] [Google Scholar]