Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, clonal, hematopoietic stem cell disorder that manifests with a complement-mediated hemolytic anemia, bone marrow failure and a propensity for thrombosis. These patients experience both intra- and extravascular hemolysis in the context of underlying complement activation. Currently eculizumab effectively blocks the intravascular hemolysis PNH. There remains an unmet clinical need for a complement inhibitor with activity early in the complement cascade to block complement at the classical and alternative pathways. C1 esterase inhibitor (C1INH) is an endogenous human plasma protein that has broad inhibitory activity in the complement pathway through inhibition of the classical pathway by binding C1r and C1s and inhibits the mannose-binding lectin-associated serine proteases in the lectin pathway. In this study, we show that commercially available plasma derived C1INH prevents lysis induced by the alternative complement pathway, of PNH erythrocytes in human serum. Importantly, C1INH was able to block the accumulation of C3 degradation products on CD55 deficient erythrocytes from PNH patient on eculizumab therapy. This could suggest a role for inhibition of earlier phases of the complement cascade than that currently inhibited by eculizumab for incomplete or non-responders to that therapy.
Keywords: PNH, bone marrow failure, C3 blockade, complement, C1 esterase inhibitor
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a stem cell disorder that manifests with a complement-mediated hemolytic anemia, marrow failure and thrombosis [1-3]. Chronic hemolytic anemia in PNH is largely mediated by the alternative pathway of complement (APC). [2]PNH cells are deficient in glycosylphosphatidylinositol (GPI) anchored proteins including the complement regulatory proteins CD55 and CD59. [4]. CD55 regulates the formation and stability of the C3 and C5 convertases [1], whereas, CD59 blocks the formation of the membrane attack complex (MAC)[5] [2].
Eculizumab is an FDA-approved humanized monoclonal antibody that binds to C5. The drug decreases intravascular hemolysis, reduces thrombosis risk, and improves quality of life in PNH [6, 7] through inhibiting formation of the MAC [8]. Eculizumab compensates for the CD59 deficiency on PNH erythrocytes, but not the CD55 deficiency. Thus, PNH patients on eculizumab accumulate C3 fragments on their CD55 deficient red cells leading to extravascular hemolysis through the accumulation of opsonins that are recognized by the reticuloendothelial system [9]. Laboratory evidence of extravascular hemolysis in eculizumab-containing patients includes reticulocytosis, persistent anemia, and often direct Coombs test positive for C3 deposition. These patients may remain asymptomatic, but others have symptomatic anemia and remain dependent on transfusions. [10]. Thus, there is need for a complement inhibitor that reduces C3 accumulation on PNH erythrocytes.
C1 esterase inhibitor (C1INH) is an endogenous human plasma protein in the family of serine protease inhibitors (SERPINs) and it has broad inhibitory activity in the complement and coagulation pathways. C1INH inhibits the classical pathway of complement by binding C1r and C1s and inhibits the mannose-binding lectin-associated serine proteases in the lectin pathway.[11, 12] Thus, C1INH could be a therapeutic for diseases of the classical complement pathway and of the lectin pathway. In fact, plasma derived formulations of C1INH (Berinert, CSL Bering; Cetor, Sanquin, NL) have been evaluated for their clinical utility in pilot studies of sepsis, ischemia-reperfusion injury and capillary leak [13-16].
One proof of concept study investigating the role of C1INH for preventing hemolysis in PNH erythrocytes ex vivo showed that a commercially manufactured plasma derived C1INH (Baxter), further purified and concentrated by the investigators, inhibited PNH cell lysis by the APC and appeared to do so by inhibiting C3 and factor B binding to erythrocytes as well as inhibiting factor B and C3 cleavage[17]. A nanofiltered plasma derived C1INH (Cinryze®; ViroPharma) is FDA approved for routine prophylaxis against angioedema attacks in adolescent and adult patients with hereditary angioedema (HAE), a disease characterized by constitutional deficiency or dysfunction of endogenous C1 esterase inhibitor. Here we demonstrate that Cinryze (C1INH) inhibits C3 deposition fragments and the APC on PNH erythrocytes treated with eculizumab.
Material and Methods
Blood Samples
Peripheral blood of all patients was obtained by protocols approved by the Johns Hopkins institutional review board. PNH type III erythrocytes were stained with anti-CD55 defined as the percentage of CD55 deficient erythrocytes in whole blood and analyzed by flow cytometry using FlowJo software (www.treestar.com)[18, 19]. Patients were ages 18 years or older with a PNH type III erythrocyte proportion >5%. Clinical parameters for hemolysis were noted at the time of the sampling. To obtain eculizumab-containing serum, 20cc of peripheral blood was obtained from an atypical hemolytic uremic syndrome (aHUS) patient 30 minutes after receiving 1200mg of eculizumab, intravenously. The eculizumab-containing serum was stored at -80°C for all experiments to demonstrate C3 deposition.
C1 Esterase Inhibitor and Antibodies
Commercial vials of Cinryze® [plasma derived C1 esterase inhibitor (human)], or C1INH, were used for C1 inhibition assays ex vivo. Vials were reconstituted with distilled water (100U/ml) following the manufacturer's instructions. Serial dilutions of C1INH were prepared for dose response curves. PNH erythrocytes from the patients were incubated with either acidified human normal serum (aHNS, pH 6.4) or acidified eculizumab human serum (aEcuHS, pH 6.4) with or without C1INH. PNH erythrocytes from the patients with heat inactivated, acidified human serum (aNHS[H]) and acidified eculizumab-containing human serum (aEcuHS[H]) were used as baselines.
To identify the PNH erythrocyte population, the pellets were resuspended and stained with PE-conjugated anti-human CD55 antibody (clone: JS11, Cat. 311308, Biolegend), FITC-conjugated anti-human C3/C3b/iC3b antibody (clone: 7C12, Cedarlane Labs), and APC-conjugated anti-human CD235 (BD Biosciences). C3 deposition assays were performed by flow cytometry (BD LSRII BD Biosciences) using FlowJo software.
Hemolysis experiments for PNH Erythrocytes
PNH erythrocytes were centrifuged, the buffy coat was aspirated, and the cells were thoroughly washed 3 times with phosphate buffered saline (PBS). The PNH erythrocytes were then re-suspended in gelatin veronal buffer (GVB). The PNH erythrocytes were prepared to a hematocrit of 20% and kept at 4°C for no more than 2 weeks. [20]. Tests for the susceptibility of erythrocytes to APC-mediated lysis followed previously described methods.[20] Briefly, the PNH erythrocytes were washed with the GVB saline (pH 7.4), incubated at a final hematocrit of 20% and then diluted (1:3) with either acidified human sera (Type AB) or the eculizumab-containing serum from the aHUS patient. To estimate dose response ex vivo, C1INH of 0 U/mL, 3 U/mL, 6 U/mL, 9 U/mL and 12 U/ mL were added and incubated at 37°C for 1 hour. After 1 hour incubation, the erythrocytes were pelleted by centrifugation at 1500 rpm for 5 minutes and 50-100 μl of supernatant was collected and measured at 415 nm using iMarktm Microplate reader (Bio-Rad). The percentage hemolysis was normalized and calculated based on 0% lysis (GVB only, pH 6.4) and 100% lysis (in distilled water).
C3 Inhibition
Flow cytometry was used to analyze deposition of C3 activation fragments on intact and lysed PNH erythrocytes (ghosts) from the patients. After 1 hour incubation with acidified human sera, GVB-EDTA was added to the erythrocytes to stop complement activity.
The pellets of ghosts and intact erythrocytes were collected by centrifugation at 1500 rpm for 5 minutes and washed three times with PBS (pH 7.4) and re-suspended in 0.5% BSA/PBS. The erythrocytes were stained with both FITC-conjugated anti-C3/C3b/iC3b antibody (1:10) and PE-conjugated anti-CD55 antibody (1:20) and C3 deposition was analyzed by flow cytometry as described above.
Results and Discussion
The clinical information for each patient is found in Table 1. These baseline data are presented to show persistent extravascular hemolysis despite long-term and ongoing inactivation of complement in patients receiving eculizumab therapy. Additionally, the data from Patient 6, who is not currently on eculizumab therapy, is shown.
Table 1. Clinical Data on Patient Samples.
| Patient Number | Age Sex | Erythrocyte Clone Size* (%) | Granulocyte Clone Size* (%) | LDH (U/L) | Hemoglobin (g/dL) | Direct CoombsC3 | Direct CoombsIgG | Absolute Reticulocyte Count (K/ cu mm) | Months on Eculizumab at q14days dosing | Days since last dose |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 F | Type II: 53 Type III: 46 TOTAL: 99 |
98 | 308 | 10.7 | Positive | Negative | 242 | 90 | 14 |
| 2 | 55 M | Type II: 0.8 Type III: 98.7 TOTAL: 99.5 |
98.7 | 318 | 10.6† | Positive | Negative | 397 | 90 | 13 |
| 3 | 49 F | Type II: 4.7 Type III: 71 TOTAL: 75.7 |
96 | 301 | 10.1† | Positive | Negative | 243 | 23 | 14 |
| 4 | 18 F | Type II: 8.7 Type III: 13 TOTAL: 21.7 |
92 | 326 | 13.2† | Positive | Negative | 103 | 4 | 11 |
| 5 | 35 M | Type II: 2 Type III: 5.9 TOTAL: 7.9 |
100 | 312 | 15.9 | Positive | Negative | 145 | 55 | 14 |
| 6 | 37 M | Type II: 15 Type III: 29 TOTAL: 43 |
87 | 885 | 11.9 | Negative | Negative | 127 | N/A | N/A |
Clinical information from patients whose PNH erythrocytes were studied.
Patients 1-5 were on therapy with eculizumab for their disease at the time of study.
Patient 6 was not on eculizumab therapy for PNH.
As measured in CLIA certified lab for clinical use
Transfused in past year
C1 inhibition is concentration dependent
To investigate whether C1INH prevents complement-mediated hemolysis of PNH erythrocytes (from patients receiving eculizumab therapy) in a concentration dependent manner, the erythrocytes of patient 1 were treated with serial concentrations of C1INH (as described above in the methods) in the presence of both acidified normal serum (aNHS) and heat-inactivated acidified normal serum (aNHS[H]) . Figure 1A shows that, in the presence of the APC activation by aNHS compared to aNHS[H], the hemolysis of PNH erythrocytes is attenuated as the concentration of C1INH is increased in the sample. A concentration of C1INH at 6U/mL prevented hemolysis nearly to the level of aNHS[H] baseline. To further determine if the PNH erythrocyte percentage (amount of Type II versus Type III cells) affected the amount of lysis or the effect of C1INH, the PNH erythrocytes from patients 2-5 were co-incubated in aNHS and aNHS[H] with 0, 3, and 6Us/ml of C1INH. The results are presented in Figure 1B. This also demonstrates that the hemolysis is blocked in a concentration dependent fashion, regardless of the percentage of Type II or Type III erythrocytes present in the sample. We next tested whether C1INH would block hemolysis in PNH patient 6 who was not receiving therapy with eculizumab. Similarly to the patients on eculizumab therapy, C1INH prevented hemolysis in vitro in a dose dependent manner (Figure 1C).
Figure 1. C1 Inhibition blocks APC-mediated Hemolysis in PNH Erythrocytes.
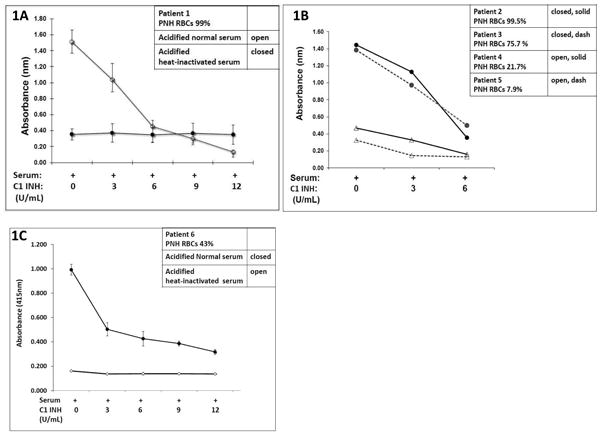
A. The erythrocytes of patient 1 were incubated for 1 hour at 37° C with acidified (pH 6.4) normal human serum(aNHS) (1:3) and acidified heat-inactivated serum (aNHS[H])(1:3) containing increasing concentrations of C1INH (0-12 Units/mL). Hemolysis was measured using the concentration of supernatant hemoglobin (determined by spectrophotometry at 415 nm).
B. The erythrocytes of patients 2-5 (treated with eculizumab) were incubated as above with increasing concentrations of C1INH (0-6 Units/mL). Hemolysis was measured again by spectrophotometry at 415 nm.
C. The erythrocytes of patient 6 (not treated with eculizumab) were incubated as above at increasing concentrations of C1INH (0-12 Units/mL) and hemolysis measured.
CI Inhibition blocks APC-mediated deposition of C3 Fragments in Patients on Eculizumab Therapy
It has been demonstrated that patients with PNH on therapy with eculizumab develop extravascular hemolysis because the drug blocks terminal complement, thus allowing the CD55/CD59-deficient cells to become opsonized with activation and degradation products of C3 as a consequence of unrestricted activation of the APC [9, 21]. To investigate whether C1INH can protect cells from this C3 deposition, we exposed PNH erythrocytes to aEcuHS, aEcuHS[H], and aEcuHS + C1 (6U/mL) and assayed for deposition of C3 fragments. PNH patients 2 & 6 are both CD55 deficient with only patient 2 currently receiving eculizumab therapy. C3 fragment deposition on the PNH erythrocytes was negligible in aEcuHS(H) (Figure 2, Top). The amount of C3 fragment deposition on the erythrocytes was increased (12.0 and 16.8%) in both subjects after incubation in aEcuHS (Figure 2, Middle). However, co-incubation of C1INH with the acidified, eculizumab-containing serum (aEcuHS + C1), C3 fragment deposition on the CD55 deficient erythrocytes was markedly attenuated (3.7% and 4.1%) (Figure 2, Lower).
Figure 2. C1 Inhibition blocks APC-mediated C3 Fragment Deposition on PNH Erythrocytes.

C3 fragment deposition was analyzed by flow cytometry LSRII (BD Biosciences) in Patient 2 (on eculizumab therapy) and Patient 6 (not on eculizumab therapy).
PNH erythrocytes were incubated at 37°C for 1 hour with heat-inactivated eculizumab-containing serum, pH 6.4 (aEcuHS[H], Top), activated (pH 6.4) eculizumab-containing serum (aEcuHS, Middle), and activated (pH 6.4) eculizumab-containing serum plus C1INH at 6U/mL (aEcuHS + C1INH, Lower).
After incubation for 1 hour at 37°C, C3 deposition and CD55 were assayed by staining with FITC-conjugated anti-C3/C3b/iC3b antibody (C3-FITC) and PE-conjugated anti-CD55 antibody (CD55-PE).
C3 fragment deposition on the PNH erythrocytes was negligible in aEcuHS(H) (Top). The amount of C3 fragment deposition on the erythrocytes was increased (12.0 and 16.8%) in all subjects after incubation in aEcuHS (Middle). However, co-incubation of C1INH with the acidified, eculizumab-containing serum markedly attenuated (3.7% and 4.1%) C3 fragment deposition on the CD55 deficient erythrocytes (aEcuHS + C1) (Lower).
Supplemental Figure 3S shows the percent hemolysis information for patients 2 and 6 using normal human serum (aNHS) as well as eculizumab-containing serum (aEcuHS). These data demonstrate near-complete hemolysis of the PNH red cells in activated normal human serum (aNHS) that is attenuated by heat inactivation, eculizumab-containing serum (aEcuHS) and additional C1INH .
We also measured C3 fragment deposition at baseline (T=0), after activation in eculizumab-containing serum and after the addition of C1INH in patients 2, 3, and 4. Supplemental Figure 4S confirms that C1INH inhibits the alternative pathway of complement by blocking the accumulation of C3 fragments on PNH erythrocytes.
In this study, we show that the commercially available plasma derived C1INH (Cinryze®) prevents PNH erythrocyte lysis induced by the alternative pathway of complement. Importantly, C1INH was able to block the accumulation of C3 degradation products on CD55 deficient erythrocytes from PNH patients on therapy with eculizumab. This activation of C3 to C3b on the surface of erythrocytes accounts for the immune-mediated, extravascular hemolysis that develops in patients on eculizumab therapy. This is clinically significant in patients treated with eculizumab who fail to achieve transfusion independence [10, 22, 23]. We and others have previously shown that breakthrough extravascular hemolysis can leave patients with only a partial response while on therapy with eculizumab [10, 22, 24]. Patients who do not respond to eculizumab therapy could theoretically respond to a C1 esterase inhibitor, either alone or in combination with C5 blockade. We hypothesize that a compound which targets the classical and the mannose complement pathways may be effective in APC-mediated hemolysis of PNH. The theory is that C1INH interacts with C3b to inhibit binding of factor B to C3b. At physiologic concentrations, this could down-regulate the activity of the alternative pathway. This could implicate a role for inhibition of earlier phases of the complement cascade than that currently inhibited by eculizumab for incomplete or non-responders to therapy with C5 blockade. A clinical trial to explore this hypothesis in vivo with patients who are suboptimal responders could be considered.
Supplementary Material
Supplemental Figure 3S: Percent Lysis Demonstration
PNH erythrocytes were incubated with acidified normal human serum without (aNHS) and with C1INH (aNHS + C1INH); heat-inactivated normal human serum (aNHS[H]); activated (pH 6.4) eculizumab-containing serum without (aEcuHS) and with C1INH (aEcuHS + C1INH) and heat-inactivated eculizumab-containing serum (pH 6.4) (aEcuHS[H]).
After 1 hour incubation, the supernatants were collected and absorbance of hemoglobin measured at 415nm by iMark microreader (Bio-Rad).
The percentages of hemolysis were normalized based on 0% lysis (GVB, PH 6.4 only) and 100% lysis (with water).
(a). The percentage of hemolysis of patient 2 and 6 were the highest lysis in aNHS (97.6 ± 1.0 and 85.7 ± 0.8). There was no hemolysis in aNHS (H) (6.5 ± 0.1 and 6.5 ± 0.1) and in aNHS + C1INH (3.8 ± 0.4 and 1.5 ± 0.1).
(b). Hemolysis was decreased when the erythrocytes were treated with EcuHS (11.7 ± 1.2 and 7.5 ± 1.0) compared to aNHS treatment; there were baseline hemolysis in aEcuHS + C1 (14.7 ± 1.1 and 9.2 ± 0.1); and aEcuHS (H) (16.2 ± 0.9 and 5.7 ± 0.1).
Error bars are standard deviations of three separate experiments.
Supplemental Figure 4S: C1 Inhibition blocks APC-mediated C3 Deposition in PNH Erythrocytes
C3 fragment deposition was analyzed by flow cytometry LSRII (BD Biosciences) in Patients 2-4 (on eculizumab therapy).
At baseline time 0, C3 fragment deposition baseline demonstrated on the PNH erythrocytes (CD55 deficient) using activated eculizumab- human serum. (aEcuHS, Top)
At time 1 hour, C3 fragment deposition was increased in all 3 subjects after incubation with 1:3 dilution of acidified (pH 6.4) eculizumab-containing human serum (aEcuHS, Middle). Then, C3 fragment deposition was decreased in all 3 subjects after co-incubation for 1 hour at 37° C with acidified (pH 6.4) eculizumab-containing human serum (1:2) and C1INH (6U/mL). (aEcuHS +C1, Lower)
At baseline (T=0) and after incubation for 1 hour at 37°C, C3 deposition and CD55 were assayed by staining with FITC-conjugated anti-C3/C3b/iC3b antibody (C3-FITC) and PE-conjugated anti-CD55 antibody (CD55-PE).
Acknowledgments
This study was sponsored by ViroPharma. The authors thank the patients who agreed to participate through the use of their blood for evaluation in this study.
Footnotes
Conflict of interest: Marc Uknis is Director of Clinical Research at ViroPharma.
JoAnne Saye is Director of Pre-Clinical Research at ViroPharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine. 1997;76(2):63–93. doi: 10.1097/00005792-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148(8):587–595. doi: 10.7326/0003-4819-148-8-200804150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Miyata T, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. The New England Journal of Medicine. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 5.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144(9):3478–3483. [PubMed] [Google Scholar]
- 6.Hillmen P, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007 doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky RA, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–7. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 8.Rother RP, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 9.Risitano AM, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009 doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 10.DeZern AE, Dorr D, Brodsky RA. Predictors of hemoglobin response to eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Eur J Haematol. 2013;90(1):16–24. doi: 10.1111/ejh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai S, et al. A direct role for C1INHibitor in regulation of leukocyte adhesion. J Immunol. 2005;174(10):6462–6. doi: 10.4049/jimmunol.174.10.6462. [DOI] [PubMed] [Google Scholar]
- 12.Beinrohr L, et al. C1INHibitor serpin domain structure reveals the likely mechanism of heparin potentiation and conformational disease. J Biol Chem. 2007;282(29):21100–9. doi: 10.1074/jbc.M700841200. [DOI] [PubMed] [Google Scholar]
- 13.Caliezi C, et al. C1INHibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit Care Med. 2002;30(8):1722–8. doi: 10.1097/00003246-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Hack CE, et al. Initial studies on the administration of C1-esterase inhibitor to patients with septic shock or with a vascular leak syndrome induced by interleukin-2 therapy. Prog Clin Biol Res. 1994;388:335–57. [PubMed] [Google Scholar]
- 15.Nurnberger W, et al. C1 esterase inhibitor concentrate for capillary leakage syndrome following bone marrow transplantation. Ann Hematol. 1997;75(3):95–101. doi: 10.1007/s002770050321. [DOI] [PubMed] [Google Scholar]
- 16.de Zwaan C, et al. Continuous 48-h C1INHibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. Eur Heart J. 2002;23(21):1670–7. doi: 10.1053/euhj.2002.3191. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, et al. Complement 1 inhibitor is a regulator of the alternative complement pathway. J Exp Med. 2001;194(11):1609–16. doi: 10.1084/jem.194.11.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodsky RA, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114(3):459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borowitz MJ, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78(4):211–230. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox LA, et al. Molecular basis of the enhanced susceptibility of the erythrocytes of paroxysmal nocturnal hemoglobinuria to hemolysis in acidified serum. Blood. 1991;78(3):820–829. [PubMed] [Google Scholar]
- 21.Lindorfer MA, et al. A novel approach to preventing the hemolysis of paroxysmal nocturnal hemoglobinuria: both complement-mediated cytolysis and C3 deposition are blocked by a monoclonal antibody specific for the alternative pathway of complement. Blood. 2010;115(11):2283–91. doi: 10.1182/blood-2009-09-244285. [DOI] [PubMed] [Google Scholar]
- 22.Hillmen P, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013 doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillmen P, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85(8):553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 24.Risitano AM, et al. Paroxysmal nocturnal hemoglobinuria--hemolysis before and after eculizumab. N Engl J Med. 2010;363(23):2270–2272. doi: 10.1056/NEJMc1010351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 3S: Percent Lysis Demonstration
PNH erythrocytes were incubated with acidified normal human serum without (aNHS) and with C1INH (aNHS + C1INH); heat-inactivated normal human serum (aNHS[H]); activated (pH 6.4) eculizumab-containing serum without (aEcuHS) and with C1INH (aEcuHS + C1INH) and heat-inactivated eculizumab-containing serum (pH 6.4) (aEcuHS[H]).
After 1 hour incubation, the supernatants were collected and absorbance of hemoglobin measured at 415nm by iMark microreader (Bio-Rad).
The percentages of hemolysis were normalized based on 0% lysis (GVB, PH 6.4 only) and 100% lysis (with water).
(a). The percentage of hemolysis of patient 2 and 6 were the highest lysis in aNHS (97.6 ± 1.0 and 85.7 ± 0.8). There was no hemolysis in aNHS (H) (6.5 ± 0.1 and 6.5 ± 0.1) and in aNHS + C1INH (3.8 ± 0.4 and 1.5 ± 0.1).
(b). Hemolysis was decreased when the erythrocytes were treated with EcuHS (11.7 ± 1.2 and 7.5 ± 1.0) compared to aNHS treatment; there were baseline hemolysis in aEcuHS + C1 (14.7 ± 1.1 and 9.2 ± 0.1); and aEcuHS (H) (16.2 ± 0.9 and 5.7 ± 0.1).
Error bars are standard deviations of three separate experiments.
Supplemental Figure 4S: C1 Inhibition blocks APC-mediated C3 Deposition in PNH Erythrocytes
C3 fragment deposition was analyzed by flow cytometry LSRII (BD Biosciences) in Patients 2-4 (on eculizumab therapy).
At baseline time 0, C3 fragment deposition baseline demonstrated on the PNH erythrocytes (CD55 deficient) using activated eculizumab- human serum. (aEcuHS, Top)
At time 1 hour, C3 fragment deposition was increased in all 3 subjects after incubation with 1:3 dilution of acidified (pH 6.4) eculizumab-containing human serum (aEcuHS, Middle). Then, C3 fragment deposition was decreased in all 3 subjects after co-incubation for 1 hour at 37° C with acidified (pH 6.4) eculizumab-containing human serum (1:2) and C1INH (6U/mL). (aEcuHS +C1, Lower)
At baseline (T=0) and after incubation for 1 hour at 37°C, C3 deposition and CD55 were assayed by staining with FITC-conjugated anti-C3/C3b/iC3b antibody (C3-FITC) and PE-conjugated anti-CD55 antibody (CD55-PE).


