Abstract
Purpose
To determine the intra- and inter-visit reproducibility of ganglion cell-inner plexiform layer thickness measures using handheld optical coherence tomography (OCT) in sedated children with optic pathway gliomas and/or Neurofibromatosis type 1 (NF1).
Design
Prospective longitudinal cohort study
Methods
Children with sporadic optic pathway gliomas and/or NF1 who had ≥ 2 volumes acquired over the macula using handheld OCT during sedation for a clinically indicated MRI were eligible for the intra-visit cohort. Children with repeat handheld OCT imaging within 6 months were eligible for the inter-visit cohort. Total retinal thickness and ganglion cell-inner plexiform layer thickness were measured using custom designed automated segmentation software. Reproducibility was compared across average and anatomic quadrant by calculating the coefficient of variation (CV) and intraclass correlation coefficient (ICC).
Results
Forty-two subjects (median age 5.4 years, range 0.8–12.7 years) contributed 45 eyes to the intra-visit cohort. Thirty-one subject eyes had normal vision and 14 had abnormal vision (decreased visual acuity and/or visual field). Average and quadrant ganglion cell-inner plexiform layer measures demonstrated CVs ≤ 4.5% with excellent ICCs (> .935). The superior quadrant CV differed between subjects with (4.4%) and without (2.1%) vision loss (P < 0.05). Twenty-five subject eyes were eligible for the inter-visit cohort, demonstrating CVs from 1.6% to 5.2%. Inter-visit ICCs were excellent (.955 – .995).
Discussion
Handheld OCT imaging in sedated children with optic pathway gliomas produces highly reproducible measures of ganglion cell-inner plexiform layer thickness.
INTRODUCTION
Optic pathway gliomas, a relatively common tumor of the anterior visual pathway in children, require surveillance and treatment most frequently between 1 and 6 years of age.1,2 Since change in tumor size is not well correlated with visual outcomes, a decline in visual acuity (VA) and or visual field (VF) are the primary indications to initiate or alter treatment.2 Due to their young age and comorbid medical conditions, children with optic pathway gliomas are frequently unable to complete standardized VA and or VF testing. 3
Recent studies have demonstrated that spectral domain optical coherence tomography (OCT) measures of the circumpapillary retinal nerve fiber layer (RNFL) thickness and ganglion cell-inner plexiform layer thickness are correlated with the magnitude of vision loss and could potentially serve as an objective biomarker of vision in children with optic pathway gliomas.4,5 For young children who cannot cooperate with traditional table-mounted devices, a handheld OCT can image young children during sedation.4–6
To date, no studies using handheld OCT have examined the intra- and inter-visit reproducibility of quantitative ganglion cell-inner plexiform layer measures. Establishing the intra- and inter-visit variance is essential to determining how much decline in ganglion cell-inner plexiform layer thickness represents a clinically significant change. We investigated the intra- and inter-visit reproducibility of handheld OCT ganglion cell-inner plexiform layer measurements in sedated children being evaluated for optic pathway gliomas.
METHODS
Subjects
Children evaluated in the Neuro-Ophthalmology, Ophthalmology or Neuro-Oncology clinics at Children’s National Medical Center were recruited to participate in a prospective longitudinal cohort study of handheld OCT. Written informed consent from the parent/guardian and written assent from the child (when applicable) was obtained before study enrollment. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Children’s National Medical Center. All data collected was HIPPA compliant.
Subjects were eligible for recruitment if they were scheduled to have a sedated MRI for their currently diagnosed optic pathway glioma and or neurofibromatosis type 1 (NF1). Subjects that did not have a clinical indication for a sedated MRI were not enrolled in the study. All subjects underwent a comprehensive ophthalmologic exam at time of enrollment and at subsequent study visits. All subjects were required to complete quantitative VA testing at each visit (i.e., qualitative measures such as fix and follow were not permitted). VA testing in preverbal children was performed using Teller acuity cards (also known as grating acuity), while older children completed age-appropriate recognition acuity tasks.7–9 Vision loss was defined as VA ≥ 0.2 logMAR above age-based norms, and or visual field (VF) loss. All subjects, based on their age and ability to cooperate, had their VF assessed by either confrontation, automated or kinetic perimetry techniques. In each eye, VF loss was defined as any appreciable defect in one or more quadrants. Subjects with decreased vision secondary to amblyopia or glaucoma, or with a past history of papilledema were not eligible for study enrollment.
A minimum of two acceptable handheld OCT macula scans acquired during a single imaging session were required for enrollment in the intra-visit cohort. Scans with motion artifact, image vignetting or low signal quality were eliminated from analysis. Subjects that underwent a second imaging session within 6 months were eligible for the inter-visit cohort analysis as long as they didn’t demonstrate any of the following compared to their initial visit; 1) progressive VA loss defined as ≥ 0.2 logMAR change; 2) new or progressive VF loss in any quadrant; and 3) new contrast enhancement or any increase in tumor size on their MRI.
Image Acquisition with the Handheld Optical Coherence Tomography
Handheld OCT acquisition was identical to the previously published protocol.4 One hour before being sedated for the MRI, mydriatic eye drops were instilled. Once sedated, handheld OCT imaging commenced using a high resolution hand-held device acquiring 36,000 A-scans per second (Bioptigen, Durham, NC). 6 × 6 × 2 mm volume scans centered on the foveola using 300 A-scans across 300 B-scans were acquired.
Handheld Optical Coherence Tomography Image Analysis
The OCT macular volume image data were segmented using an automated custom-made software program.10 For each volume image data, the foveola position was manually selected by looking for the largest separation between the junction of the inner and outer segments of the photoreceptors and retinal pigment epithelium as appearing on the horizontal and vertical cross-sectional B-scans. The selected foveola position was then used as the center for thickness measurements within various regions: center (region within 1mm diameter circle centered on the foveola), temporal (0°–45° and 315°–360°), superior (45°–135°), nasal (135°–225°), inferior (225°–315°) quadrants (regions outside 1mm diameter circle and within 3mm diameter circle), and global average thickness (region within 3mm diameter circle centered on the foveola). Segmentation measured the total retinal thickness and ganglion cell-inner plexiform layer thickness (see Figure 1). A graphic description of the image analysis algorithm has previously been published.10 Algorithm errors were detected in 2 steps: (1) failed frames were defined as an obvious disruption of the detected border, and/or border wandering (detected border jumping to and from different anatomic structures) for larger than 15% consecutive or 20% cumulative of each horizontal frame of a given volume image data, and (2) failed analysis was defined as 15% consecutive or 20% cumulative “failed” frames within a given volume image data.
Figure 1.
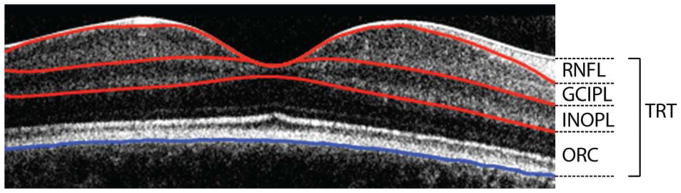
Handheld OCT scan through the fovea demonstrating the segmentation of the retinal nerve fiber layer (RNFL), ganglion cell – inner plexiform layer (GCIPL), inner-nuclear outer-plexiform layer, and outer retinal complex (ORC). All layers combine to calculate the total retinal thickness (TRT).
To assess signal quality, we used the previously described method to calculate the quality index (QI).11 Two histogram parameters, namely the intensity ratio, comparable to signal to noise ratio, and the tissue signal ratio, representative of the ratio of tissue signal pixels versus background noise, are both used to calculate the quality index.11
All data were de-identified, not including clinical information, and processed by the same investigator (C-LC). Image quality was as previously described and scans with a quality index value less than 20 were considered to be of poor image quality and discarded.11
Statistics
Demographic and clinical characteristics were summarized by standard descriptive statistics. The coefficient of variation (CV) and intraclass correlation coefficient (ICC, two-way mixed-effects model) was calculated for the global average and anatomic quadrant total retinal thickness and ganglion cell-inner plexiform layer thickness of the intra- and inter-visit cohorts. Since the number of scans acquired beyond the required 2 could vary, the two scans with the highest quality index were selected for the intra-visit ICC calculation. The average of all quality qualified scans from visit 1 and visit 2 were used to calculate the inter-visit ICC. Wilcoxon rank-sum test were used to compare CV between vision groups. Each subject with normal vision contributed only one eye which was determined by a random number generator. If the optic pathway glioma was isolated to the optic nerve, the contralateral and unaffected eye was not included in the analysis. Children with abnormal vision in both eyes could contribute 2 eyes to the analysis, but given the potential impact of the inter-eye correlation despite disparate patterns of vision loss in each eye, the intravisit CV analysis results were confirmed by repeating the analysis using only 1 subject eye. A post-hoc analysis using a multivariable linear regression model investigated the influence of patient age and diagnosis (optic pathway glioma secondary to NF1 versus sporadic optic pathway glioma) on CV measures. Data were analyzed using commercially available software (STATA, version 13; StataCorp, College Station, Texas).
RESULTS
Intra-visit Cohort
Thirty-one subjects contributed a single eye with normal vision and 11 subjects contributed 14 eyes with abnormal vision. The clinical and demographic features of the intra-visit cohort are listed in Table 1. One-hundred twenty-eight acquisitions met inclusion criteria and were included in the analysis. CV, ICC and ICC 95th percentile confidence of total retinal thickness and ganglion cell-inner plexiform layer thickness are listed in Table 2 for the intra-visit cohort. The CV of total retinal thickness was significantly different between vision groups (P < 0.05) only in the inferior quadrant, although it neared significance for the superior quadrant (z = 1.8, P = 0.069). Ganglion cell-inner plexiform layer CV was significantly different between groups only in the superior quadrant (P < 0.05), but also neared significance in the inferior quadrant (z = −1.9, P = 0.055). The CV between groups did not significantly change if the vision loss group restricted subjects to contributing only 1 eye (P > 0.05, all comparisons). All ICC values for the total retinal thickness and ganglion cell-inner plexiform layer were above 0.950 except for the temporal quadrant of the normal vision group (ICC = 0.936). Using multivariable linear regression, patient age and diagnosis (optic pathway glioma secondary to Neurofibromatosis type 1 versus sporadic optic pathway glioma) failed to demonstrate a statistically significant influence on CV.
Table 1.
Demographic and Clinical Characteristics of the Intra-visit Cohort Subjects with Optic Pathway Gliomas Undergoing Handheld Optical Coherence Tomography During Sedation.
| Characteristics | Abnormal Vision (N = 11) b | Normal Vision (N = 31) |
|---|---|---|
| Age (median)a | 5.3 | 5.4 |
| Range | 1.0 – 8.2 | 0.79 – 13.0 |
| Female sex, no. (%) | 7(64) | 20(65) |
| Race, no. (%) | ||
| Asian | 1 (9) | – |
| Black | 1 (9) | 5 (16) |
| Multiracial | – | 4 (13) |
| White | 9 (82) | 22 (71) |
| Diagnosis, no. (%) | ||
| NF1with optic pathway glioma | 4 (36) | 21 (68) |
| Sporadic optic pathway glioma | 7 (64) | 5 (16) |
| NF1 without optic pathway glioma | – | 5 (16) |
N = number; NF1 = Neurofibromatosis type 1.
Years.
Fourteen study eyes analyzed.
Table 2.
Intra-visit Reproducibility of Total Retinal Thickness and Ganglion Cell – Inner Plexiform Layer Thickness Measures Using Handheld Optical Coherence Tomography in Children with Optic Pathway Gliomas.
| Abnormal Vision (N = 14) | Normal Vision (N = 31) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| TRT | Thickness ± SD | CV (%) ± SD | ICC (95% CI) | Thickness ± SD | CV (%) ± SD | ICC (95% CI) |
| Average | 397±25 | 0.3±0.1 | .998 | 436±21 | 0.3±0.2 | .996 |
| .99, .99 | .99, .99 | |||||
| Temporal | 387±23 | 0.5±0.3 | .994 | 423±22 | 0.7±0.6 | .988 |
| .98, .99 | .97, .99 | |||||
| Superior | 399±23 | 0.6±0.3 | .993 | 441±20 | 0.5±0.3 | .991 |
| .98,.99 | .98, .99 | |||||
| Nasal | 397±30 | 0.3±0.2 | .997 | 437±22 | 0.5±0.4 | .990 |
| .99, .99 | .97, .99 | |||||
| Inferior a | 406±27 | 0.4±0.4 | .995 | 445±21 | 0.6±0.4 | .988 |
| .98, .99 | .97, .99 | |||||
|
| ||||||
|
GCIPL
| ||||||
| Average | 93±12 | 2.1±1.5 | .993 | 130±12 | 1.5±0.9 | .981 |
| .97, .99 | .96, .99 | |||||
| Temporal | 90±17 | 3.8±2.1 | .983 | 124±12 | 3.1±2.1 | .936 |
| .95, .99 | .86, .96 | |||||
| Superior a | 95±19 | 4.4±2.8 | .973 | 134±13 | 2.1±1.3 | .983 |
| .91, .99 | .96, .99 | |||||
| Nasal | 91±24 | 3.9±3.1 | .986 | 129±15 | 2.3±2.4 | .955 |
| .95, .99 | .90, .97 | |||||
| Inferior | 95±21 | 4.5±3.2 | .968 | 132±13 | 2.7±2.1 | .951 |
| .90, .98 | .90, .97 | |||||
N = number, TRT = total retinal thickness; GCIPL = ganglion cell layer-inner plexiform layer; CV = coefficient of variation; ICC = intraclass correlation coefficient; SD = standard deviation; CI = confidence interval.
P < 0.05.
Inter-visit Cohort
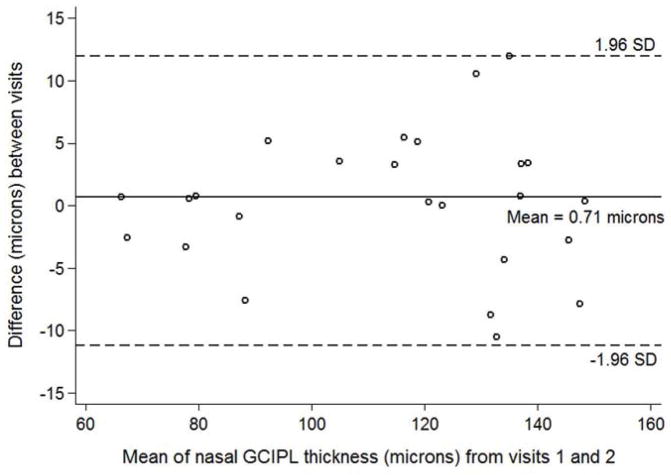
144 volumes from 25 eyes (21 unique subjects) comprised the inter-visit cohort. One subject from the intra-visit cohort was not eligible for inclusion due to progressive vision loss and tumor growth. Table 3 lists CV, ICC and ICC 95th percentile confidence interval for total retinal thickness and ganglion cell-inner plexiform layer measures for the inter-visit cohort. The CV of total retinal thickness was not significantly different in any quadrant between vision groups. The ganglion cell-inner plexiform layer CV was not statistically different between groups for the average and all quadrants (P > 0.05 for all comparisons), except for the inferior quadrant (Z = 1.98, P = 0.047). The magnitude of differences between vision loss groups was relatively small as demonstrated in Bland-Altman plots for ganglion cell-inner plexiform layer average and quadrants (See Figures 2 – 6).
Table 3.
Inter-visit Reproducibility of Total Retinal Thickness and Ganglion Cell – Inner Plexiform Layer Thickness Measures Using Handheld Optical Coherence Tomography in Children with Optic Pathway Gliomas.
| Abnormal Vision (N = 8) | Normal Vision (N = 17) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| TRT | Thickness ± SD | CV (%) ± SD | ICC (95% CI) | Thickness ± SD | CV (%) ± SD | ICC (95% CI) |
| Average | 402±27 | 0.9±0.8 | .985 | 433±25 | 0.7±0.6 | .987 |
| .93, .99 | .96, .99 | |||||
| Temporal | 393±23 | 1.2±0.9 | .970 | 422±25 | 0.7±0.6 | .986 |
| .84, .99 | .96, .99 | |||||
| Superior | 401±24 | 0.9±0.7 | .982 | 437±25 | 0.5±0.4 | .991 |
| .91, .99 | .97, .99 | |||||
| Nasal | 399±33 | 1.1±0.5 | .989 | 432±27 | 0.8±0.6 | .985 |
| .97, .99 | .96, .99 | |||||
| Inferior | 412±28 | 1.1±0.9 | .980 | 441±26 | 0.9±0.8 | .975 |
| .90, .99 | .93, .99 | |||||
|
| ||||||
|
GCIPL
| ||||||
| Average | 94±20 | 2.3±1.4 | .994 | 127±16 | 1.6±1.0 | .988 |
| .97, .99 | .97, .99 | |||||
| Temporal | 93±17 | 2.6±1.8 | .987 | 123±14 | 2.7±2.2 | .959 |
| .93, .99 | .88, .98 | |||||
| Superior | 95±22 | 3.7±4.2 | .980 | 132±17 | 2.4±1.9 | .971 |
| .90, .99 | .92, .98 | |||||
| Nasal | 90±25 | 1.9±1.9 | .995 | 124±19 | 2.7±2.0 | .974 |
| .98, .99 | .93, .99 | |||||
| Inferior a | 97±21 | 5.2±2.8 | .971 | 129±16 | 2.4±2.1 | .972 |
| .85, .99 | .92, .98 | |||||
N = number, TRT = total retinal thickness; GCIPL = ganglion cell layer-inner plexiform layer; CV = coefficient of variation; ICC = intraclass correlation coefficient; SD = standard deviation; CI = confidence interval.
P < 0.05.
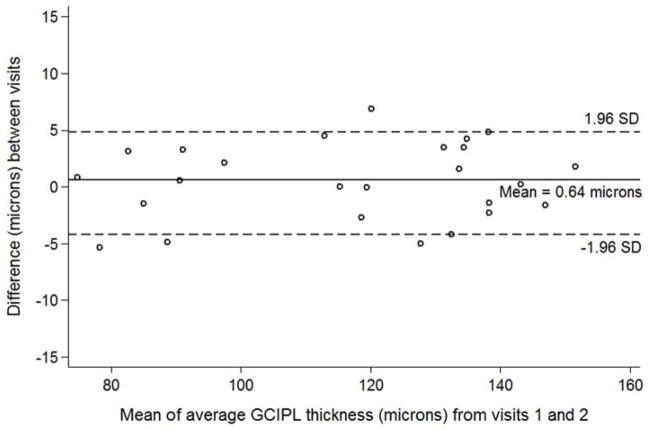
Figure 2.
Bland-Altman plot with inter-visit change in total ganglion cell – inner plexiform layer thickness plotted on the y-axis and average total ganglion cell – inner plexiform layer (GCIPL) thickness from both visits plotted on the x-axis in children with optic pathway gliomas.
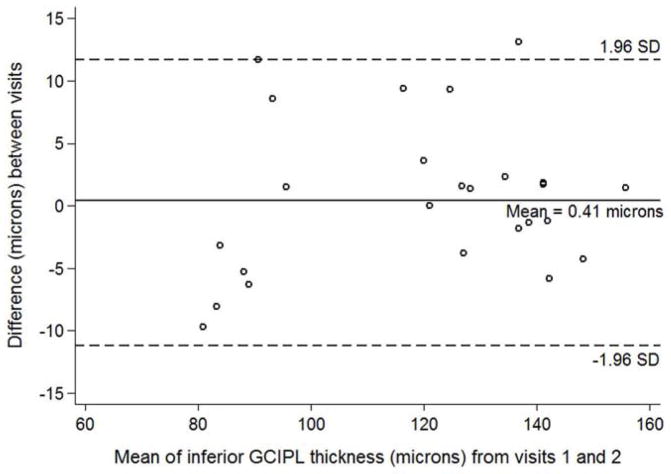
Figure 6.
Bland-Altman plot with inter-visit change in inferior ganglion cell – inner plexiform layer thickness plotted on the y-axis and average inferior ganglion cell – inner plexiform layer (GCIPL) thickness from both visits plotted on the x-axis in children with optic pathway gliomas.
DISCUSSION
Clinicians caring for infant and young children now have the ability to obtain high resolution spectral-domain OCT images in young children using a handheld OCT. As demonstrated in this study, the portability of the handheld OCT permits acquisition during sedation for other procedures, as well as in the clinic or intensive care unit.4,6,12–20 Most importantly, we demonstrated that macular volumes acquired with a handheld OCT and analyzed using custom designed automated segmentation software demonstrated excellent reproducibility of total retinal thickness and ganglion cell-inner plexiform layer thickness measures. Subjects who experienced VA and or VF loss from their optic pathway glioma demonstrated slightly higher CVs than subjects who had normal VA/VF, although the ICC values remained excellent regardless of the presence or absence of vision loss. Even though total retinal thickness measures had a lower CV than ganglion cell-inner plexiform layer measures, the latter is more clinically relevant to our patient population.
The reproducibility of total retinal thickness and ganglion cell-inner plexiform layer measures using current generation table-mounted OCT devices in adults with glaucoma has been evaluated by a number of investigators.21–25 Mwanza and colleagues demonstrated inter-visit CV values ranging from 1.8–3.6% using a 2.0mm (vertical) by 2.4 mm (horizontal) annulus centered over the fovea in adult patients with mild, moderate and severe glaucoma.23 Using the same device, Garvin et al22 demonstrated worse CV measures than the Mwanza23 study using the manufacturer supplied software, however they were able to significantly reduce the CV values using their custom designed software. To date, Francoz et al.,24 has reported the lowest CV values for ganglion cell-inner plexiform layer measures as all areas were below 1.75%. Using a different OCT device, intersession reproducibility of the ganglion cell complex above and below the horizontal meridian has also demonstrated CV values below 3%.21
Two studies have examined the reproducibility of total retinal thickness using FD-OCT or SD-OCT in children.26,27 Similar to our total retinal thickness measures, both studies demonstrated CV values below 1% for the mean macular thickness, but did not have software to segment the inner retinal layers.26,27 The ability to segment multiple inner retinal layers using commercially available software has only recently become available for some, but not all SD-OCT devices. Our custom software and analysis capabilities, which have also demonstrated excellent circumpapillary RNFL diagnostic4 and reproducibility20 results in children with optic pathway gliomas, can be made available to other investigators as part of research collaboration.10,11
A number of factors could be responsible for the slightly higher CV values reported in our study compared to others. 21–25 Many of the current generation of OCT devices have eye tracking/registration software that reduces between visit variability.28 It is conceivable that between visit differences in the angle and location of acquisition could vary using handheld OCT. Currently, our custom designed segmentation software does not perform co-registration of previously acquired images to guarantee the analysis at the same location. Nonetheless, our results still demonstrated excellent reproducibility for the intra-visit and inter-visit cohorts. Another possible explanation for the slightly higher CV values is that some of our patients may have been experiencing ganglion cell loss without clinical findings. Clinical variables did not contribute to the variance, as we performed a post-hoc multivariable regression analysis and found no difference in CV measures based on patient age or between subjects with and without NF1. It is also possible that the magnitude of optic atrophy differs between our subjects and those with mild or moderate glaucoma. Lastly, differences in device software and how images are segmented could certainly contribute to variability between studies.
Two recent studies have sought to establish reference ranges for macular thickness and retinal layers in healthy children using SD-OCT with real time eye tracking software.29,30 Our total retinal thickness measures were higher than both studies,24, 25 likely due to differences in segmentation algorithms. Similar to these two studies, we also used the 3mm diameter annulus of the classic ETDRS grid rather than the 6mm. This size allows the peak density of ganglion cells and macula ridge in both horizontal and vertical planes to be captured within our annulus.31,32 Our smaller annulus likely improves reproducibility by reducing the effects of less than perfectly centered scans.33 Using custom segmentation software and a similar annulus diameter, Lee and colleagues also demonstrated excellent reproducibility in adults with macular edema.34
Establishing a reference range and reproducibility measure of ganglion cell-inner plexiform layer thickness measures using the handheld OCT in normal/healthy children would be ideal, although this may not be entirely necessary for our study population. A normative reference range is helpful in classifying a single measure as normal or abnormal, yet the more relevant metric in this population is the longitudinal change, or lack thereof, in the ganglion cell-inner plexiform layer thickness measure. Furthermore, clinicians are faced with the challenge of determining which child with an optic pathway glioma requires observation versus treatment. Therefore, our subjects with optic pathway gliomas and normal vision are a more appropriate reference group as they may have small, but relevant differences in their ganglion cell-inner plexiform layer thickness measures as compared to normal/healthy children without optic pathway gliomas. Nonetheless, our handheld OCT results as well as the development of a normative reference range derived from normal/healthy children could be applied to other pediatric ophthalmologic conditions such as glaucoma, retinopathy of prematurity and inherited retinal dystrophies.
The use of handheld OCT in sedated children has a number of important limitations. Despite being able to acquire a single volume in 3 seconds, the quantitative assessment of the image/signal quality and retinal segmentation using our custom automated software cannot be performed until after the imaging session has ended. The operator must visually inspect the acquired images and subjectively determine whether the image quality may be sufficient for successful segmentation. When the operator suspects poor image quality, that volume is frequently discarded and additional acquisitions are performed, time permitting. In cases when the operator is unsure of the image quality, the volume is frequently saved and segmentation is attempted. Under ideal circumstances, we estimate that 80–90% of macular volumes subjectively interpreted as having good image quality will be successfully segmented. Inadequate mydriasis, poor corneal lubrication, and aberrant eye alignment during handheld OCT acquisition are factors that can contribute to poor image/signal quality.
Our results suggest that a change of at least 10% in the ganglion cell-inner plexiform layer thickness should be considered clinically significant. Further support of this estimate of change will need to be evaluated in longitudinal studies which specifically examine children who experience VA and VF decline, while also exploring other important and potentially influential factors such tumor location, tumor biology, type of chemotherapy used and magnitude of visual acuity/visual field loss at presentation.2,35 Similar to RNFL measures in glaucoma, there may be a “tipping point” in which a reduction in ganglion cell-inner plexiform layer results in vision loss.36 Additionally, normal and or stable ganglion cell-inner plexiform layer measures may have the potential to provide reassurance that the tumor is not causing progressive damage to the visual pathway—which would be especially helpful for children who cannot reliably complete VA and VF testing. While our reproducibility results are encouraging, we do not recommend using handheld OCT measures to make clinical decisions in children with optic pathway gliomas until a large multi-center study can validate the usefulness of these measures. In children who are cooperative, VA and VF measures remain paramount in deciding whether to initiate or defer treatment.37
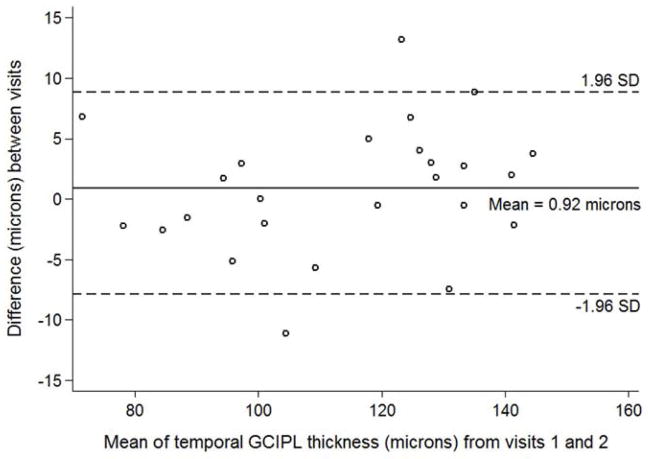
Figure 3.
Bland-Altman plot with inter-visit change in temporal ganglion cell – inner plexiform layer thickness plotted on the y-axis and average temporal ganglion cell – inner plexiform layer (GCIPL) thickness from both visits plotted on the x-axis in children with optic pathway gliomas.
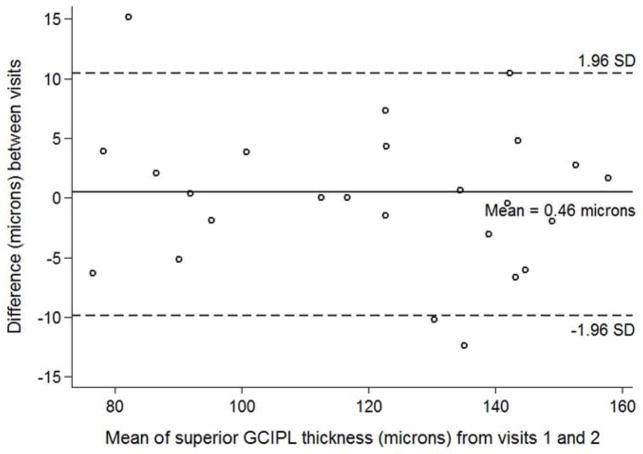
Figure 4.
Bland-Altman plot with inter-visit change in superior ganglion cell – inner plexiform layer thickness plotted on the y-axis and average superior ganglion cell – inner plexiform layer (GCIPL) thickness from both visits plotted on the x-axis in children with optic pathway gliomas.
Figure 5.
Bland-Altman plot with inter-visit change in nasal ganglion cell – inner plexiform layer thickness plotted on the y-axis and average nasal ganglion cell – inner plexiform layer (GCIPL) thickness from both visits plotted on the x-axis in children with optic pathway gliomas.
Acknowledgments
Funding/Support: This article was supported by grants from the National Eye Institute/National Institutes of Health, Bethesda, Maryland (K23-EY022673, RAA), the National Institutes of Health/National Eye Institute Pediatric Research Loan repayment program (RAA), “Clinical Research Award” from the Children’s Tumor Foundation, New York, New York (RAA), the Gilbert Family Neurofibromatosis Institute, Washington, DC (RAA, RJP), the National Center for Advancing Translational Sciences/National Institutes of Health UL1TR000075 (AC), Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health, Bethesda, Maryland P30 HD040677 (AC), NIH R01-EY013178 and P30-008098 (HI, JSS), The Eye and Ear Foundation of Pittsburgh, Pittsburgh, PA (HI, JSS), and an unrestricted grant from Research to Prevent Blindness, New York, New York (HI, JSS).
Biography

Dr. Robert A. Avery completed his Neuro-ophthalmology fellowship at the Children’s Hospital of Philadelphia/University of Pennsylvania and a Master’s degree in Clinical Epidemiology at the University of Pennsylvania. Dr. Avery is an assistant professor of Neurology, Ophthalmology and Pediatrics at Children’s National Medical Center in Washington DC where he has a dedicated pediatric neuroophthalmology practice and clinical research program.
Footnotes
Financial Disclosures: Dr. Schuman receives royalties for intellectual property licensed by Massachusetts Institute of Technology and Massachusetts Eye and Ear Infirmary to Zeiss.
Contribution of Author: Design of the study (RAA, AC, GEQ, HI), Conduct of the study (RAA, C-LC, NCG, HI); Collection, management, analysis and interpretation of the data (RAA, AC, JSS, C-LC, NCG, RJP, GEQ, HI); preparation, approval, and review of the manuscript (RAA, AC, JSS, C-LC, NCG, RJP, GEQ, HI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31(3):269–278. doi: 10.1097/WNO.0b013e31822aef82. [DOI] [PubMed] [Google Scholar]
- 2.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54(2):1034–1038. doi: 10.1167/iovs.12-11385. [DOI] [PubMed] [Google Scholar]
- 4.Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmology. 2014;132(3):265–271. doi: 10.1001/jamaophthalmol.2013.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55(3):1402–1408. doi: 10.1167/iovs.13-13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson V, Quinn GE, Biglan AW, Tung B, Flynn JT, Palmer EA. Acuity card assessment of visual function in the cryotherapy for retinopathy of prematurity trial. Invest Ophthalmol Vis Sci. 1990;31(9):1702–1708. [PubMed] [Google Scholar]
- 8.McDonald MA, Dobson V, Sebris SL, Baitch L, Varner D, Teller DY. The acuity card procedure: a rapid test of infant acuity. Invest Ophthalmol Vis Sci. 1985;26(8):1158–1162. [PubMed] [Google Scholar]
- 9.Mayer DL, Dobson V. Assessment of vision in young children: a new operant approach yields estimates of acuity. Invest Ophthalmol Vis Sci. 1980;19(5):566–570. [PubMed] [Google Scholar]
- 10.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46(6):2012–2017. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein DM, Ishikawa H, Hariprasad R, et al. A new quality assessment parameter for optical coherence tomography. Br J Ophthalmol. 2006;90(2):186–190. doi: 10.1136/bjo.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allingham MJ, Cabrera MT, O’Connell RV, et al. Racial variation in optic nerve head parameters quantified in healthy newborns by handheld spectral domain optical coherence tomography. J AAPOS. 2013;17(5):501–506. doi: 10.1016/j.jaapos.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera MT, Maldonado RS, Toth CA, et al. Subfoveal fluid in healthy full-term newborns observed by handheld spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153(1):167–175. doi: 10.1016/j.ajo.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera MT, O’Connell RV, Toth CA, et al. Macular findings in healthy full-term Hispanic newborns observed by hand-held spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2013;44(5):448–454. doi: 10.3928/23258160-20130801-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012;130(5):569–578. doi: 10.1001/archopthalmol.2011.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315–2325. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno TA, O’Connell RV, Chiu SJ, et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54(6):4140–4147. doi: 10.1167/iovs.12-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott AW, Farsiu S, Enyedi LB, Wallace DK, Toth CA. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147(2):364–373. doi: 10.1016/j.ajo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Avery RA, Cnaan A, Schuman JS, et al. Reproducibility of Circumpapillary Retinal Nerve Fiber Layer Measurements Using Handheld Optical Coherence Tomography in Sedated Children. Am J Ophthalmol. doi: 10.1016/j.ajo.2014.06.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garas A, Vargha P, Hollo G. Reproducibility of retinal nerve fiber layer and macular thickness measurement with the RTVue-100 optical coherence tomograph. Ophthalmology. 2010;117(4):738–746. doi: 10.1016/j.ophtha.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Garvin MK, Lee K, Burns TL, Abramoff MD, Sonka M, Kwon YH. Reproducibility of SD-OCT-Based Ganglion Cell-Layer Thickness in Glaucoma Using Two Different Segmentation Algorithms. Invest Ophthalmol Vis Sci. 2013;54(10):6998–7004. doi: 10.1167/iovs.13-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(11):8323–8329. doi: 10.1167/iovs.11-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francoz M, Fenolland JR, Giraud JM, et al. Reproducibility of macular ganglion cell-inner plexiform layer thickness measurement with cirrus HD-OCT in normal, hypertensive and glaucomatous eyes. Br J Ophthalmol. 2014;98(3):322–328. doi: 10.1136/bjophthalmol-2012-302242. [DOI] [PubMed] [Google Scholar]
- 25.Hirasawa H, Araie M, Tomidokoro A, et al. Reproducibility of Thickness Measurements of Macular Inner Retinal Layers Using SD-OCT With or Without Correction of Ocular Rotation. Invest Ophthalmol Vis Sci. 2013;54(4):2562–2570. doi: 10.1167/iovs.12-10552. [DOI] [PubMed] [Google Scholar]
- 26.Altemir I, Pueyo V, Elia N, Polo V, Larrosa JM, Oros D. Reproducibility of optical coherence tomography measurements in children. Am J Ophthalmol. 2013;155(1):171–176. doi: 10.1016/j.ajo.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Ghasia FF, El-Dairi M, Freedman SF, Rajani A, Asrani S. Reproducibility of Spectral-Domain Optical Coherence Tomography Measurements in Adult and Pediatric Glaucoma. J Glaucoma. doi: 10.1097/IJG.0b013e31829521db. in press. [DOI] [PubMed] [Google Scholar]
- 28.Menke MN, Dabov S, Knecht P, Sturm V. Reproducibility of retinal thickness measurements in healthy subjects using spectralis optical coherence tomography. Am J Ophthalmol. 2009;147(3):467–472. doi: 10.1016/j.ajo.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Turk A, Ceylan OM, Arici C, et al. Evaluation of the nerve fiber layer and macula in the eyes of healthy children using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153(3):552–559. doi: 10.1016/j.ajo.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Yanni SE, Wang J, Cheng CS, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155(2):354–360. doi: 10.1016/j.ajo.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knighton RW, Gregori G. The shape of the ganglion cell plus inner plexiform layers of the normal human macula. Invest Ophthalmol Vis Sci. 2012;53(11):7412–7420. doi: 10.1167/iovs.12-10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knighton RW, Gregori G, Budenz DL. Variance reduction in a dataset of normal macular ganglion cell plus inner plexiform layer thickness maps with application to glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2012;53(7):3653–3661. doi: 10.1167/iovs.12-9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotowski J, Folio LS, Wollstein G, et al. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol. 2012;96(11):1420–1425. doi: 10.1136/bjophthalmol-2011-301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Chiu SJ, Srinivasan PP, et al. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci. 2013;54(12):7595–7602. doi: 10.1167/iovs.13-11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–24. doi: 10.1212/01.wnl.0000435745.95155.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96(1):47–52. doi: 10.1136/bjo.2010.196907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110(1):1–7. doi: 10.1007/s11060-012-0944-y. [DOI] [PubMed] [Google Scholar]