Abstract
Despite the introduction almost a century ago of Mycobacterium bovis BCG (BCG), an attenuated form of M. bovis that is used as a vaccine against Mycobacterium tuberculosis, tuberculosis remains a global health threat and kills more than 1.5 million people each year. This is mostly because BCG fails to prevent pulmonary disease – the contagious form of tuberculosis. Although there have been significant advances in understanding how the immune system responds to infection, the qualities that define protective immunity against M. tuberculosis remain poorly characterized. The ability to predict who will maintain control over the infection and who will succumb to clinical disease would revolutionize our approach to surveillance, control, and treatment. Here we review the current understanding of pulmonary T cell responses following M. tuberculosis infection. While infection elicits a strong immune response that contains infection, M. tuberculosis evades eradication. Traditionally, its intracellular lifestyle and alteration of macrophage function are viewed as the dominant mechanisms of evasion. Now we appreciate that chronic inflammation leads to T cell dysfunction. While this may arise as the host balances the goals of bacterial sterilization and avoidance of tissue damage, it is becoming clear that T cell dysfunction impairs host resistance. Defining the mechanisms that lead to T cell dysfunction is crucial as memory T cell responses are likely to be subject to the same subject to the same pressures. Thus, success of T cell based vaccines is predicated on memory T cells avoiding exhaustion while at the same time not promoting overt tissue damage.
Keywords: Tuberculosis, T cell, Priming, Cytokine, Exhaustion, Memory
1. Introduction
The mammalian immune system evolved in parallel with the host’s adaptation to colonization by polymicrobial communities. Consequently, an important function of the immune system is to distinguish beneficial bacterial microbiota from invading pathogens. Bacterial invasion of sterile tissue can lead to acute or chronic disease. Acute infection has a time course of days and is resolved by the immune system as exemplified Rhinovirus and other viral upper respiratory infections. Alternatively, acute infection can result in death, as typically happens following pneumonic plague (Yersinia pestis). Other pathogens have intermediate virulence and are capable of evading or subverting the immune response, which makes their eradication difficult. These pathogens establish chronic infection with a time course of years to decades, often resulting in persistent inflammation and disease. For bacterial pathogens that cause chronic infection, there is none more “successful” than Mycobacterium tuberculosis, the causative agent of the human disease tuberculosis. Despite concerted eradication efforts, there are 8-10 million new cases of active tuberculosis each year.
Immunologists are concerned with two general questions regarding tuberculosis. First, how does the immune system control M. tuberculosis infection? Second, can vaccination prevent, or at least ameliorate, infection and disease. The number of people that develop active disease is a small fraction of the estimated two billion people that have been infected by the bacterium but have no signs of disease. Based on epidemiological studies, individuals with immunological evidence of prior infection (e.g., positive tuberculin skin test) are at an increased risk of developing active tuberculosis [1], and from these data we infer that the human immune system can control bacterial replication although it cannot efficiently sterilize infected tissue. This has given rise to the concept of latent (or asymptomatic) infection. An important question is how the immune systems of asymptomatic infected people control M. tuberculosis infection and whether some are capable of sterilizing immunity. Ascertaining why immunity fails to control M. tuberculosis infection in people that develop the disease tuberculosis is essential for ongoing efforts to develop a vaccine to protect vulnerable individuals. The old axiom “an ounce of prevention is worth a pound of cure” is particularly relevant as drug resistant strains of M. tuberculosis emerge and disseminate across the globe. The development and evaluation of vaccines against tuberculosis has been much more difficult than initially anticipated. By definition, individuals that develop active disease have failed immunity to M. tuberculosis, but it is unclear whether this failure is based on an acquired or hereditary defect in immunity. The importance of microbial and environmental factors also needs to be considered. Whatever the defect, one must consider the possibility that the factors increasing susceptibility to tuberculosis may also impair the generation or function of vaccine-induced immunity in the very people that are at highest risk for developing active disease.
Most clinical and experimental work in the area of immunity to M. tuberculosis has focused on T cells because of their dominant role in mediating immunity during primary infection and their role in vaccine-elicited protection in experimental models. This review highlights areas of progress and unanswered questions in our pursuit of knowledge regarding the capacity of T cell immunity to control M. tuberculosis infection.
2. Dissemination of bacteria to the draining LN leads to initiation of the T cell response
There is an unanticipated delay in T cell priming and recruitment of immune T cells to the lung after M. tuberculosis infection. While this phenomenon is best described in the murine model, it has been verified in other animal models as well [2]. As there is evidence that delay in initiation of T cell immunity correlates with host susceptibility, understanding why immunity is delayed is important [3,4]. Indeed, M. tuberculosis may inhibit T cell priming as a virulence strategy. Similarly, delay in recall of memory responses may hamper vaccine efficacy. Why T cell priming only occurs late after M. tuberculosis infection is incompletely understood. One idea is that M. tuberculosis inhibits maturation of dendritic cells (DCs), which prevents their trafficking to the lymph nodes (LN) draining the lung [5,6]. A second possibility is that early following infection, there are so few bacteria, there is simply not enough antigen to be detected by the immune system. However, increasing the inoculum delivered to the lung does not significantly change the timing of when the endogenous T cell response is primed [7]. One way that DCs acquire mycobacterial antigens is by engulfing infected apoptotic cells, a process important for T cell priming [8-11]. The ability of M. tuberculosis to inhibit apoptosis and instead induce necrosis can delay T cell priming [3,6,10-17] (Figure 1).
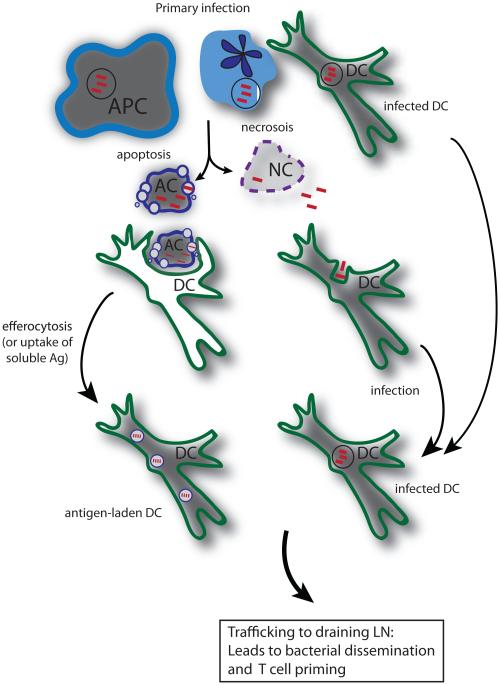
Figure 1. Complex interactions between resident and recruited innate leukocytes lead to bacterial dissemination and acquisition of antigen by DC for T cell priming.
Multiple cell types are initially infected including macrophages, PMNs and DCs. Both apoptotic and necrotic types of cell death can occur following primary M. tuberculosis infection. Apoptosis is associated with control of infection and can lead to acquisition of antigen by APC by the process of efferocytosis. In contrast, necrosis facilitates dispersal of bacteria and re-infection of other cell types. DC, whether they have acquired antigen via efferocytosis or by infection, traffic to the lung draining LN where they have a vital role in T cell priming.
Our current understanding of T cell priming is that it occurs primarily in the lung draining LN, although under certain conditions it can occur in the lung [3,6,10-17]. DCs are essential for T cell priming following M. tuberculosis infection [4,13]. Temporally, bacterial dissemination via the lymphatics occurs before T cell priming can be detected, and it is likely that bacteria are carried within DC, a cell type that is specialized to traffic from inflamed peripheral tissues to draining LNs [3,11,14]. One possibility is that pulmonary DCs ingest apoptotic vesicles containing mycobacterial antigens derived from dying infected cells and traffic to the LN where they prime naïve T cells. Alternately, infected pulmonary cells migrate to the LN, where they undergo apoptosis, and their apoptotic vesicles are taken up by local DC, processed and presented. Support for such a model comes from work showing that CCR2+ inflammatory monocytes play an important role in transfer of M. tuberculosis from the lung to the draining LN [18]. The delay in bacterial dissemination associated with transient ablation of CCR2+ inflammatory monocytes is associated also with delay in T cell priming. Interestingly, while CCR2+ inflammatory monocytes transport bacteria to the LN, they are not required for T cell priming, a role that is served by classical DC [18]. More recently, a pathway for the transfer of mycobacterial antigens from infected migratory DCs to uninfected LN resident DCs has been described [19]. These mechanisms for transferring antigen to uninfected DCs may be particularly important, because it has been demonstrated that directly infected DCs have a diminished ability to activate T cells [13] (Figure 1).
Inhibition of apoptotic death is a virulence strategy of M. tuberculosis that predisposes infected macrophages to a necrotic death. This enables bacteria to disperse and infect other macrophages, delay T cell priming and impair host immunity [8-10,20-23]. However, infected cells undergo different forms of cell death because of heterogeneity among bacteria, host cells, and the lung microenvironment, leading to a discrete frequency of apoptosis following infection of macrophages by virulent M. tuberculosis [3,4,24]. For example, changes in the ratio of the lipid mediators LXA4 and PGE2, that favor PGE2, shift the balance towards apoptosis, leading to earlier T cell priming, and enhanced host immunity against infection [10,22,23].
Apoptosis of infected cells generates vesicles that contain the contents of the dying cells including mycobacterial antigens [8,9]. Uptake of these vesicles can lead to presentation and cross-presentation of mycobacterial antigens to class I MHC-restricted CD8+ T cells, class II MHC-restricted CD4+ T cells, and CD1-restricted T cells, both in vitro and in vivo [8-10,15]. In fact, the enhanced ability of the live recombinant BCG vaccine [rBCG ΔureC::hly(+)] to induce T cell responses and protection is based on its ability to induce apoptosis [25]. Thus, packaging of mycobacterial antigens by apoptotic cells and delivery to professional APC is a mechanism that leads to T cell priming following infection with M. tuberculosis and certain engineered vaccines.
While alveolar macrophages have been considered to be the cell principally infected following exposure to M. tuberculosis, many different cell types are infected including various types of DC, recruited macrophages, and neutrophils [6,11]. While apoptosis of macrophages has been shown to facilitate priming of naïve T cells [8-10], there is new evidence that neutrophils, which play an important role in the pathogenesis of tuberculosis [26,27], are infected early after infection [11,13]. Infected neutrophils affect priming of CD4+ T cells by producing chemokines that attract DC, transferring bacteria and bacterial antigens to DC, and promoting DC migration to the draining LN, all with the end result of helping DC to activate naïve CD4+ T cells [15]. Indeed, just like with infected macrophages, virulent M. tuberculosis inhibits neutrophil apoptosis, and this too impairs T cell priming [15].
3. M. tuberculosis antigens and understanding “protective” immunity
3.1 Antigen processing
Activation of M. tuberculosis-specific naïve T cells requires presentation of mycobacterial antigens by professional APCs such as DCs. The class II or class I MHC processing pathway samples the extracellular/endosomal or cytosolic cell compartments, respectively. Mycobacterial proteins in these compartments are degraded into peptides, and if they have affinity for the class II or class I MHC molecules of that particular individual, can bind and ultimately be expressed on the cell surface of the APC and potentially recognized by MHC class II-restricted CD4+ and class I-restricted CD8+ T cells, respectively. In addition to the well-characterized MHC-restricted CD4+ and CD8+ T cells, there are other T cells that that recognize distinct antigens presented by other antigen-presenting molecules. For example, the family of CD1 antigen presenting molecules can present lipid, glycolipid, and lipopeptides derived from M. tuberculosis to human T cells [28,29]. Which antigens are ultimately presented depends on their access to, and affinity for, the different antigen presenting molecules. There is important heterogeneity among different cell types in their expression of antigen-presenting molecules. MHC class II is generally restricted to myeloid lineage cells and B cells, while MHC class I can be expressed by all nucleated cells. Group 1 CD1 is highly expressed by mature DCs and less frequently by other cells. Furthermore, most antigen-presenting molecules are regulated by exogenous signals including cytokines and danger signals such as TLR ligands. Which antigens are presented also depends on their relative abundance, whether they are secreted, and how the antigens were acquired by the APC.
3.2 What are protective antigens?
The hunt for mycobacterial antigens has important practical applications for vaccine targets, diagnostic tests, and drug therapy monitoring. There is also crucial scientific benefit for monitoring M. tuberculosis-specific T cell responses to native antigens, which can improve our understanding of the host-pathogen interaction and how T cell diversity contributes to host resistance. Key among the pertinent questions is how to define a protective antigen. For humoral immunity, only a subset of the total microbial epitopes that can be recognized elicit a protective antibody response. In part, this is because not all antibodies block essential pathogen functions. For T cells, the antigens recognized, whether they are peptide fragments or lipids, are physically dissociated from the pathogen, and hence have no intrinsic biological function. Thus, why some antigens generate protective T cell responses while others do not is a complicated question. Whether the elicited T cells express effector functions capable of killing or activating the infected cell, to deprive the pathogen of its intracellular niche, may be more important than which antigen is recognized.
3.3 Which antigens are presented where?
An important consideration is whether there is a difference between the pools of antigens that prime T cells in the LN and the antigens that are presented by infected cells in the lung. For example, mycobacterial antigens acquired by DCs through the engulfment of apoptotic cells or from the transfer of soluble antigens, may differ from those presented by infected macrophages. A potential consequence is that T cells primed in the LN may not recognize infected cells in the lung. Similarly, DCs express certain antigen-presenting molecules, such as CD1b, which are not expressed by all macrophages. This might allow M. tuberculosis to evade detection by CD1b-restricted T cells. Another important issue is the heterogeneity of antigens during infection. Mycobacterial antigens differ in their temporal regulation and in how much is produced by the bacterium, and both factors affect the relative abundance of antigen that can be presented by infected cells [30,31]. Finally, antigens vary in their access to the antigen presentation machinery. Antigens that are secreted by M. tuberculosis appear to be more immunogenic. The best-documented case is for proteins secreted by the type VII secretion system [32,33]. ESAT6 and CFP10, which form a heterodimer and are important for the virulence of the bacterium, are both very immunogenic, which is dependent upon their secretion [34-36].
3.4 Which epitopes are important?
All of the above factors contribute to strength of the T cell response to certain epitopes. One of the unforeseen characteristics of the T cell response to M. tuberculosis is the extreme immunodominance observed for certain mycobacterial antigens. This is best described for the CD8+ T cell response. In three different strains of mice with diverse MHC haplotypes, a single mycobacterial epitope can be identified that is recognized by between 25-50% of the CD8+ T cells in the lungs of infected mice [37-41]. These T cell expansions develop early and are long-lived responses that persist for the duration of the infection [38]. Immunodominant responses are also detected in M. tuberculosis infected people [42-44]. In most patients that have been studied in detail, an immunodominant T cell responses can be identified in peripheral blood [45,46]. Finally, the recognition of the immunodominance of CFP10 and ESAT6 has led to their usefulness diagnostically, despite geographic, ethnic and MHC differences [47-49]. The basis for immunodominance is not clear. In non-infectious models, the magnitude of the response is generally proportional to the precursor frequency for a given epitope; however, during infection the bacterial factors discussed above may have a larger effect. Another factor is the promiscuity of certain mycobacterial epitopes in binding to different MHC alleles, which may explain their immunodominance across MHC types [50,51]. An important question is whether these immunodominant antigens are good vaccine candidates. Data from animal models demonstrates that while vaccination with some of the major antigens, such as ESAT6 and Ag85B induce CD4+ T cell mediated protection, vaccination with immunodominant CD8+ epitopes has had mixed results [52,53]. Analysis of M. tuberculosis strains from across the world has shown that the genes encoding T cell epitopes are more highly conserved than other genetic elements [54]. Although the number of known epitopes is limited, these data suggest that the evolutionary success of M. tuberculosis is linked to its recognition by T cells – it may be that immunological recognition of M. tuberculosis benefits its survival [54]. Finally, T cells elicited by vaccination using peptides for cryptic epitopes, not normally elicited by infection, recognize infected cells, possess a distinct phenotype after activation, and lower bacterial CFU in the murine model [55]. This highlights the point that M. tuberculosis may elicit larger T cell responses specific for certain epitopes in order to maintain a population of T cells with a specific phenotype that is beneficial for pathogenesis.
4. Integration of multiple signals during T cell priming determines the quality of immunity
4.1 Signal One
Once the antigen is presented on the cell surface, naïve T cells and DC can interact. For this to occur, naïve T cells in the LN need to express a T cell receptor (TCR) that recognizes mycobacterial antigen in the context of antigen-presenting molecules on the cell surface of the DC. If other signals are also present (see sections 4.2 and 4.3), the T cell will become activated, expand and traffic from the LN to the lung. This process ensures that the T cell’s effector functions are directed at target cells expressing the cognate antigen, in the context of this review, M. tuberculosis infected cells. While many signals must be integrated for activation of a naïve T cells (i.e., priming) to occur, the chief signal is mediated by the T cell receptor (TCR) (Figure 2).
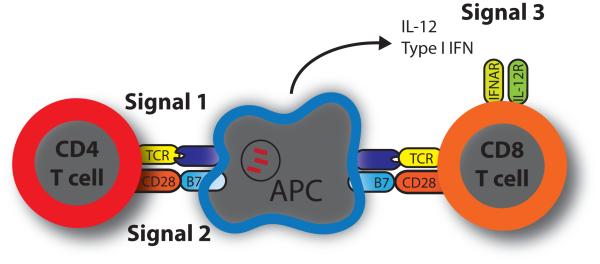
Figure 2. Up to three signals are required for optimal T cell priming.
Classically, priming of naïve CD4+ and CD8+ T cells requires two signals. Signal 1 is from the engagement of the T cell receptor (TCR) by cognate peptide presented in the context of major histocompatibility complex (MHC) molecules on the surface of antigen presenting cells (APC). Signal 2 is provided by the interaction of costimulatory receptors, here CD28, with the appropriate ligand, here B7.1 or B7.2. More recently, the model of CD8+ T cell priming has been expanded to include a third signal. Signal 3 is generated by an inflammatory cytokine, usually IL-12 or type 1 interferons (Type 1 IFN). These cytokines bind receptors on the CD8+ T cell and affect expansion, differentiation, effector functions, and memory formation. Additional abbreviations: IL-12R, IL-12 receptor; IFNAR, interferon alpha/beta receptor.
The TCR repertoire is very diverse. It is estimated that people have the potential to generate >1014 different TCR specificities [56]. In practice, thymic selection constrains the repertoire, as T cells need to be MHC-restricted and high affinity TCRs that recognize self are eliminated. A person only has approximately 1010 T cells, and of these, it is estimated that the an individual’s naïve T cell repertoire consists of 2.5 × 107 unique TCRs [57,58]. Thus, based on sampling, it is unlikely that there is much sharing of TCRs between people. Nevertheless, such ‘public’ TCRs are detected and may be of special significance [59]. Although there is no affinity maturation via somatic mutation as is the case for immunoglobulin genes, there can be affinity selection. Higher affinity T cells have a more durable interaction with peptide/MHC complexes on the surface of APC (particularly when antigen is limiting) and receive signals that sustain their proliferation and survival. A higher affinity T cells is predicted to outcompete lower affinity T cells and because the TCR signal leads to proliferation, ultimately it will become a more dominant part of the T cell response. This phenomenon has been observed for memory responses to viral pathogens such as flu [60], and we have also observed such selection during chronic M. tuberculosis infection [unpublished observation].
At a very basic level, the TCRs used by T cells specific for M. tuberculosis contain information about the quality of the immune response. For example, if the TCR repertoire is largely unshared between individuals, variations in the susceptibility or resistance to disease could be determined by differences in the quality (e.g. affinity) or quantity (e.g., number of epitopes recognized). TCR diversity is ultimately a surrogate for the quality of the immune response, which raises the possibility that clonotypic diversity (or bias) correlates with T cell effectiveness. While we are far from being able to test such hypotheses, new studies highlight the value of studying TCRs used by M. tuberculosis specific T cells. Du et al identified TCRβ sequences from PPD-reactive CD4+ and CD8+ T cells from BCG vaccinated macaques [61]. While these sequences were diverse, when challenged with M. tuberculosis, several but not all TCRβs underwent dramatic clonal expansion. What determines which T cells, presumably all specific for mycobacterial antigens, expand? Are the antigens differentially expressed between BCG and M. tuberculosis? Since the specificity of the T cells is unknown, we cannot be certain, but these data suggest that infection elicits a fundamentally different T cell response to antigen than vaccination. The TCR repertoire has also been queried in people with tuberculosis. Spectratyping of peripheral blood CD4+ and CD8+ T cells from pediatric and adult tuberculosis patients found skewing of the TCR repertoires compared to healthy controls [62-64]. Extreme TCR bias (e.g., clonality) was noted primarily in the setting of severe clinical disease, raising the possibility that TCR bias is associated with disease progression [62,65]. Arguing against this interpretation is the presence of highly skewed TCR repertoires in lung granulomas from patients with latent tuberculosis, with as many as 27% of CD4s and 17% of CD8s being clonal [66]. While it is provocative that TCR bias may be associated with the clinical severity of tuberculosis, the inability to study structure-function correlates of the antigen-specific T cells, and the lack of longitudinal studies, hinders drawing conclusions from these few studies. Although the relationship between TCR breadth and depth and T cell protection is not yet clear, it is a question that should be considered both in the context of primary infection and vaccination.
4.2 Signal Two
Classically, T cell activation is thought to require two signals. In addition to TCR ligation (signal 1), naïve T cells require signals from cell surface co-stimulatory receptors (signal 2) (Figure 2). CD28 is the prototypical co-stimulatory receptor and is constitutively expressed on both naïve CD4+ and CD8+ T cells. Its ligands, CD80 (B7.1) and CD86 (B7.2), are upregulated on the surface of APC upon their activation. Ligation of CD28 triggers downstream signaling events that synergize with TCR signaling to promote T cell activation. CD28 co-stimulation also enhances T cell survival, proliferation, and the production of key cytokines such as IL-2. Beyond promoting naïve T cell responses, CD28 signaling remains important for activated T cells in part by promoting IL-2 production [67]. Evidence suggests that for CD8+ T cells, continued interactions with APC promote effector T cell survival in the periphery in a manner involving CD28 and IL-2 [68]. The role of continued CD28 co-stimulation during chronic infections is unclear, but it is interesting to speculate that continued interactions with APC might be necessary for effector T cell survival during the extended course of infection.
During M. tuberculosis infection, mice lacking either CD80 or CD86 alone have no phenotype; however, mice lacking both co-stimulatory ligands show delayed T cell priming and have fewer CD4+ and CD8+ T cells producing IFNγ in their lungs [69,70]. These mice are still capable of mounting a modest T cell response, suggesting that other co-stimulatory receptors or ligands may provide a signal 2 during tuberculosis. Intriguingly, there is evidence suggesting some microbial products directly co-stimulate T cells. The M. tuberculosis lipoprotein LprG can serve as a co-stimulatory signal for human CD4+ T cells in a manner dependent on TLR1 and TLR2 [71], and the TLR2 agonist P3CSK4 is sufficient to co-stimulate transgenic CD4+ T cells specific for Ag85B [72]. Rv2468c, a protein primarily found in the M. tuberculosis cell wall, can also serve as a co-stimulatory molecule by interacting with the integrin VLA-5 (α5β1) on CD4+ T cells [73]. Co-stimulatory receptors and ligands have a profound influence on T cell biology, and we are only beginning to address the roles these molecules during tuberculosis.
4.3 Signal Three
More recently, the two-signal model of T cell activation has been expanded for CD8+ T cells to include a necessary third signal (Figure 2). Work with artificial antigen presenting cells reveals that optimal CD8+ T cell responses require input from inflammatory cytokines in addition to TCR engagement and co-stimulation during priming [74].The predominant “signal 3” cytokines are IL-12 and type 1 interferons (type 1 IFN), and they have pivotal roles in influencing CD8+ T cell expansion, differentiation, effector functions, and memory formation. The relative importance of either IL-12 or type 1 IFN during infection is determined by the pathogen, depending on which cytokines are elicited during CD8+ T cell priming [74]. During tuberculosis, both IL-12 and type 1 IFN are elicited and have profound effects on disease outcome. Mice lacking IL-12 p40 are more susceptible to tuberculosis and are incapable of producing IFNγ [75]. In contrast, mice lacking the interferon αβ receptor are more resistant to tuberculosis, potentially due to an enhanced Th1 response [76]. Given the dramatic and disparate effects of these cytokines during tuberculosis, it will be important to dissect their role in modulating CD8+ T cell responses. Such understanding will undoubtedly have implications for rational vaccine design and the development of immunotherapies.
5.0 Migration of M. tuberculosis-specific T cells into the infected lung
During the clonal expansion phase following primary antigen recognition, responding CD4+ T cells exit the lung-draining lymph node, enter the peripheral circulation, and then migrate out of the lung-associated blood vasculature into the lung parenchyma. Using Ag85B [14] or ESAT-6 [16,17] specific TCR transgenic CD4+ T cells to carefully track the very early events in the response against M. tuberculosis, it was shown that following low dose aerosol infection CD4+ T cells first appear in the lungs between days 14 and 16, approximately 2-3 days after the first evidence of antigen recognition in the lymph node, and then rapidly accumulate in situ thereafter. CD4+ T cells must directly interact with MHC class II on M. tuberculosis-infected macrophages and DC in the lungs in order to induce containment of the infection, so CD4+ T cell localization into the granuloma is likely critical for control [77]. However, the pathways that govern lymphocyte trafficking in M. tuberculosis infection and migratory capability of different M. tuberculosis-specific CD4+ T cell subsets are only partially understood. Here we will discuss the negative and more recent positive data on the role of selectins, integrins and chemokine receptors in protective CD4+ T cell responses in M. tuberculosis infection, as well as the heterogeneity of CD4+ T cell subsets with lung-homing capacity.
5.1 Selectins
The first interactions between leukocytes and inflamed endothelium involve selectin / selectin ligand binding which causes the tethering and rolling behavior that initiates the process of trans-endothelial migration. Interestingly, M. tuberculosis-infected mice singly or doubly deficient in Fuct-IV or Fuct-VII, which generate functional selectin counter-receptors, display normal accumulation of activated CD4+ T cells in their lungs, indicating that fucosyltransferase dependent E- and P-selectin ligands may not be required for CD4+ T cell entry into the lungs during pulmonary M. tuberculosis infection [78]. This is in strong contrast to the major role for Fuct-VII in the delayed type hypersensitivity reaction elicited by placing PPD in the skin of M. tuberculosis infected mice. Therefore, the very early events that mediate the interaction between M. tuberculosis-specific CD4+ T cells and the inflamed lung vascular endothelium may be redundant and need clarification.
5.2 Integrins
Integrins play major role in trafficking of CD4+ T cells into peripheral sites of infection by mediating firm adhesion and transmigration across the endothelium, and several integrins have been examined in the context of M. tuberculosis infection. Mice deficient in CD49a (α1 integrin) display alterations in granuloma structure, but normal bacterial loads and normal numbers of activated CD44hiCD62Llo CD4+ T cells in their lungs [79]. Similarly, mice deficient in αE or β7 are normally resistant to aerosol M. tuberculosis infection indicating that the αEβ7 and α4β7 heterodimers are not critically required for resistance to pulmonary M. tuberculosis infection [80]. Mice deficient in either CD11a or CD18 (β2), however, are unable to control M. tuberculosis infection and succumb earlier compared to WT mice, while CD11b−/− mice display normal resistance [80,81]. These data indicate that LFA-1 (CD11a/CD18 heterodimers) is critical for immunity to pulmonary M. tuberculosis. Short-term, competitive migration experiments, where WT and CD11a−/− CD4+ T cells from the lymph nodes of infected mice were mixed and adoptively co-transferred back into congenic marker disparate M. tuberculosis-infected mice, have shown that CD11a-deficient CD4+ T cells are impaired in their ability to home to the lung [80]. This indicates that the susceptibility of the CD11a−/− mice may at least in part be explained by defects in CD4+ T cell migration into the lungs. Interestingly, mice deficient in ICAM-1, a major ligand for LFA-1, are able to control low-dose aerosol infection with M. tuberculosis similar to WT mice, although antigen-specific T cell responses in the lung were not measured in this earlier study [82]. Therefore, the LFA-1 binding partner on the lung vascular endothelium mediating CD4+ T cell recruitment during M. tuberculosis infection is still not clear.
5.3 Chemokines
Chemokine/chemokine receptor interactions are essential for transmigration across the endothelium and proper localization of T cells to discrete areas within tissues, and the roles of several chemokine pathways have been examined in M. tuberculosis infection. Interestingly, several of the major Th1 associated inflammatory chemokine pathways have been found to be dispensable for control of M. tuberculosis infection in the experimental mouse model. CXCR3 expression is T-bet dependent [83] and accordingly is characteristic of both mouse [84] and human [45] M. tuberculosis-specific CD4+ T cells. While CXCR3 has been shown to be critical for migration of T cells into peripheral sites of inflammation in several settings, CXCR3 gene-targeted mice do not show increased susceptibility to M. tuberculosis infection on a C57Bl/6 background [85]. On the BALB/c background they display higher numbers of CD4+ T cells and lower bacterial loads in the lungs following M. tuberculosis infection compared to WT animals [86]. Therefore, CXCR3 signals are likely uninvolved or redundant in T cell migration and may even impair control of M. tuberculosis infection in the murine model. Likewise, CCR5 gene targeted mice display higher numbers of T cells and normal bacterial loads [87], indicating that this pathway alone may also not be essential for host resistance to M. tuberculosis infection. However, these two chemokine receptors may have some role in regulating CD8+ T cell activation within the lung. Following aerosol M. tuberculosis infection, CXCR3/CCR5 double knock-out mice generate increased numbers of KbTB10.3/44-11 specific CD8+ T cells compared to WT mice but display dramatically reduced frequencies of CD69+ and increased frequencies of CD127+ KbTB10.3/44-11 specific CD8+ T cells [88]. This indicates that in the absence of these two chemokine receptors CD8+ T cells in the lungs may not efficiently interact with antigen presenting cells. Mice deficient in CX3CR1, which is expressed on subsets of monocytes and activated T cells and binds the unique transmembrane chemokine CX3CL1 on vascular endothelium, are also normally resistant to low dose aerosol M. tuberculosis infection [89].
Data on the role of homeostatic chemokine pathways is also available in the context of M. tuberculosis infection. CCR7 plays a major role in the migration of CD4+ T cells into secondary lymphoid organs (SLO), however, it has been shown that CD4+ T cell priming can also occur within the lung tissue itself if forced to do so in mice lacking SLO [90]. Indeed, the CCR7 ligand, CCL19 is produced in M. tuberculosis infected lungs [91] and naïve CD4+ T cells have been shown to localize into the lung tissue parenchyma and airways in surprising numbers during M. tuberculosis infection in mice [84]. Interestingly, CCR7−/− mice are still able to control pulmonary M. tuberculosis infection similar to WT mice, and they even have slightly reduced bacterial loads in their spleens, indicating that normal CD4+ T cell priming in the lymph node or ectopically in the lung may not require CCR7 alone [91].
Although most of the work on CXCR5 has focused on its homeostatic role in regulating B cell and T follicular helper cell migration within secondary lymphoid tissue, a major functional role for CXCR5 on CD4+ T cells in mediating host resistance to M. tuberculosis infection in the lungs has been demonstrated recently [92,93]. Following low-dose aerosol exposure to M. tuberculosis, mice deficient in CXCR5 or its ligand CXCL13 display defective CD4+ T cell localization into ectopic lymphoid structures in lung, reduced activation of lung macrophages and higher bacterial burdens. At high doses of bacteria, both CXCR5−/− and CXCL13−/− mice even succumb early following infection. Importantly, adoptive transfer of WT but not CXCR5−/− CD4+ T cells into CXCR5−/− mice reduced bacterial loads back down to normal, indicating that expression of CXCR5 by CD4+ T cells themselves is important for control of pulmonary M. tuberculosis infection. Yet to be determined is whether CXCR5 expression by CD4+ T cells is preferentially required for the extravasation across the lung blood vascular endothelium, the efficient co-localization of CD4+ T cells with other immune cells subsets once they have exited the circulation, or both. Nonetheless, these data are major step forward as they identify the fist non-redundant chemokine receptor and ligand required specifically for optimal CD4+ T cell-dependent containment of M. tuberculosis infection.
5.4 Trafficking into the lung parenchyma
Recently two studies have shown that different subsets of M. tuberculosis-specific CD4+ and CD8+ T cells dramatically differ in their ability to migrate into the lung tissue parenchyma of infected mice. Using an intravascular staining approach to carefully distinguish between CD4+ T cells that are in the lung vasculature or lung parenchyma by flow cytometry (described in detail by Anderson et al [94]), the M. tuberculosis-specific CD4+ T cell response was found to be comprised of two major subsets that either rapidly migrate into the lung parenchyma or do not and are preferentially retained in the lung blood vessels [84] (Figure 3). Interestingly, the CD4+ T cells that produce the highest amounts of IFNγ in vivo were found to be KLRG1hiCX3CR1hiT-bethi cells that displayed little lung homing capacity, are therefore enriched within lung blood vessels, and are poorly protective against M. tuberculosis infection. In contrast, CD4+ T cells that express high levels of CXCR3, intermediate levels of T-bet and relatively low amounts of IFNγ, are highly enriched in the lung parenchyma and are highly protective against M. tuberculosis infection. Therefore, the protective capacity of M. tuberculosis-specific CD4+ T cells against pulmonary M. tuberculosis infection correlates with the ability to rapidly home to the lung parenchyma rather than produce extremely high amounts of IFNγ. These data further emphasize the importance of understanding the pathways that control effector CD4+ T cell entry into M. tuberculosis infected lungs and, moreover, the relationship between CD4+ T cell migratory capacity and differentiation state during M. tuberculosis infection.
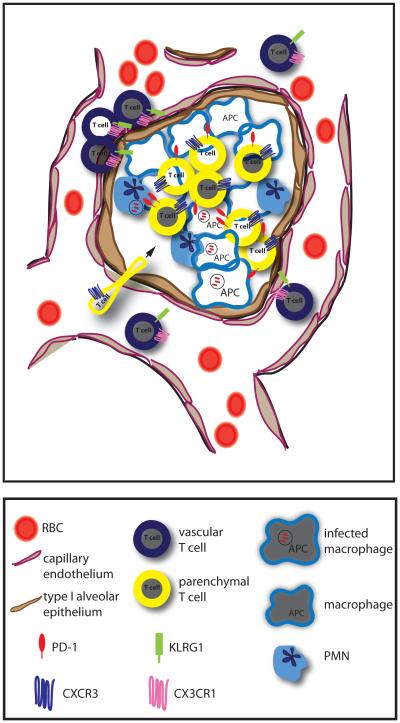
Figure 3. Migration of M. tuberculosis-specific T cells into the lung.
To contain bacterial replication, antigen-specific effector T cells migrate from blood into the infected lung tissue, where they directly interact with MHC/peptide complexes on bacilli-laden macrophages. M. tuberculosis-specific CD4+ T cells that express low levels of KLRG1 and high levels of CXCR3 migrate efficiently into the lung parenchyma where they upregulate PD-1, while CD4+ T cells that express high levels of KLRG1 and CX3CR1 are highly enriched in the lung vasculature.
6.0 The effector T cell phase
After low dose aerosol infection of mice with M. tuberculosis, there is a 10,000-fold increase in the pulmonary bacterial load within the first two weeks of infection before T cell immunity is initiated. Thus, the delay in T cell priming is detrimental as during this interval, the bacteria find their intracellular niche, multiply, and disseminate. Nevertheless, once activated, T cells are effective at preventing further increases in bacterial numbers. Indeed, onset of T cell immunity correlates with the transition from an exponential increase in bacterial numbers to a plateau phase, in which the bacterial burden remains steady for months [3,14]. The transition to the plateau phase does not occur in the absence of T cells. For example, there is progressive bacterial multiplication and premature death of the infected RAG knockout mice, which lack mature B and T cells [95,96]. The requirement for T cells has most definitively been shown in the mouse model, in which T cells and T cell subsets can be ablated by a variety of methods (e.g., genetically or using depleting antibodies), as well as in NHP, in which depletion of both CD4+ and CD8+ T cells leads to greater bacterial dissemination, growth and disease [97-100]. Similarly, the attrition of CD4+ T cells during HIV infection is one of the most important risk factors known for the development of active tuberculosis in people [101]. Following priming, T cell proliferation, acquisition of effector function, and trafficking to sites of infection, allows the host to control infection. Why immunity is effective at limiting growth but is unable to sterilize infected tissue is an important question that needs to be considered for the development of vaccines and host-directed therapies.
As immunologists divide and subdivide T cells into different lineages and subsets, defining which T cell populations mediate protection against M. tuberculosis is of great practical importance. This is crucial for developing immunization strategies and evaluating vaccine efficacy. For example, which antigens are administered, how they are delivered, and subsequently processed, determines which T cell subsets are activated. Different T cell subsets recognize different antigens types. For example, protein-based subunit vaccines preferentially stimulating CD4+ T cells while they fail to elicit CD1-restricted T cells or class I MHC-restricted CD8+ T cells. Soluble antigens, which appear to be important targets of the immune response following infection, are not as immunogenic after immunization with dead bacteria [102]. Similarly, the differentiation and acquisition of effector function by T cells is greatly influenced by the inflammatory milieu, which can be modulated by adjuvants. Evaluation of people with tuberculosis and experimentally infected animals finds that the types of T cell subsets that participate in the immune response to M. tuberculosis are exceedingly diverse. T cells expressing both αβTCRs and γδTCRs are activated following infection or can be recalled after simulation in vitro with mycobacterial antigen [103-105]. Among αβTCR+ T cells, MHC-restricted CD4+ and CD8+ account for the majority of T cells detected at the site of disease. Among these, several subsets of MHC-restricted CD4+ T cells participate in the immunity to M. tuberculosis: predominantly these are Th1, but Th17 and Treg cells are also detected. CD8+ T cells that predominantly produce cytokines as well as those with CTL activity are present [106-110]. In addition, Group 1 (e.g., CD1a, -b, and –c) and Group 2 (e.g., CD1d-restricted, a.k.a. NKT cells) CD1-restricted T cells can recognize M. tuberculosis infected macrophages and/or are detected in people infected with M. tuberculosis. Finally, there is a great deal of interest in MAIT cells, which are detected in people with tuberculosis [111,112].
By ablating various T cells subset, CD4+ T cells and to a lesser extent, CD8+ T cells, have been shown to be required for optimal host resistance to infection [113]. However, several caveats should be considered. First, there is a paucity of data concerning T cell subsets not found in mice; hence, while group 1 CD1-restricted T cells or MAIT cells can be detected in certain patients or even in biopsy or autopsy material, we have little information about how they participate in the response to infection. This T cell diversity is also confusing, particular for vaccine developers that would like to elicit T cells that can prevent tuberculosis. Studies performed in non-human primates (NHP) are valuable because they share with humans certain T cell subsets that rodent’s lack, and develop pathology that more closely resembles the spectrum of human disease. T cell studies in NHP models confirm an important role for CD4+ T cells [97,114] as well as an essential role for CD8+ T cells [115]. Even γδ T cells appear to play a more substantial role in NHPs than in mice [103,113]. We do not find this surprising since γδ T cells in mice differ significantly from those in people. Furthermore, more susceptible hosts (e.g., NHP), may have a greater dependence on T cell subsets other than CD4+ T cells for immunity. We view the rodent model as being somewhat CD4+ T cell-centric.
Still, all this is somewhat unsatisfactory as most approaches are still rather crude and are biased to detecting large effects. While we would not argue against the importance of these effects, there may be other elements required for immunity that are not as easily detected. Consider that the T cell response to M. tuberculosis is a dynamic process. For example, ‘innate’ T cells, including activated iNKT cells and γδ T cells are detected early after infection, followed by a wave of Th17 cells, before Th1 cells become established as the dominant CD4+ T cell subset present in the infected lung [116]. Different T cell subsets may be important at different times and under different conditions, for different reasons. For example, Th17 cells produce IL-17 and are important in orchestrating recruitment of PMNs and other cells. While Th17 cells were not initially thought to play an important role in protective immunity, it now appreciated that Th17 is important early during the immune response to M. tuberculosis, but can become detrimental to the host if they persist beyond a couple of weeks [117-119]. Furthermore, early IL-17 production can be made by γδ T cells or Th17 cells [116]. The Th17 response is generally inhibited by IFNγ, which is produced by Th1 cells [26,120]. CD8+ T cells are also important for immunity to tuberculosis [37]. Initially an important role was ascribed to CD8+ T cells based on the mouse model following high dose IV infections [121,122]. As the field switched to low dose aerosol model, the role for CD8+ T cells, while still important for survival, became less dramatic [113]. CD8+ T cells may be more important during latency [123]. In contrast to the mouse model, CD8+ T cells appear to have a more important role in controlling bacterial growth in the NHP model [115]. Importantly, efficient acquisition of CTL effector function by CD8+ T cells requires help from CD4+ T cells [124-126]. Thus, these two examples show how CD4+ T cells can promote host resistance to M. tuberculosis through immunoregulatory effects (inhibition of TH17 cells and promotion of CD8+ CTL function) in addition to their own direct antimicrobial function.
7.0 Mechanisms of protection
It is not entirely clear how T cells control bacterial replication and disease. Several functions of T cells have been associated with killing of intracellular bacterial in vitro or associated with a beneficial outcome in animal models. A correlate of T cell mediated protection would greatly accelerate vaccine development. However, an important confounder is that the majority of experiments that address the protection issue pose an “all or none” hypothesis – a particular mechanism is either required or not. Hence, many of the mechanisms that have been identified may be essential for host defense – but may not be sufficient. One needs to consider that quantitative differences in mediators could be the difference between resistance and susceptibility. Unfortunately, the evidence that any one T cell function correlates with disease outcome following vaccination is either weak or non-existent.
7.1 T cell production of cytokines
A major function of all types of T cells is their cytokine production. Several cytokines made by T cells directly activate macrophages to kill M. tuberculosis or have other effects on immunity. Different T cell subsets differ in their cytokine production. This is especially true for CD4+ T cell subsets that are defined by distinct master transcription factors, which regulate cytokine expression. Although there exist exceptions, IFNγ is typical of Th1 cells; IL-4, -5, and -13 are characteristic of Th2 cells; IL-17 is produced by Th17 cells; and so on. Polyfunctional or multifunctional are terms given to T cells that can produce multiple cytokines and/or other molecules including chemokines or those involved in CTL activity. Most T cells, such as those elicited by vaccination, can be classified as being polyfunctional [127]. Under various circumstances, cytokine production is lost, which can be an indicator of T cell exhaustion such as occurs during chronic LCMV infection in mice (see section 9) [128,129]. Loss of polyfunctional T cells occurs during M. tuberculosis infection both in mice and in people [130-134]. It is hoped that T cell polyfunctionality may correlate with resistance to tuberculosis and can be used as a biological marker for vaccine efficacy.
The immune response to M. tuberculosis is predominantly a type 1 cytokine response and most CD4+ T cells elicited by infection are Th1 cells that secrete IFNγ (Figure 4). Both experimental and clinical data clearly indicate that IFNγ is essential for host defense against M. tuberculosis in vivo. However, the various functions of IFNγ during infection and which ones are most important need to be clarified. For example, although IFNγ is a classic activator of macrophages, low doses of IFNγ are not particularly effective at activating macrophages, particularly human macrophages, to kill M. tuberculosis [135,136]. This issue is exceedingly complex and depends on when the IFNγ is added, its dose, the type of macrophages, the strain of M. tuberculosis, and the duration of the culture. Even in the mouse model, where IFNγ regulates inducible nitric oxide synthase (iNOS) and the production of nitric oxide (NO) by macrophages, a molecule that can kill M. tuberculosis, it is not clear whether the main function of IFNγ is to activate infected macrophages to kill M. tuberculosis in vivo. A competing hypothesis is that the immunoregulatory actions of IFNγ modulate host susceptibility (see section 7.3). Nevertheless, since T cells are an important source of IFNγ, and T cells are required for immunity to tuberculosis, the dogma is that T cell production of IFNγ is required for immunity to M. tuberculosis. Surprisingly, there is little data that IFNγ production is required for protection mediated by T cells. Recently, Flynn’s group showed that long-term survival conferred by the transfer of CD4+ T cells required IFNγ production [124]. On the other hand, several studies have shown that effector CD4+ T cells and memory CD4+ T cells can mediate protection independently of IFNγ [26,124,137]. Under these conditions, IFNγ production by other cell types was not sufficient to mediate long-term protection indicating that while other cell types can produce IFNγ, there is something unique about CD4+ T cells as has been shown previously [138].
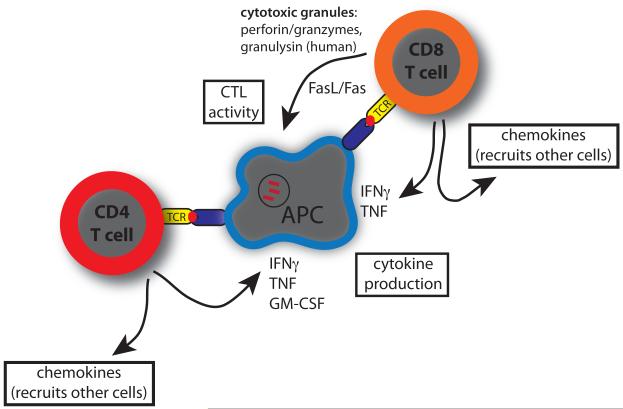
Figure 4. T cell effector functions that activate macrophages to kill M. tuberculosis.
Specific recognition of infected target cells, as indicated by TCR signaling, leads to the expression of several effector function that can activate macrophages to kill intracellular M. tuberculosis. The best characterized for rodent models and people are cytokines including IFNγ, TNF, and GM-CSF. There is also evidence for humans and a number of other species that CTL activity, generally in a perforin-dependent manner, also leads to a reduction of intracellular bacterial growth. Finally, T cells indirectly promote host resistance through their immunoregulatory actions – most relevant here is the production of chemokines that recruit other cells to the infectious focus, promote granuloma formation, and infection containment.
These data highlight a problem in how protection is studied in the mouse model. Many studies have taken advantage of knockout mice in which the cytokine gene (e.g., IFNγ) has been genetically ablated (e.g., IFNγ−/− mice). These are powerful models. However, evaluation of cytokine knockout mice can only lead to the conclusion that a particular cytokine is essential. One cannot conclude whether the cytokine is sufficient to confer immunity; nor does this approach inform us about the mechanism. Newer genetic techniques of cell-specific knockouts or the use of cell transfer studies can address whether T cell production of different mediators is required for protection, and such strategies are beginning to be applied to the murine tuberculosis model. For example, Gallegos et al systematically assessed which soluble mediators are required for CD4+ T cell protection. Using a transgenic TCR specific for the class II MHC-restricted epitope of the immunodominant antigen ESAT6, they evaluated the requirement for different cytokines known to be important in immunity to tuberculosis [137]. Surprisingly, these transgenic T cells conferred protection independently of IFNγ, TNF, perforin or fasL [137]. Even CD4+ T cells unable to make both IFNγ and TNF were able to transfer protection. One possibility is that these different cytokines are redundant in the pathways they activate in infected macrophages. Alternatively, there may be important mediators that remain to be implicated in anti-mycobacterial immunity. Such novel T cell effector functions may be revealed in environments that are not completely devoid of cytokines such as IFNγ, as is true in the model used by Gallegos [137] and Green [124]. It will be interesting to see if these models can be exploited to identify novel effector pathways that are active against M. tuberculosis.
In the meantime, we are still learning about effector molecules that are well-established in their ability to activate macrophages and suppress intracellular bacterial growth. Two of the best characterized are TNF and GM-CSF (Figure 4). Like IFNγ, TNF has multiple actions that could contribute to host defense. TNF can directly activate macrophages to restrict M. tuberculosis growth, and this activity is particularly potent in combination with IFNγ. TNF can also induce apoptosis of infected macrophages, which can lead to killing of M. tuberculosis [139,140]. Finally, TNF has a variety of immunoregulatory actions including promoting granuloma integrity. Elucidating how TNF contributes to anti-mycobacterial defense is complicated because multiple cell types produce it [141]. Allie et al investigated cell type specific TNF−/− mice and found that while myeloid cell expression of TNF is important for cell recruitment to the lung, initiation of T cell immunity and establishment of lung lesions appeared normal [141]. In contrast, mice with a T cell-specific TNF−/− were unable to control the infection during the chronic phase of the infection and died prematurely.
GM-CSF is another cytokine previously shown to restrict the intracellular growth of M. tuberculosis and M. avium in human macrophages [142,143]. Subsequently, it was discovered that GM-CSF−/− mice are highly susceptible to M. tuberculosis [144,145]. GM-CSF is also produced by many different cell types; however, T cells are potentially an important source and could act to target GM-CSF to infected cells. GM-CSF may be a marker of polyfunctional effector memory CD4+ T cells [146] and CD8+ T cells capable of producing IFNγ and GM-CSF can be detected in the granulomas of patients with LTBI [66]. Although it is not known whether T cell production of GM-CSF has an important antimicrobial effect in vivo, this possibility is supported by our work on iNKT cells [109]. iNKT cells recognize M. tuberculosis infected macrophages in vitro and inhibit intracellular bacterial growth. Restriction of bacterial replication is independent of IFNγ and instead depends on their production of GM-CSF [110]. Indeed, iNKT cells become activated during pulmonary M. tuberculosis infection and produce GM-CSF in a CD1d-restricted manner. We have observed that numerous T cell subsets produce GM-CSF early during infection, but ultimately, CD4+ T cells are the predominant T cell subset that secretes GM-CSF. Importantly, the presence of anti-GM-CSF autoantibodies that block GM-CSF function has been linked to both cryptococcal meningitis and pulmonary tuberculosis in otherwise healthy subjects indicating that GM-CSF has an important role in host defense against infection in people [147].
7.2 CTL activity
In addition to cytokine production, most T cells, but particularly CD8+ T cells, have the capacity to kill cells that they recognize. In contrast to NK cell mediated killing, T cell killing is generally TCR-dependent. CD8+ T cells with the capacity to kill target cells are called cytotoxic T lymphocytes (CTLs). CD8+ T cells are elicited during M. tuberculosis infection in people and animal models and these CD8+ T cells behave as CTLs in vivo [41,148,149] (Figure 4). There exist three dominant molecular pathways that mediate CTL activity: 1) cytotoxic granule exocytosis; 2) Fas/FasL (CD95/CD95L); and 3) TNF secretion. All three of these mechanisms are used in a hierarchical manner to kill target cells in M. tuberculosis infected mice [150]. The increased susceptibility of Fas−/−, FasL−/− and perforin−/− mice to M. tuberculosis corroborate the importance of these pathways for immunity [151,152]. Importantly, perforin is required for protection mediated by CTLs [150]. Human CD8+ T cells require perforin to restrict M. tuberculosis growth in vitro, with granulysin being an important granule constituent [153]. Other than perforin, the crucial effector molecules for murine CD8+ T cells are unknown. How killing of infected macrophages by CD8+ T cells impairs M. tuberculosis survival is an active area of investigation. All three killing mechanisms used by CTL induce target cell apoptosis, which is associated with reduced bacterial viability [154]. Following apoptosis, the engulfment of apoptotic, infected cells by uninfected macrophages – a process known as efferocytosis – targets bacteria trapped in the phagocytosed apoptotic cell (the ‘efferosome’) to lysosomes, which leads to killing of M. tuberculosis [24]. We hypothesize that a beneficial effect of CTL activity on host immunity is the ability of CD8+ T cells to kill infected cells by apoptosis, which enables the infected cells to be cleared by efferocytosis and the bacteria killed.
7.3 Immunoregulatory effects of T cells
Finding a biomarker of T cell immunity is complicated because T cells activate macrophages to kill M. tuberculosis, but they also perform other complex functions that modulate disease activity during infection. Such complexity is illustrated by the many functions of IFNγ during infection that benefit host defense. While IFNγ activates macrophages, by itself it has limited ability to induce microbicidal activity. Nevertheless, IFNγ is essential for host immunity against tuberculosis. In its absence, both people and experimentally infected animals are unable to limit disease. IFNγ production by CD4+ T cells modulates various components of the T cell response. CD4+ T cells make CD8+ T cells better CTLs and facilitate their production of IFNγ [124-126]. Other cytokines produced by T cells enhance T cell function as well. IL-2 regulates perforin expression [155] and expands effector T cells [156]. IL-21, a cytokine produced exclusively by CD4+ T cells, also regulates CD8+ CTL activity.
A second function of T cells is their key role in granuloma formation. T cell-derived cytokines (such as TNF) and chemokines (such as CCL3) recruit inflammatory macrophages, neutrophils and B cells to the granuloma [157,158]. IFNγ and TNF maintain granuloma architecture in mice and people. The importance of CD4+ T cells in shaping the granuloma microenvironment is inferred from HIV+ subjects who form dysfunctional granulomas that fail to contain M. tuberculosis [159] and by studies in guinea pigs and rabbits. Recent imaging studies in people and NHP indicate that granulomas behave autonomously and are more dynamic than previously appreciated. Granulomas change over time independently of each other with respect to size and metabolic activity – some shrink whereas others expand. Although CD4+ T cells promote granuloma formation early after M. tuberculosis infection, they also contribute to transmission by promoting granuloma necrosis accompanied by erosion into airways during later disease stages [160].
Finally, T cells have an anti-inflammatory function, which may be important in ameliorating disease. We discussed previously the importance of IFNγ in terminating the Th17 response, inhibiting neutrophil infiltration, preventing tissue destruction pathology [26]. Both IFNγ and the effector molecule NO, which can kill TB, have important anti-proliferative and immune modulating effects [27,161,162]. T cell production of IL-2 is important in expanding Tregs [156]. Although there can be other sources of IL-10, T cell production of IL-10 is associated with increased susceptibility as IL-10 inhibits effector T cell responses and leads to worse immunopathology. Too much IL-10 may be detrimental, particularly under conditions of impaired M. tuberculosis control. However, the presence or absence of IL-10 did little to alter the bacterial load in studies using inherently resistant strains of mice [163-165]. Under such conditions, it would be interesting to know whether IL-10 altered the degree of tissue damage. These anti-inflammatory effects of T cells can lessen tissue damage, but may do so at the expense of more vigorous immunity, which could more effectively eliminate M. tuberculosis.
8.0 Memory T cell responses
Vaccines have proven to be the most cost-effective intervention against infectious diseases in history, yet the development of a vaccine that reliably prevents tuberculosis has eluded scientists for nearly a century. Classic vaccine approaches using heat-killed or live-attenuated pathogens have almost exclusively been used to generate protective antibodies by relying on the immune system to develop memory B cell and possibly CD4+ T cell responses to a broad array of antigens [166]. However, the protection conferred by memory T cells elicited by vaccination with an attenuated pathogen has been disappointing for TB. The only tuberculosis vaccine approved for use in humans, BCG, is administered by intradermal or subcutaneous inoculation with an attenuated strain of Mycobacterium bovis. BCG vaccination has been widely used outside of the U.S. but does not reliably prevent pulmonary tuberculosis in adults despite reducing bacterial growth in animal models [167]. New vaccines currently being tested in clinical trials include five strains of live-attenuated mycobacteria and several different subunit vaccines delivered together with adjuvants or in viral vectors [168,169]. However, the first phase 2B clinical trial of a tuberculosis vaccine, a modified Vaccinia Ankara incorporating M. tuberculosis antigen 85A (MVA85A) failed to reduce the incidence of tuberculosis when used to boost BCG in South African infants [170]. Although environmental non-tuberculous mycobacteria (NTM) and chronic helminth infection are potential confounders to BCG vaccine efficacy in tuberculosis endemic areas [171-173], they do not explain the overall high rates of tuberculosis in endemic areas despite environmental NTM exposure, BCG vaccination, or prior M. tuberculosis infection, all of which elicit memory T cells. These observations imply a failure of “natural” memory T cells to prevent or optimally control active TB.
Classically, most effector CD4+ and CD8+ αβTCR+ T cells undergo apoptosis after antigen clearance, but a subset of ~10% survive for years after resolution of infection. These T cells are long-lived memory T cells and are found in people after antibiotic clearance of tuberculosis [174-178]. Memory T cells are additionally described by their expression of high levels of IL7-R (CD127) and CD44, and low KLRG1 expression. Central memory T cells, the subset attributed with the greatest proliferative potential, express high levels of surface CD62L and CCR7 [179-181]. Other, recently identified subsets of memory T cells, including tissue resident memory (CD69+, CD103+) and stem cell-like memory T cells (CD44lo, CD62LHi) are largely uncharacterized in the context of tuberculosis, but may also be protective [182]. Memory T cells are advantageous in host defense against infection because: 1) antigen-specific T cells have a higher frequency in the memory compartment than in the naïve repertoire; 2) memory T cells circulate through non-lymphoid tissue and independently respond to antigen at the site of infection; 3) they differentiate more rapidly into effector T cells with CTL activity and cytokine production; and 4) they have a lower threshold for activation and a decreased dependence on co-stimulation [60,177,183,184]. Despite these functional advantages, generating M. tuberculosis-specific memory T cells has shown only modest protection, if any, in animal models. Although BCG, rBCG, and protein subunit vaccination are able to modestly prolong survival and lower bacterial loads in guinea pig, macaque, and mouse models of infection with variable efficacy, these tuberculosis vaccines do not eradicate M. tuberculosis and untreated animals ultimately succumb to disease [185-188]. Furthermore, at least 2 studies have evaluated the protective capacity of memory T cells from resolved natural infection with M. tuberculosis in a mouse model by prolonged treatment with antibiotics, and found that although bacterial numbers were initially lower in “natural memory” mice, there was no significant difference in survival, or bacterial numbers at a later stage of infection, implying a transient benefit of memory T cells derived from infection [189-191]. People treated for tuberculosis actually have an increased risk of subsequent re-infection, independent of reactivation, even in low-incidence settings [192-195]. Although memory T cells should become activated and begin killing bacteria immediately after infection, IFNγ-producing CD4+ T cells are detected only three days earlier in the lung of natural-memory mice compared to previously naive mice after aerosol challenge [190]. Thus, the full benefit of potent memory T cells may not be fully realized if they are not able to respond to infection early. Once the bacterial load has risen exponentially, both naïve and memory T cells are subject to the negative effects of chronic antigenic stimulation, including terminal differentiation with decreased proliferative potential [196,197] and T cell exhaustion [128] and may become dysfunctional.
Finally, whether memory T cells develop during tuberculosis is a point of controversy. Although cells with an IL-7Rhi phenotype are elicited during infection, none express markers of central memory such as CD62L. Even if these cells could be considered “effector memory”, a large proportion of them show decreased cytokine production, indicating dysfunction [198]. Although memory T cells may not be optimally elicited by tuberculosis or LTBI, early control of M. tuberculosis infection may provide conditions under which functional memory T cells develop. A meta-analysis by Andrews, et al. showed a lower incidence of active pulmonary tuberculosis in patients with LTBI, attributable to newly-acquired infection [199]. Furthermore, memory CD4+ T cells are found to have different cell surface receptor phenotypes in patients with LTBI compared with resolved pulmonary tuberculosis [200]. Assuming an equal susceptibility to disease, these data imply that memory T cells may be more protective if generated in the context of controlled, rather than chronic, uncontrolled infection.
Control of tuberculosis is correlated with functional, IFNγ-secreting CD4+ and CD8+ T cell responses. Therefore, a logical strategy for tuberculosis vaccine development is to elicit large numbers of T cells specific for a diverse array of M. tuberculosis antigens, poised to kill bacteria within infected cells early after inoculation. One strategy that could meet this goal is to develop a vaccine that elicits effector memory CD4+ and CD8+ T cells in the lung, as well as a cohort of circulating central memory T cells. Both the rapid and potent CTL and cytokine-producing functions of effector memory T cells as well as the sustainable, high proliferative capacity of central memory T cells are believed to be important for a potentially successful tuberculosis vaccine [201-203]. Although significant effort has been placed on eliciting “polyfunctional” T cells, no study has shown that vaccine-elicited cells able to secrete multiple cytokines during infection actually develop into long-lived memory T cells that are more capable of controlling infection, as evidenced by the clinical failure of MVA85A [170]. Finally, it is possible that preventative vaccination may not be enough. If early T cell recognition of infected cells cannot be accomplished by memory, therapeutic vaccines or administration of small molecules that can rejuvenate dysfunctional T cells, activate memory T cells, or redirect antigen-specificity without causing excessive inflammation may be more strategic interventions to clear infection.
9.0 T cell exhaustion and complexities of imbalanced responses during chronic M. tuberculosis infection
Peripheral tolerance of T cells can occur in several different settings. Classical T cell clonal anergy refers to the non-responsive state of CD4+T cell clones stimulated in vitro with strong TCR signals in the absence of costimulation [204]. In vivo, antigen-specific T cells can also become intrinsically hyporesponsive in both non-infectious settings and during certain chronic infections as a result of prolonged exposed to their cognate peptide, rather than lack of proper costimulation. For example, when TCR Tg CD8+ T cells specific for the male HY antigen are adoptively transferred into male recipients, the donor T cells persist long-term but display defects in their ability to proliferate and make IL-2 in response to restimulation and lose the ability to kill male target cells [205]. Using a similar experimental strategy, pigeon cytochrome C (PCC) specific CD4+ T cells that are adoptively transferred into hosts Tg for PCC fail to become functional memory cells and instead persist in a hyporesponsive state referred to as adaptive tolerance [206]. The first example of in vivo functional inactivation of pathogen-specific T cells was observed during chronic infection with LCMV clone-13. Using MHC class I tetramers (only newly available at the time) to quantify epitope-specific CD8+ T cells, it was found that large numbers of virus-specific T cells were present throughout the duration of the infection but lacked effector functions, a state commonly referred to as T cell exhaustion [207]. It is important to point out that impaired responses of T cells in exhaustion refers to intrinsic defects in the functional quality of T cells, rather than extrinsic inhibitory signals (e.g. Tregs or myeloid cell derived IL-10), which can also play a major role in inhibiting T cell responses during infections.
T cell exhaustion is a now well-defined feature of CD8+ T cell responses to many chronic pathogens and is well studied in HIV, HCV, and HBV and in malignances [208]. Indeed, most of what we understand about peripheral tolerance of pathogen-specific T cells during persistent infections is derived from studies on CD8+ T cell exhaustion during chronic viral infection. Rather than a simple on/off, CD8+ T cells display a range of suboptimal capacities during chronic infection when compared to highly functional memory CD8+ T cells. The degree of exhaustion can be measured by comparing the percentage of antigen-specific T cells as measured by MHC tetramer staining with the percentage of cells that can respond to high-dose peptide restimulation by making the cytokine of interest. This type of quantitative approach has shown that exhaustion represents a progressive and hierarchical loss of T cell effector functions [209]. The ability of antigen-specific CD8+ T cells to proliferate and produce IL-2 is among the first effector functions to become impaired, followed by reduction in TNF production. The ability to secrete IFNγ and kill infected target cells is preserved longer during chronic infections, and their loss indicates the end stages of exhaustion. Cells that have progressed through to the most severe forms of exhaustion may become deleted. Importantly, the extent and rapidity of functional impairment and clonal deletion correlates with the degree of ongoing antigen-recognition, i.e. in the same host CD8+ T cells specific for highly abundant antigens undergo more rapid exhaustion and are then deleted, while cells that recognize lower amounts of antigen are found in intermediate states of impairment.
Much less is understood about the features of functional exhaustion in CD4+T cells. In part this is due to inherent differences in effector CD4+ and CD8+ T cells. Describing an effector function as impaired implies that it was supposed to be there in the first place, and the diversity of polarized effector CD4+T cell responses makes it difficult to disentangle CD4+T cell exhaustion from differentiation state. The lack of IL-4 production by a Th1 cell would not imply exhaustion. Although this is clear, the concept is broadly applicable to less obvious settings. For example, resting memory CD4+T cells can produce IL-2, but in highly polarized Th1 CD4+T cells that produce high levels of IFNγ, T-bet itself suppresses the transcription of IL-2 [210,211]. Therefore, the lack of IL-2 cannot necessarily be thought of as a feature of Th1 cell exhaustion. Quantifying exhaustion in CD4+T cells is further confounded by the fact that CD4+ T cells specific for the same epitope can diverge into several distinct effector fates, such as Tfh and Th1 and Th17 cells, during the same immune response. Indeed, it is likely CD4+ T cell responses to most infections are comprised of simultaneously generated populations of distinct polarized effector fates. Defects in the production of IFNγ by a population of antigen-specific CD4+ T cells could either reflect functional impairment or altered Th1 cell production. The problem is compounded by the fact that antigen-specific CD4+ T cell responses are notoriously more difficult to accurately quantify with MHC tetramers and in general far fewer pathogen-derived class II restricted epitopes have been identified. However, a recent study of anti-viral T helper cells in chronic LCMV infection has described distinct immune signaling networks associated with CD4+ T cell exhaustion [212]. These data suggest that exhausted CD4+ T cells share some transcriptional “modules’ with exhausted CD8+ T cells but largely displayed a molecular profile that distinguished them from exhausted CD8+ T cells as well as other CD4+ T cell effector fates. Importantly, given that this study used the well defined system of LCMV infection that elicits clear Th1 responses and tracked a population of CD4+ T cells of a single antigen specificity, it was able to show that exhausted antiviral CD4+ T cells are surprisingly heterogeneous in their expression of lineage-specific transcription factors and did not display the clear Th1 polarization found in memory CD4+ T cells. In all, this study showed that MHC class II restricted CD4+ T cell exhaustion is not the same molecular process as in MHC class I restricted CTL responses.
The degree of functional impairment of M. tuberculosis-specific T cells during active tuberculosis is less clear. Another approach to evaluate T cell functional capacity has been to quantify the simultaneous production of multiple cytokines by intracellular cytokine staining. Highly functional memory cells are often found to produce many different cytokines while the functional impairment of exhausted cells is characterized by reduced polyfunctionality. CD8+ T cells in C57Bl/6 mice display differential alterations in their ability to co-produce IFNγ and TNF or IL-2 over the course of infection, and the extent of the changes varies with their epitope specificity [196]. DbMtb32c93-102 specific CD8+ T cells displayed greater loss of IFNγ/TNF co-production compared to KbTB10.3/43-11 and ESAT-617-25 specific CD8+ T cells. When DbMtb32c93-102 specific CD8+ T cells were directly compared with memory DbGP33-41 specific CD8+ T cells generated after acute LCMV-Armstrong infection (a gold standard of highly functional memory cells), it was found that the M. tuberculosis-specific CD8+ T cells produced lower levels of IFNγ and TNF compared to LCMV-specific cells. In humans, recent studies show no major differences in effector cytokine profile of M. tuberculosis-specific CD8+ T cells in LTBI and tuberculosis patients [131]. Nevertheless, M. tuberculosis-specific CD8+ T cells of LTBI subjects contained an increased proportion of triple cytokine (IFNγ+/TNF+/IL-2+) producing CD8+ T cells compared to active tuberculosis patients. The observation that the majority of T cells in both LTBI and active tuberculosis patients were either dual IFNγ /TNF or single IFNγ producing CD8+ T cells provides clinical evidence of loss of other effector functions such as IL-2 in dual IFNγ /TNF or IL-2, TNF expression in single IFNγ producing CD8+ T cells. The extent of functional impairment that develops in CD4+ T cell responses in M. tuberculosis infection is also unclear. However, in humans it was shown that there is a predominance of CD4+ T cells that produce TNF but not IFNγ in the peripheral blood of patients with tuberculosis disease compared to individuals with latent infection [213]. This was carefully confirmed in another study where it was found that M. tuberculosis-specific CD4+ T cells produce TNF but not IFNγ, IL-17 or IL-2 in active disease [214]. Collectively, these data indicate that there is likely some degree of functional impairment of CD4+ and CD8+ T cells during active tuberculosis disease.
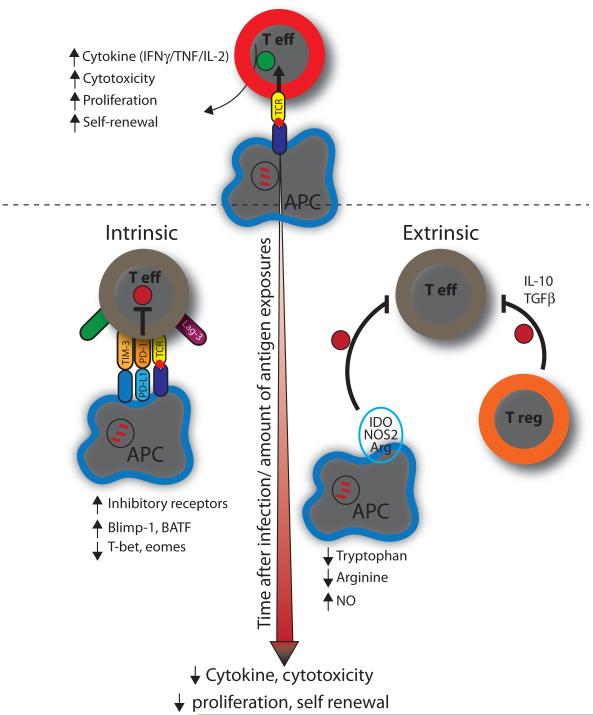
9.1 Nature vs. nurture - the role of intrinsic and extrinsic factors that drive T cell exhaustion
A complex network of immunoregulatory pathways exists to actively inhibit excessive immune responses during chronic infections and inflammation. Immunoregulatory pathways can be broadly divided into extrinsic or intrinsic pathways. Intrinsic pathways derive from within the T cell and typically involve (1) down-regulation of activating receptors such as cytokine receptors, antigen receptors and degradation of downstream signaling molecules and (2) upregulation of inhibitory receptors. Extrinsic pathways usually involve the environment in which the effector T cell is present and how its function might be nurtured by other cells, which either exert direct regulatory effect or via release of suppressive cytokines and biochemical compounds that inhibit effector functions.
9.1.1 T cell inhibitory receptors in regulating immunity to M. tuberculosis
Studies of T cell regulation in chronic viral infections as well as cancer have shown that T cell exhaustion is partially due to the upregulation of numerous inhibitory receptors that block T cell effector functions such as PD-1, Lag3, Tim-3, and 2B4 [215]. Accordingly, there has been great interest in targeting these receptors for therapeutic intervention in different settings, and several recent clinical trials have reported exciting anti-tumor efficacy of PD-1 pathway blockade [216,217]. This has led many investigators to examine the role of T cell inhibitory receptors during M. tuberculosis infection, and there is mounting evidence that host responses to M. tuberculosis are controlled by numerous cell-surface inhibitory receptors. PD-1, Tim-3 and 2B4 expression is upregulated on PBMCs, pleural fluid mononuclear cells, NK cells, and mucosal-associated invariant T cells during from patients with tuberculosis [218-222].
9.1.1.1 PD1
In mice, PD-1 is expressed at high levels on CD4+ T cells during M. tuberculosis infection, but PD-1 deficiency leads to extremely high bacterial loads and rapid mortality following low-dose aerosol infection with M. tuberculosis, rather than the enhanced resistance one might expect in the absence of an inhibitory pathway [223,224]. This is due to CD4+ T cell driven immunopathology, as CD4 depletion could prevent the early wasting and death of M. tuberculosis-infected PD-1−/− mice and adoptive transfer of PD-1−/− CD4+ T cells into M. tuberculosis infected T cell deficient mice results in early mortality [224]. The effect was associated with large increases in I-AbESAT-6 specific CD4+ T cells, profoundly upregulated inflammatory cytokine responses in the lungs, and focal necrotic areas, suggesting that the PD-1 pathway is required to control excessive inflammatory responses after M. tuberculosis infection [224]. In contrast to the critical importance of PD-1 dependent regulation in CD8+ T cells during viral infections, there was little effect of PD-1 in regulating KbTB10.3/43-11 or DbMtb32c93-102 specific CD8+ T cells and CD8+ T cells had little contribution to immunopathology in PD-1−/− M. tuberculosis infected mice. A recent study by Singh et al demonstrated that expression of PD-1 and its ligands on a per cell basis directly correlates with sputum-bacillary burden in newly diagnosed tuberculosis patients. In this study, in vitro PD-1 blockade rescued M. tuberculosis-specific T cells by increasing IFNγ and IL-2 secretion as well preventing antigen-specific T cells from undergoing apoptosis. Consistent with the known role for TCR stimulation in the induction of PD-1 expression, the number of PD-1–expressing T cells decreases significantly during therapy in both mice [225] and humans [226], suggesting that PD-1 may serve as a potential biomarker to monitor host immunity among tuberculosis patients during therapy and vaccine studies. All together, the data indicate that PD-1 does regulate CD4+ T cell function in mice and humans, but the extreme susceptibility of PD-1−/− mice to M. tuberculosis infection illustrates the complexity of the balance between T cell-driven immunopathology and host resistance to M. tuberculosis infection.
9.1.1.2 Tim-3
Like PD-1 and other inhibitory receptors, Tim-3 has been shown to be involved in T cell exhaustion during chronic viral infections and cancer [227-229]. Tim-3 expression is significantly upregulated on PBMC-derived T cells from active tuberculosis patients [218,230]. Two clinical studies from China attribute disparate functions to Tim-3+ T cells [218,230]. One study reported Tim-3-expressing T cells have an effector or effector-memory phenotype, which was associated with higher cytokine expression [218]. A second study found that Tim-3-expressing T cells were exhausted, and Tim-3 blocking antibodies could improve T cell function in vitro [230]. Thus, despite a role for Tim-3 in mediating T cell exhaustion during other chronic infections, it is not yet clear whether Tim-3 alters T cell function during tuberculosis. In the mouse model, lung-derived antigen-specific T cells express Tim-3 [231]. Interestingly, while Tim-3 may signal T cell exhaustion, its interaction with Galectin-9, its best-described ligand, activates microbicidal activity in both murine and human macrophages. Thus, Tim-3+ T cells can restrict M. tuberculosis growth in macrophages by inducing IL-1β expression and regulating cell death [139,218,231,232].
9.1.1.3 2B4
A recent study showed that tuberculosis patients that fail therapy had significantly elevated expression of 2B4 on antigen-specific CD4+ T cells compared with those that were newly-diagnosed with tuberculosis [219]. 2B4 expression may modulate effector cytokine production as 2B4int and 2B4lo had higher cytokine expression than to 2B4hi T cells. Crosslinking 2B4 on T cell surface significantly down-regulated IFNγ expression while 2B4 blockade reinvigorated IFNγ expression suggesting an intrinsic role for 2B4 in T cell exhaustion following M. tuberculosis infection [219].
9.1.2 Extrinsic inhibitory signals in M. tuberculosis infection
In mice, regulatory T cells (Treg) play an important role in inhibiting control of M. tuberculosis infection [233]. Pathogen-specific Tregs are induced early in the LN and delay T cell priming and impede the timely expansion of effector T cells in the lungs of infected mice [234,235]. In humans, Tregs are significantly increased in the peripheral blood of pulmonary tuberculosis patients compared to healthy controls [236-238]. Furthermore in vitro co-culture of CD4+CD25+FoxP3+T cells with effector T cells resulted in dramatic down-regulation of IFNγ. Depletion of CD4+CD25+FoxP3+ T cell subset enhanced T cell derived IFNγ production suggesting Treg has a direct contact-dependent inhibitory mechanism [238]. In agreement, it has been shown recently in HIV-infection and tumor models that Tim-3+ Tregs have a greater immunosuppressive capacity than Tim-3− Tregs in down-regulating IFNγ responses [239,240].
Cytokines such as IL-10 have been shown to attenuate M. tuberculosis-specific T cell responses. IL-10, secreted by Tregs, DCs, M. tuberculosis-infected macrophages, B cells and CD8+ T cells all of whom are found within the granulomatous lesion will have profound effects on T cell function [241] [242]. IL-10 has multiple pleiotropic effects and has been shown to reduce pro-inflammatory cytokine production by macrophages and T cells, impede the function of APCs, directly-dampen T cell responses [243]. Interestingly, activating T cells in the presence of IL-10 can induce nonresponsiveness/anergy that cannot be reversed by IL-2 or stimulation by anti-CD3 and anti-CD28 [243]. Susceptibility to chronic M. tuberculosis infection, in particular the susceptible CBA/J murine strain, is associated with increased pulmonary production of IL-10 [163]. It has been shown previously that IL-10-producing T cells can directly suppress immune responses in tuberculosis patients [242]. Blocking the action of IL-10 can improve outcome of M. tuberculosis infection strongly suggesting that negative role of IL-10 in the generation and maintenance of protective immunity against M. tuberculosis infection [163].
In addition to immunosuppressive cytokines, the amino acid L-arginine is found in the granulomas of infected mice and PBMCs lysates of tuberculosis patients and has a central role in regulating the expression level of CD3ζ, thereby directly influencing T cell function [244,245]. Alterations in signal transduction molecules such as changes in the expression of CD3ζ and p56lck and the absence of NF-κB p65 in the nucleus are more predominant in patients with lepromatous leprosy than in either patients with tuberculoid leprosy or healthy control subjects and might be a reflection of immunological incompetence [245]. Likewise, the signal-transduction alterations seen in patients with pulmonary tuberculosis may reflect both the different stages of immune competence and T cell–function impairments that may result in the induction of anergy, as is commonly seen in miliary tuberculosis [242]. Other extrinsic factors involve the metabolic activity within each granuloma. Recent imaging studies in people and NHPs indicate that granulomas can function autonomously and can change in size and metabolic activity independently of one another [246,247]. It is unclear whether in uncontrolled granulomatous lesions T cell exhaustion occurs or in lesion that decrease in size, whether T cell function is augmented and does not undergo functional impairment. Understanding how T cell function is regulated in these distinct foci would help development immunotherapeutic strategies to counter T cell exhaustion.
Summary.
Numerous studies describe the failure of adaptive immunity to eradicate M. tuberculosis, yet host survival depends on a functional T cell response to active infection. Furthermore, to successfully develop a tuberculosis vaccine for prevention or therapy, we need to understand why effector T cell responses do not eradicate M. tuberculosis, and why functional memory T cells cannot prevent (re-)infection. M. tuberculosis clearly evades normal T cell function, as evidenced by the substantial delay observed in the priming of antigen-specific T cells, defective T cell trafficking in the lungs, and the development of T cell dysfunction and exhaustion. However, the CD4+ and CD8+ T cell responses initially seem to develop normally after priming of naïve T cells in the lung-draining lymph nodes, perhaps this occurs too late after the onset of exponential bacterial growth to eradicate the infection. Unfortunately, memory T cells are not recalled much earlier than the endogenous, naïve T cell response, nor is it unclear where memory T cells become reactivated. We find the selective exclusion of memory CD8+ T cells during aerosol M. tuberculosis infection in the mouse model (unpublished), as does another group using the LCMV clone-13 mouse model [248], implying that precursor memory T cells might become irrelevant during chronic infection. As we begin to better understand T cell function during tuberculosis, vaccine adjuvants and small molecule therapies that address defects in the regulation of T cell function will undoubtedly be developed and will help us to understand the clinical importance of each pathway of T cell dysfunction elicited by mycobacterial infection. Developing a comprehensive understanding T cell functional deficits and their mechanisms is critical, including how to optimize memory T cell function during tuberculosis. Continuing efforts in vaccine development without this understanding may lead to additional ineffective strategies to combat the disease.
Highlights.
T cell priming is delayed following tuberculosis infection.
A balance of positive and negative costimulatory signals controls T cell responses.
Distinct T cell subsets infiltrate the lung parenchyma while others remain in the lung vasculature.
Inhibitory receptors such as PD-1 prevent CD4-T cell-driven immunopathology.
Memory T cells respond only slightly earlier than naive T cells and are unable to prevent infection
Figure 5. Chronic M. tuberculosis infection leads to functional impairment of T cells.
As M. tuberculosis establishes a chronic infection, the composition of T cell population changes and more polyfunctional T cells are lost. T cell exhaustion develops in a step-wise and progressive loss of IFNγ, TNF and IL-2. Both intrinsic T cell factors and extrinsic factors in the microenvironment mold the exhaustion signature. Exhausted T cells express an array of inhibitory receptors such as PD-1, Tim-3, Lag-3 and 2B4 and distinctive patterns of cytokine receptors and transcription factors (Blimp-1), which distinguish them from conventional effector T cells. While inhibitory receptors such as PD-1 may impair bacterial control during chronic M. tuberculosis infection, PD-1 is also prevents CD4+ T cell-driven tissue destruction.
Acknowledgments
D.L.B is supported by the Intramural Research Program of the NIH/NIAID. S.M.B. is supported by the following NIH grants: AI106725, AI098637, AI085669, and AI100766
Abbreviations
- Ag
antigen
- APC
antigen presenting cell
- BCG
bacillus Calmette-Guerin
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HIV
human immunodeficiency virus
- HPV
human papillomavirus
- HBV
hepatitis B virus
- IFN
interferon
- LCMV
lymphocytic choriomeningitis virus
- LN
lymph node
- MAIT
mucosa-associated invariant T
- MHC
major histocompatibility complex
- NHP
non-human primate
- NTM
non-tuberculous mycobacteria
- SLO
secondary lymphoid organ
- TCR
T-cell receptor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
D.L.B. has patents and receives patent royalties related to PD-1.
References
- [1].Horsburgh CR, Jr., Rubin EJ. Latent Tuberculosis Infection in the United States. N Engl J Med. 2011;364:1441–8. doi: 10.1056/NEJMcp1005750. [DOI] [PubMed] [Google Scholar]
- [2].North RJ, Jung Y-J. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- [3].Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar S. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tian T, Woodworth J, Sköld M, Behar S. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–72. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- [5].Hanekom WA, Mendillo M, Manca C, Haslett PAJ, Siddiqui MR, Barry C, et al. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J Infect Dis. 2003;188:257–66. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- [6].Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- [7].Myers AJ, Marino S, Kirschner DE, Flynn JL. Inoculation dose of Mycobacterium tuberculosis does not influence priming of T cell responses in lymph nodes. J Immunol. 2013;190:4707–16. doi: 10.4049/jimmunol.1203465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–17. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- [9].Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–46. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- [10].Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar S. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010;11:751–8. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blomgran R, Ernst JD. Lung Neutrophils Facilitate Activation of Naive Antigen-Specific CD4+ T Cells during Mycobacterium tuberculosis Infection. J Immunol. 2011;186:7110–9. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Olmos S, Stukes S, Ernst JD. Ectopic Activation of Mycobacterium tuberculosis-Specific CD4+ T Cells in Lungs of CCR7−/− Mice. J Immunol. 2010;184:895–901. doi: 10.4049/jimmunol.0901230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- [14].Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–15. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis Inhibits Neutrophil Apoptosis, Leading to Delayed Activation of Naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci USA. 2008;105:10961–6. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–68. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Samstein M, Schreiber HA, Leiner IM, Sušac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Srivastava S, Ernst JD. Cell-to-Cell Transfer of M. tuberculosis Antigens Optimizes CD4 T Cell Priming. Cell Host Microbe. 2014;15:741–52. doi: 10.1016/j.chom.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117:2279–88. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen M, Divangahi M, Gan H, Shin DSJ, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. Journal of Experimental Medicine. 2008;205:2791–801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, et al. Efferocytosis Is an Innate Antibacterial Mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farinacci M, Weber S, Kaufmann SHE. The recombinant tuberculosis vaccine rBCG ΔureC::hly+ induces apoptotic vesicles for improved priming of CD4+ and CD8+ T cells. Vaccine. 2012;30:7608–14. doi: 10.1016/j.vaccine.2012.10.031. [DOI] [PubMed] [Google Scholar]
- [26].Nandi B, Behar S. Regulation of neutrophils by interferon-limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome–dependent processing of IL-1β. Nat Immunol. 2012;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Seshadri C, Turner MT, Lewinsohn DM, Moody DB, van Rhijn I. Lipoproteins Are Major Targets of the Polyclonal Human T Cell Response to Mycobacterium tuberculosis. J Immunol. 2012;190:278–84. doi: 10.4049/jimmunol.1201667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- [30].Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal Activation of Antigen-Specific CD4+ Effector Cells Enables Persistence of M. tuberculosis In Vivo. PLoS Pathog. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aagaard CS, Hoang T, Dietrich J, Cardona P-J, Izzo A, Dolganov G, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–94. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- [32].Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [33].Sayes F, Sun L, Di Luca M, Simeone R, Degaiffier N, Fiette L, et al. Strong Immunogenicity and Cross-Reactivity of Mycobacterium tuberculosis ESX-5 Type VII Secretion -Encoded PE-PPE Proteins Predicts Vaccine Potential. Cell Host Microbe. 2012;11:352–63. doi: 10.1016/j.chom.2012.03.003. [DOI] [PubMed] [Google Scholar]
- [34].Garces A, Atmakuri K, Chase MR, Woodworth JS, Krastins B, Rothchild AC, et al. EspA Acts as a Critical Mediator of ESX1-Dependent Virulence in Mycobacterium tuberculosis by Affecting Bacterial Cell Wall Integrity. PLoS Pathog. 2010;6:e1000957. doi: 10.1371/journal.ppat.1000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Woodworth JS, Fortune SM, Behar S. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun. 2008;76:4199–205. doi: 10.1128/IAI.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kunnath-Velayudhan S, Porcelli SA. Recent Advances in Defining the Immunoproteome of Mycobacterium tuberculosis. Front Immunol. 2013;4:335. doi: 10.3389/fimmu.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Woodworth JSM, Behar S. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006;26:317–52. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Woodworth JS, Shin D, Volman M, Nunes-Alves C, Fortune SM, Behar S. Mycobacterium tuberculosis Directs Immunofocusing of CD8+ T Cell Responses Despite Vaccination. J Immunol. 2011;186:1627–37. doi: 10.4049/jimmunol.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoang TTKT, Nansen A, Roy S, Billeskov R, Aagaard CS, Elvang T, et al. Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS ONE. 2009;4:e5928. doi: 10.1371/journal.pone.0005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kamath A, Woodworth JSM, Behar S. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. 2006;177:6361–9. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar S. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. 2004;200:1479–89. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Skjøt RLV, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff THM, et al. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70:5446–53. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Axelsson-Robertson R, Weichold F, Sizemore D, Wulf M, Skeiky YAW, Sadoff J, et al. Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8+ T-cell responses. Immunology. 2009;129:496–505. doi: 10.1111/j.1365-2567.2009.03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Axelsson-Robertson R, Ahmed RK, Weichold FF, Ehlers MM, Kock MM, Sizemore D, et al. Human Leukocyte Antigens A*3001 and A*3002 Show Distinct Peptide-Binding Patterns of the Mycobacterium tuberculosis Protein TB10.4: Consequences for Immune Recognition. Clinical and Vaccine Immunology. 2011;18:125–34. doi: 10.1128/CVI.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, et al. Memory T Cells in Latent Mycobacterium tuberculosis Infection Are Directed against Three Antigenic Islands and Largely Contained in a CXCR3+CCR6+ Th1 Subset. PLoS Pathog. 2013;9:e1003130. doi: 10.1371/journal.ppat.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lewinsohn DA, Winata E, Swarbrick GM, Tanner KE, Cook MS, Null MD, et al. Immunodominant Tuberculosis CD8 Antigens Preferentially Restricted by HLA-B. PLoS Pathog. 2007;3:e127–49. doi: 10.1371/journal.ppat.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24:529–36. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- [48].Pottumarthy S, Morris AJ, Harrison AC, Wells VC. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J Clin Microbiol. 1999;37:3229–32. doi: 10.1128/jcm.37.10.3229-3232.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bellete B, Coberly J, Barnes GL, Ko C, Chaisson RE, Comstock GW, et al. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin Infect Dis. 2002;34:1449–56. doi: 10.1086/340397. [DOI] [PubMed] [Google Scholar]
- [50].Shams H, Klucar P, Weis SE, Lalvani A, Moonan PK, Safi H, et al. Characterization of a Mycobacterium tuberculosis peptide that is recognized by human CD4+ and CD8+ T cells in the context of multiple HLA alleles. J Immunol. 2004;173:1966–77. doi: 10.4049/jimmunol.173.3.1966. [DOI] [PubMed] [Google Scholar]
- [51].Lindestam Arlehamn CS, Sidney J, Henderson R, Greenbaum JA, James EA, Moutaftsi M, et al. Dissecting Mechanisms of Immunodominance to the Common Tuberculosis Antigens ESAT-6, CFP10, Rv2031c (hspX), Rv2654c (TB7.7), and Rv1038c (EsxJ) J Immunol. 2012;188:5020–31. doi: 10.4049/jimmunol.1103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wu Y, Woodworth JS, Shin DS, Morris S, Behar S. Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+ T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection. Infect Immun. 2008;76:2249–55. doi: 10.1128/IAI.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Korsholm KS, Hansen J, Karlsen K, Filskov J, Mikkelsen M, Lindenstrøm T, et al. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32:3927–35. doi: 10.1016/j.vaccine.2014.05.050. [DOI] [PubMed] [Google Scholar]
- [54].Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. Protective CD4 T Cells Targeting Cryptic Epitopes of Mycobacterium tuberculosis Resist Infection-Driven Terminal Differentiation. J Immunol. 2014;192:3247–58. doi: 10.4049/jimmunol.1300283. [DOI] [PubMed] [Google Scholar]
- [56].Murugan A, Mora T, Walczak AM, Callan CG. Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proc Natl Acad Sci USA. 2012;109:16161–6. doi: 10.1073/pnas.1212755109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Arstila TPT, Casrouge AA, Baron VV, Even JJ, Kanellopoulos JJ, Kourilsky PP. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- [58].Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the Composition of the Preimmune Repertoire of T Cells Specific for Peptide–Major Histocompatibility Complex Ligands. Annu Rev Immunol. 2010;28:275–94. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- [59].Li H, Ye C, Ji G, Han J. Determinants of public T cell responses. Cell Res. 2012;22:33–42. doi: 10.1038/cr.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–10. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Du G, Chen CY, Shen Y, Qiu L, Huang D, Wang R, et al. TCR Repertoire, Clonal Dominance, and Pulmonary Trafficking of Mycobacterium-Specific CD4+ and CD8+ T Effector Cells in Immunity Against Tuberculosis. J Immunol. 2010;185:3940–7. doi: 10.4049/jimmunol.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, Wahn U, et al. Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J Immunol. 2007;179:1331–9. doi: 10.4049/jimmunol.179.2.1331. [DOI] [PubMed] [Google Scholar]
- [63].Yang J, Xu K, Zheng J, Wei L, Fan J, Li L. Limited T cell receptor beta variable repertoire responses to ESAT-6 and CFP-10 in subjects infected with Mycobacterium tuberculosis. Tuberculosis. 2013;93:529–37. doi: 10.1016/j.tube.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [64].Yang J, He J, Huang H, Ji Z, Wei L, Ye P, et al. Molecular characterization of T cell receptor beta variable in the peripheral blood T cell repertoire in subjects with active tuberculosis or latent tuberculosis infection. BMC Infect Dis. 2013;13:423. doi: 10.1186/1471-2334-13-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Luo W, Su J, Zhang X-B, Yang Z, Zhou M-Q, Jiang Z-M, et al. Limited T cell receptor repertoire diversity in tuberculosis patients correlates with clinical severity. PLoS ONE. 2012;7:e48117. doi: 10.1371/journal.pone.0048117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tully G, Kortsik C, Höhn H, Zehbe I, Hitzler WE, Neukirch C, et al. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol. 2005;174:2174–84. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- [67].Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- [68].Dolfi DV, Duttagupta PA, Boesteanu AC, Mueller YM, Oliai CH, Borowski AB, et al. Dendritic Cells and CD28 Costimulation Are Required To Sustain Virus-Specific CD8+ T Cell Responses during the Effector Phase In Vivo. J Immunol. 2011;186:4599–608. doi: 10.4049/jimmunol.1001972. [DOI] [PubMed] [Google Scholar]
- [69].Bhatt K, Uzelac A, Mathur S, McBride A, Potian J, Salgame P. B7 costimulation is critical for host control of chronic Mycobacterium tuberculosis infection. The Journal of Immunology. 2009;182:3793–800. doi: 10.4049/jimmunol.0802996. [DOI] [PubMed] [Google Scholar]
- [70].Bhatt K, Kim A, Kim A, Mathur S, Salgame P. Equivalent functions for B7.1 and B7.2 costimulation in mediating host resistance to Mycobacterium tuberculosis. Cell Immunol. 2013;285:69–75. doi: 10.1016/j.cellimm.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [71].Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, et al. Mycobacterium tuberculosis Lipoproteins Directly Regulate Human Memory CD4+ T Cell Activation via Toll-Like Receptors 1 and 2. Infect Immun. 2011;79:663–73. doi: 10.1128/IAI.00806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Reba SM, Li Q, Onwuzulike S, Ding X, Karim AF, Hernandez Y, et al. TLR2 engagement on CD4 +T cells enhances effector functions and protective responses to Mycobacterium tuberculosis. Eur J Immunol. 2014;44:1410–21. doi: 10.1002/eji.201344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li Q, Ding X, Thomas JJ, Harding CV, Pecora ND, Ziady AG, et al. Rv2468c, a novel Mycobacterium tuberculosis protein that costimulates human CD4+ T cells through VLA-5. J Leukoc Biol. 2012;91:311–20. doi: 10.1189/jlb.0711364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–62. [PubMed] [Google Scholar]
- [75].Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- [77].Srivastava S, Ernst JD. Cutting Edge: Direct Recognition of Infected Cells by CD4 T Cells Is Required for Control of Intracellular Mycobacterium tuberculosis In Vivo. J Immunol. 2013;191:1016–20. doi: 10.4049/jimmunol.1301236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schreiber T, Ehlers S, Aly S, Hölscher A, Hartmann S, Lipp M, et al. Selectin ligand-independent priming and maintenance of T cell immunity during airborne tuberculosis. J Immunol. 2006;176:1131–40. doi: 10.4049/jimmunol.176.2.1131. [DOI] [PubMed] [Google Scholar]
- [79].Taylor JL, Bielefeldt-Ohmann H, Pozzi A, Izzo AA. Lack of alpha-1 integrin alters lesion morphology during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2008;88:444–52. doi: 10.1016/j.tube.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ghosh S, Chackerian AA, Parker CM, Ballantyne CM, Behar S. The LFA-1 adhesion molecule is required for protective immunity during pulmonary Mycobacterium tuberculosis infection. J Immunol. 2006;176:4914–22. doi: 10.4049/jimmunol.176.8.4914. [DOI] [PubMed] [Google Scholar]
- [81].Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol. 2000;165:2596–602. doi: 10.4049/jimmunol.165.5.2596. [DOI] [PubMed] [Google Scholar]
- [82].Johnson CM, Cooper AM, Frank AA, Orme IM. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–70. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–9. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, et al. Cutting Edge: Control of Mycobacterium tuberculosis Infection by a Subset of Lung Parenchyma-Homing CD4 T Cells. J Immunol. 2014;192:2965–9. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Seiler P, Aichele P, Bandermann S, Hauser AE, Lu B, Gerard NP, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33:2676–86. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- [86].Chakravarty SD, Xu J, Lu B, Gerard C, Flynn J, Chan J. The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. J Immunol. 2007;178:1723–35. doi: 10.4049/jimmunol.178.3.1723. [DOI] [PubMed] [Google Scholar]
- [87].Algood HMS, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol. 2004;173:3287–96. doi: 10.4049/jimmunol.173.5.3287. [DOI] [PubMed] [Google Scholar]
- [88].Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, et al. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J Exp Med. 2011;208:1621–34. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hall JD, Kurtz SL, Rigel NW, Gunn BM, Taft-Benz S, Morrison JP, et al. The impact of chemokine receptor CX3CR1 deficiency during respiratory infections with Mycobacterium tuberculosisor Francisella tularensis. Clin Exp Immunol. 2009;156:278–84. doi: 10.1111/j.1365-2249.2009.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Day TA, Koch M, Nouailles G, Jacobsen M, Kosmiadi GA, Miekley D, et al. Secondary lymphoid organs are dispensable for the development of T-cell-mediated immunity during tuberculosis. Eur J Immunol. 2010;40:1663–73. doi: 10.1002/eji.201040299. [DOI] [PubMed] [Google Scholar]
- [91].Kahnert A, Höpken UE, Stein M, Bandermann S, Lipp M, Kaufmann SHE. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- [92].Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine–induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–84. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Slight SR, Rangel Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–26. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–22. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ehlers S, Benini J, Held HD, Roeck C, Alber G, Uhlig S. Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J Exp Med. 2001;194:1847–59. doi: 10.1084/jem.194.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185–92. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- [97].Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T Cell Depletion Exacerbates Acute Mycobacterium tuberculosisWhile Reactivation of Latent Infection Is Dependent on Severity of Tissue Depletion in Cynomolgus Macaques. AIDS Res Hum Retroviruses. 2012;28:1693–702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–58. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, et al. Reactivation of Latent Tuberculosis in Cynomolgus Macaques Infected with SIV Is Associated with Early Peripheral T Cell Depletion and Not Virus Load. PLoS ONE. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Flory CM, Hubbard RD, Collins FM. Effects of in vivo T lymphocyte subset depletion on mycobacterial infections in mice. J Leukoc Biol. 1992;51:225–9. doi: 10.1002/jlb.51.3.225. [DOI] [PubMed] [Google Scholar]
- [101].Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7:268–75. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- [102].Weiss DW, Dubos RJ. Antituberculous immunity induced in mice by vaccination with killed tubercle bacilli or with a soluble bacillary extract. J Exp Med. 1955;101:313–30. doi: 10.1084/jem.101.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shen Y, Zhou D, Zhou D, Qiu L, Lai X, Lai X, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, et al. Phosphoantigen/IL2 Expansion and Differentiation of Vγ2Vδ2 T Cells Increase Resistance to Tuberculosis in Nonhuman Primates. PLoS Pathog. 2013;9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Behar S, Boom WH. Handbook of Tuberculosis. Wiley-VCH; Weinheim: 2008. Unconventional T cells. [Google Scholar]
- [106].Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77:4621–30. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Behar S, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–50. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- [108].Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature Publishing Group. 2013;14:706–13. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar S. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rothchild AC, Jayaraman P, Nunes-Alves C, Behar S. iNKT Cell Production of GM-CSF Controls Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1003805. doi: 10.1371/journal.ppat.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gold MC, Ehlinger HD, Cook MS, Smyk-Pearson SK, Wille PT, Ungerleider RM, et al. Human innate Mycobacterium tuberculosis-reactive alphabetaTCR+ thymocytes. PLoS Pathog. 2008;4:e39. doi: 10.1371/journal.ppat.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T Cells Contain Early Extrapulmonary Tuberculosis (TB) Dissemination and Rapid TB Progression and Sustain Multieffector Functions of CD8+ T and CD3− Lymphocytes: Mechanisms of CD4+ T Cell Immunity. J Immunol. 2014;192:2120–32. doi: 10.4049/jimmunol.1301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- [117].Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–16. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-Specific Th17 Cells Confer Partial Protection against Mycobacterium tuberculosis Infection in the Absence of Gamma Interferon. Infect Immun. 2010;78:4187–94. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, et al. Essential Role of IL-17A in the Formation of a Mycobacterial Infection-Induced Granuloma in the Lung. J Immunol. 2010;184:4414–22. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- [120].Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–20. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- [121].Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Behar S, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–98. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [124].Green AM, DiFazio R, Flynn JL. IFN- from CD4 T Cells Is Essential for Host Survival and Enhances CD8 T Cell Function during Mycobacterium tuberculosis Infection. J Immunol. 2012;190:270–7. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167:6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- [126].Bold TD, Ernst JD. CD4+ T Cell-Dependent IFN- Production by CD8+ Effector T Cells in Mycobacterium tuberculosis Infection. J Immunol. 2012;189:2530–6. doi: 10.4049/jimmunol.1200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [129].Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- [130].Qiu Z, Zhang M, Zhu Y, Zheng F, Lu P, Liu H, et al. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci Rep. 2012;2:216. doi: 10.1038/srep00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, et al. Mycobacterium tuberculosis-specific CD8 +T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43:1568–77. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–9. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'rie T, et al. Functional Capacity of Mycobacterium tuberculosis-Specific T Cell Responses in Humans Is Associated with Mycobacterial Load. J Immunol. 2011;187:2222–32. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Day CL, Moshi ND, Abrahams D-A, van Rooyen M, O'rie T, de Kock M, et al. Patients with Tuberculosis Disease Have Mycobacterium tuberculosis-Specific CD8 T Cells with a Pro-Apoptotic Phenotype and Impaired Proliferative Capacity, Which Is Not Restored following Treatment. PLoS ONE. 2014;9:e94949. doi: 10.1371/journal.pone.0094949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Douvas GS, Looker DL, Vatter AE, Crowle AJ. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hu X, Herrero C, Li W-P, Antoniv TT, Falck-Pedersen E, Koch AE, et al. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–66. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- [137].Gallegos AM, Van Heijst JWJ, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–16. [PubMed] [Google Scholar]
- [139].Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, et al. IL-1 Promotes Antimicrobial Immunity in Macrophages by Regulating TNFR Signaling and Caspase-3 Activation. J Immunol. 2013;190:4196–204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–41. [PubMed] [Google Scholar]
- [141].Allie N, Grivennikov SI, Keeton R, Hsu N-J, Bourigault M-L, Court N, et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection. Sci Rep. 2013;3:1809. doi: 10.1038/srep01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Denis M, Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol Lett. 1990;24:203–6. doi: 10.1016/0165-2478(90)90049-v. [DOI] [PubMed] [Google Scholar]
- [143].Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–7. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- [144].Szeliga J, Daniel DS, Yang C-H, Sever-Chroneos Z, Jagannath C, Chroneos ZC. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb) 2008;88:7–20. doi: 10.1016/j.tube.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–22. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- [146].Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, et al. Mycobacterium tuberculosis-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. Cytokine. 2008;43:143–8. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [147].Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF Autoantibodies in Patients with Cryptococcal Meningitis. J Immunol. 2013;190:3959–66. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Skinner MA, Parlane N, McCarthy A, Buddle BM. Cytotoxic T-cell responses to Mycobacterium bovis during experimental infection of cattle with bovine tuberculosis. Immunology. 2003;110:234–41. doi: 10.1046/j.1365-2567.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Pathan AA, Wilkinson KA, Wilkinson RJ, Latif M, Mcshane H, Pasvol G, et al. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000;30:2713–21. doi: 10.1002/1521-4141(200009)30:9<2713::AID-IMMU2713>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [150].Woodworth JS, Wu Y, Behar S. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Turner J, D'Souza CD, Pearl JE, Marietta P, Noel M, Frank AA, et al. CD8− and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am J Respir Cell Mol Biol. 2001;24:203–9. doi: 10.1165/ajrcmb.24.2.4370. [DOI] [PubMed] [Google Scholar]
- [152].Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci USA. 2000;97:4204–8. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- [154].Behar S, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010 doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and Inflammation Induce Distinct Transcriptional Programs that Promote the Differentiation of Effector Cytolytic T Cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC, et al. IL-2 Simultaneously Expands Foxp3+ T Regulatory and T Effector Cells and Confers Resistance to Severe Tuberculosis (TB): Implicative Treg-T Effector Cooperation in Immunity to TB. J Immunol. 2012;188:4278–88. doi: 10.4049/jimmunol.1101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- [158].Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103–11. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- [159].Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–46. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- [160].Kwan CK, Ernst JD. HIV and Tuberculosis: a Deadly Human Syndemic. Clin Microbiol Rev. 2011;24:351–76. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Pearl JE, Torrado E, Tighe M, Fountain JJ, Solache A, Strutt T, et al. Nitric oxide inhibits the accumulation of CD4 +CD44 hiTbet +CD69 loT cells in mycobacterial infection. Eur J Immunol. 2012;42:3267–79. doi: 10.1002/eji.201142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Cooper A, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN-gamma and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10:221–6. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- [163].Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–50. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].North RJ. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–8. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 Signaling during Bacillus Calmette-Guerin Vaccination Enhances and Sustains Th1, Th17, and Innate Lymphoid IFN- and IL-17 Responses and Increases Protection to Mycobacterium tuberculosis Infection. J Immunol. 2012;189:4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. The Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- [168].Ottenhoff THM, Kaufmann SHE. Vaccines against Tuberculosis: Where Are We and Where Do We Need to Go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–58. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. The Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Black GF, Dockrell HM, Crampin AC, Floyd S, Weir RE, Bliss L, et al. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis. 2001;184:322–9. doi: 10.1086/322042. [DOI] [PubMed] [Google Scholar]
- [172].Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70:672–8. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine. 2008;26:3897–902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- [174].Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–60. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- [175].Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- [176].Orme IM. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988;140:3589–93. [PubMed] [Google Scholar]
- [177].Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–8. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- [178].Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- [179].Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and Functional Profiling of Memory CD8 T Cell Differentiation. Cell. 2002;111:837–51. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- [180].Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Henao-Tamayo M, Ordway DJ, Orme IM. Memory T cell subsets in tuberculosis: What should we be targeting? Tuberculosis. 2014 doi: 10.1016/j.tube.2014.05.001. [DOI] [PubMed] [Google Scholar]
- [183].Jameson SC, Masopust D. Diversity in T Cell Memory: An Embarrassment of Riches. Immunity. 2009;31:859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Weng N-P, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–15. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective Effect of a Tuberculosis Subunit Vaccine Based on a Fusion of Antigen 85B and ESAT-6 in the Aerosol Guinea Pig Model. Infect Immun. 2004;72:6148–50. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SHE. Recombinant BCG ureC hly+ Induces Superior Protection Over Parental BCG by Stimulating a Balanced Combination of Type 1 and Type 17 Cytokine Responses. J Infect Dis. 2011;204:1573–84. doi: 10.1093/infdis/jir592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. 2012;122:303–14. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A Defined Tuberculosis Vaccine Candidate Boosts BCG and Protects Against Multidrug-Resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74–4. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Cooper AM, Callahan JE, Keen M, Belisle JT, Orme IM. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- [190].Jung Y-J, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–24. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Kamath AB, Behar S. Anamnestic responses of mice following Mycobacterium tuberculosis infection. Infect Immun. 2005;73:6110–8. doi: 10.1128/IAI.73.9.6110-6118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Millet J-P, Shaw E, Orcau A, Casals M, Miró JM, Caylà JA, et al. Tuberculosis Recurrence after Completion Treatment in a European City: Reinfection or Relapse? PLoS ONE. 2013;8:e64898. doi: 10.1371/journal.pone.0064898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Crofts JP, Andrews NJ, Barker RD, Delpech V, Abubakar I. Risk factors for recurrent tuberculosis in England and Wales, 1998-2005. Thorax. 2010;65:310–4. doi: 10.1136/thx.2009.124677. [DOI] [PubMed] [Google Scholar]
- [194].Dobler CC, Crawford ABH, Jelfs PJ, Gilbert GL, Marks GB. Recurrence of tuberculosis in a low-incidence setting. Eur Respir J. 2009;33:160–7. doi: 10.1183/09031936.00104108. [DOI] [PubMed] [Google Scholar]
- [195].van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- [196].Jeong YH, Jeon BY, Gu SH, Cho SN, Shin S-J, Chang J, et al. Differentiation of Antigen-Specific T Cells with Limited Functional Capacity during Mycobacterium tuberculosis Infection. Infect Immun. 2013;82:132–9. doi: 10.1128/IAI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Jeong YH, Jeon BY, Gu SH, Cho SN, Shin S-J, Chang J, et al. Differentiation of Antigen-Specific T Cells with Limited Functional Capacity during Mycobacterium tuberculosis Infection. Infect Immun. 2014;82:3514–4. doi: 10.1128/IAI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54:784–91. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct Effector Memory CD4+ T Cell Signatures in Latent Mycobacterium tuberculosis Infection, BCG Vaccination and Clinically Resolved Tuberculosis. PLoS ONE. 2012;7:e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Henao-Tamayo M, Ordway DJ, Orme IM. Memory T cell subsets in tuberculosis: What should we be targeting? Tuberculosis (Edinb) 2014 doi: 10.1016/j.tube.2014.05.001. [DOI] [PubMed] [Google Scholar]
- [202].Andersen P, Woodworth JS. Tuberculosis vaccines--rethinking the current paradigm. Trends Immunol. 2014;35:387–95. doi: 10.1016/j.it.2014.04.006. [DOI] [PubMed] [Google Scholar]
- [203].Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar S. In search of a new paradigm for protective immunity to TB. Nature Publishing Group. 2014;12:289–99. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- [205].Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, et al. Modifications of CD8+T Cell Function during In Vivo Memory or Tolerance Induction. Immunity. 1998;8:581–90. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- [206].Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–9. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- [207].Kalia V, Sarkar S, Ahmed R. Memory T Cells. Vol. 684. Springer New York; New York, NY: 2010. CD8 T-Cell Memory Differentiation during Acute and Chronic Viral Infections; pp. 79–95. [DOI] [PubMed] [Google Scholar]
- [208].Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Wherry EJ. T cell exhaustion. Nat Immunol. 2011;131:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- [210].Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- [211].Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- [212].Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, et al. Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, et al. Dominant TNF-α+ Mycobacterium tuberculosis–specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–6. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, et al. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4 +T cells in active disease. Eur J Immunol. 2013;43:939–48. doi: 10.1002/eji.201243090. [DOI] [PubMed] [Google Scholar]
- [215].Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–7. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, et al. Tim-3-Expressing CD4+ and CD8+ T Cells in Human Tuberculosis (TB) Exhibit Polarized Effector Memory Phenotypes and Stronger Anti-TB Effector Functions. PLoS Pathog. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Yang B, Wang X, Jiang J, Cheng X. Involvement of CD244 in Regulating CD4+ T Cell Immunity in Patients with Active Tuberculosis. PLoS ONE. 2013;8:e63261. doi: 10.1371/journal.pone.0063261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Jurado JO, Alvarez IB, Pasquinelli V, Martínez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–25. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- [221].Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, la Barrera de SS, et al. Role Played by the Programmed Death-1–Programmed Death Ligand Pathway during Innate Immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202:524–32. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- [222].Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med. 2014;190:329–39. doi: 10.1164/rccm.201401-0106OC. [DOI] [PubMed] [Google Scholar]
- [223].Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA. 2010;107:13402–7. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T Cells Promote Rather than Control Tuberculosis in the Absence of PD-1-Mediated Inhibition. J Immunol. 2011;186:1598–607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Henao-Tamayo M, Irwin SM, Shang S, Ordway D, Orme IM. T lymphocyte surface expression of exhaustion markers as biomarkers of the efficacy of chemotherapy for tuberculosis. Tuberculosis. 2011;91:308–13. doi: 10.1016/j.tube.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the Programmed Death 1 Pathway Rescues Mycobacterium tuberculosis-Specific Interferon -Producing T Cells From Apoptosis in Patients With Pulmonary Tuberculosis. J Infect Dis. 2013;208:603–15. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- [227].Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, Mcmahon BJ, et al. Negative Immune Regulator Tim-3 Is Overexpressed on T Cells in Hepatitis C Virus Infection and Its Blockade Rescues Dysfunctional CD4+ and CD8+ T Cells. J Virol. 2009;83:9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Wang X, Cao Z, Jiang J, Li Y, Dong M, Ostrowski M, et al. Elevated expression of Tim-3 on CD8 T cells correlates with disease severity of pulmonary tuberculosis. Journal of Infection. 2011;62:292–300. doi: 10.1016/j.jinf.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [231].Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343–54. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Sada-Ovalle I, Chávez-Galán L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, et al. The Tim3-Galectin 9 Pathway Induces Antibacterial Activity in Human Macrophages Infected with Mycobacterium tuberculosis. J Immunol. 2012;189:5896–902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–93. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, et al. Pathogen-Specific Treg Cells Expand Early during Mycobacterium tuberculosis Infection but Are Later Eliminated in Response to Interleukin-12. Immunity. 2013;38:1261–70. doi: 10.1016/j.immuni.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T Cells Are Expanded in Blood and Disease Sites in Patients with Tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- [237].Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Chen X, Zhou B, Li M, Deng Q, Wu X, Le X, et al. CD4+CD25+FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clinical Immunology. 2007;123:50–9. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- [239].Elahi S, Dinges WL, Lejarcegui N, Laing KJ, Collier AC, Koelle DM, et al. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011;17:989–95. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, et al. TIM3+FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- [242].Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–25. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [244].Modolell M, Choi B-S, Ryan RO, Hancock M, Titus RG, Abebe T, et al. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3:e480–0. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Zea AH, Culotta KS, Ali J, Mason C, Park H-J, Zabaleta J, et al. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–93. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- [246].Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med. 2013;20:75–9. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–40. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].West EE, Youngblood B, Tan WG, Jin H-T, Araki K, Alexe G, et al. Tight Regulation of Memory CD8+ T Cells Limits Their Effectiveness during Sustained High Viral Load. Immunity. 2011;35:285–98. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]