Abstract
Purpose
To review the progress made in understanding the genetic basis, molecular pathology, and treatment of retinoblastoma since the previous Jackson lecture on the topic was published 50 years ago.
Design
Perspective based on personal experience and the literature.
Methods
The literature regarding retinoblastoma was reviewed since 1963. Advances in understanding the biology and treatment of retinoblastoma provided context through the author’s clinical, pathological and research experiences.
Results
Retinoblastoma was first identified in the 1500s and defined as a unique clinicopathologic entity in 1809. Until the mid-1900s, knowledge advanced sporadically, with technological developments of ophthalmoscopy and light microscopy, and with the introduction of surgical enucleation, chemotherapy and radiation therapy. During the last 50 years, research and treatment have progressed at an unprecedented rate due to innovations in molecular biology and the development of targeted therapies. During this time period, the retinoblastoma gene was discovered; techniques for genetic testing for retinoblastoma were developed; and plaque brachytherapy, chemoreduction, intraarterial chemotherapy, and intraocular injections of chemotherapeutic agents were successfully introduced.
Conclusions
Nearly all patients with retinoblastoma in developed countries can now be cured of their primary cancer- a remarkable achievement for a childhood cancer that once was uniformly fatal. Much of this success is owed to deciphering the role of the Rb gene, and the benefits of targeted therapies, such as chemoreduction with consolidation as well as intra-arterial and intravitreal chemotherapies. Going forward, the main challenge will be ensuring that access to care is available for all children, particularly those in developing countries.
Introduction
It is an honor to deliver the LXXI Edward Jackson Memorial Lecture. Although Dr. Jackson is probably best known for the Jackson cross cylinder, he profoundly influenced the entire course of modern ophthalmology. Doctor Jackson was a major initiator of the predecessor society of the American Academy of Ophthalmology, founder of the American Board of Ophthalmology and the founding editor of the American Journal of Ophthalmology.1 In addition to being an educator and clinician, Dr. Jackson was known for his grace and kindness1, qualities that serve as an example for today’s ophthalmologists.
When Dunphy delivered the XX Jackson Memorial Lecture in 19632, he called his lecture “The Story of Retinoblastoma”. In this lecture, he recounted the history of retinoblastoma, from the 1500s to the mid1960s. Many advances have been made in the fifty years since then; for instance, the Knudson two-hit hypothesis, cloning the retinoblastoma gene, chemoreduction therapy, and intra-arterial chemotherapy, to list just a few. Dunphy divided the history of retinoblastoma into four general periods: the prehistologic, histologic, enucleation, and irradiation/chemotherapy periods.2 The retinoblastoma story revolved around numerous personalities, including ophthalmologists, pathologists and researchers. It is time for an update of that story. I propose that there have been three additional periods since Dunphy’s lecture: 1) the period of molecular biology; 2) the period of targeted therapy;3) and the period of global health awareness. As in Dunphy’s time, the current era is made possible because of the contributions of ophthalmologists, pathologists and researchers. Although there have been tremendous advances in understanding the biology and treatment of retinoblastoma, a major challenge persists today: that of ensuring access to care in many parts of the world.
History
In addition to Dunphy’s lecture2, Albert has provided an historic review of retinoblastoma.3 Summarizing briefly, Pawius of Amsterdam is credited as the first to recognize retinoblastoma in an autopsy report of a young child published in 1597.4 Wardrop of Edinburgh established retinoblastoma as a distinct entity in 1809 and advocated enucleation as preferred treatment.55 Steven at the New York Hospital is believed to have reported the first case in the American literature in 1818.6 In those days, retinoblastoma was known as fungus haematodes, a term coined by Hey of Leeds, England.5 In 1854, the famous German pathologist, Virchow, introduced the term glioma of the retina.7 Hirshberg later divided these gliomas into the exophytum and endophytum types8, known today as exophytic and endophytic growth patterns. In 1891, Flexner of Johns Hopkins described the histologic finding of cellular rosettes in the tumor9; in 1897, Wintersteiner of Vienna, who was apparently unaware of Flexner’s paper, described the structure’s lumen, a component which now permits subclassification as the Flexner-Wintersteiner rosette.10 This clear-center rosette is due to a recapitulation of the external limiting membrane of the retina. The central portion of the Homer Wright rosette, which can be found in retinoblastoma as well, is filled with neuropil. This feature can be seen in medulloblastoma and neuroblastoma, thus it lacks the specificity that the Flexner-Wintersteiner rosette has for retinoblastoma.3 Robin and Langenbeck noted in the early 1800s that the tumor microscopically arose in the retina.11;12 Histologic resemblance of the tumor to undifferentiated embryonic retina prompted the famous American ophthalmic pathologist, Verhoeff, to name the tumor retinoblastoma, a term that was adopted by the American Ophthalmological Society in 1926.13 Cases of spontaneously regressed retinoblastoma in phthisical eyes began to appear in the literature in the early 1900s.3 Gallie and colleagues described a clinically benign variant of retinoblastoma they called retinoma in 1982.14 The following year, Margo and colleagues at the Armed Forces Institute of Pathology reported the histologic features of this benign variant that they called retinocyoma. 15 Trilateral retinoblastoma was described in the early 1980s.16;17 This entity includes bilateral retinoblastoma and a third primary intracranial tumor16;18, which may be a primitive neuroectodermal tumor, pinealblastoma or ependymoblastoma.19 Another unusual variant of retinoblastoma is anterior diffuse retinoblastoma.20;21 This tumor arises in the far peripheral retina as a small, inconspicuous intraretinal mass and seeds the anterior chamber via the aqueous causing a pseudohypopyon.
Clinical Features
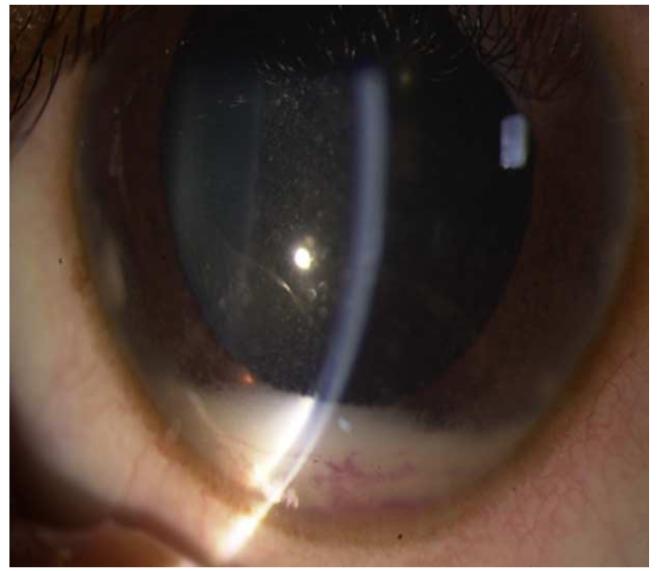
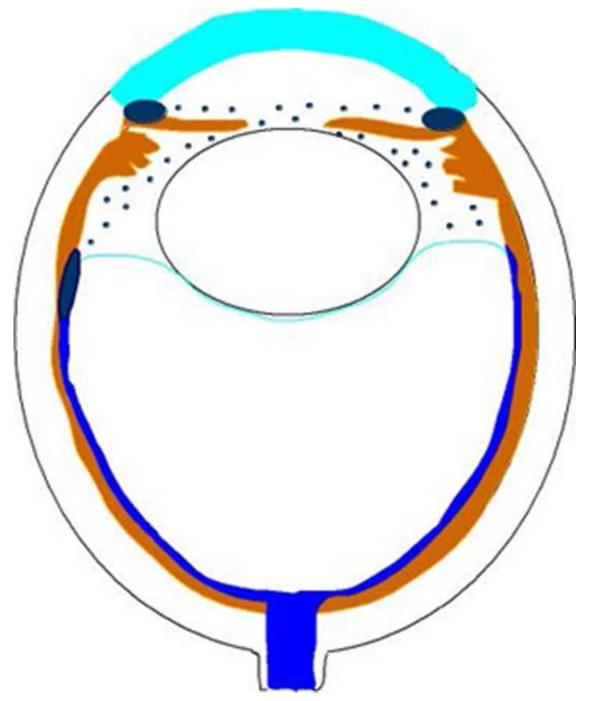
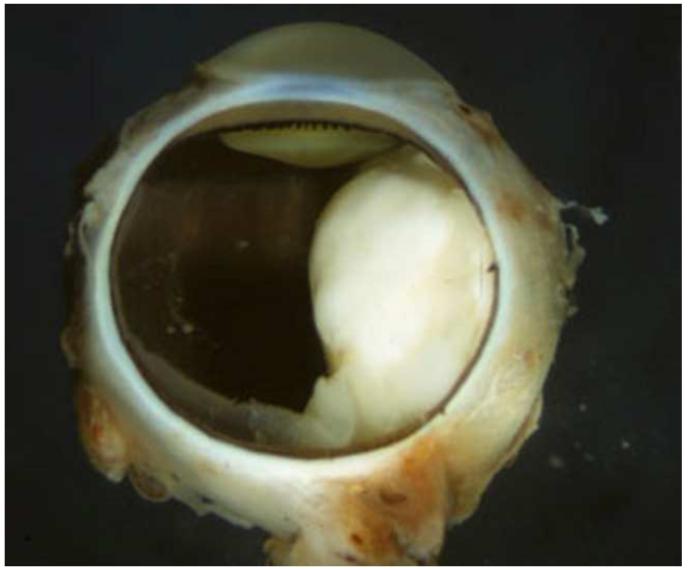
Retinoblastoma, the most common intraocular malignancy in childhood, comprises 4% of all pediatric cancers.22 The incidence of retinoblastoma is approximately 1 in 20,000 live births23 and there are about 200-300 new cases of retinoblastoma in the United States each year.22 In more populated countries, such as India and China, there are about 1,000 new cases of retinoblastoma annually.22 Leukocoria is the most common presenting sign (60%) followed by strabismus (20%).23 Tumor growth patterns include endophytic (Figure 1), in which the tumor grows into the vitreous; exophytic (Figure 2), in which the tumor grows under the retina toward the choroid; mixed (both endophytic and exophytic); and diffuse infiltrating which forms a greyish plaque on the retinal surface.23-25 A particular variant of the diffuse growth pattern is anterior diffuse (Figure 3). As mentioned above, a small primary tumor arises in the peripheral retina in this variant then seeds the anterior chamber via the aqueous .26
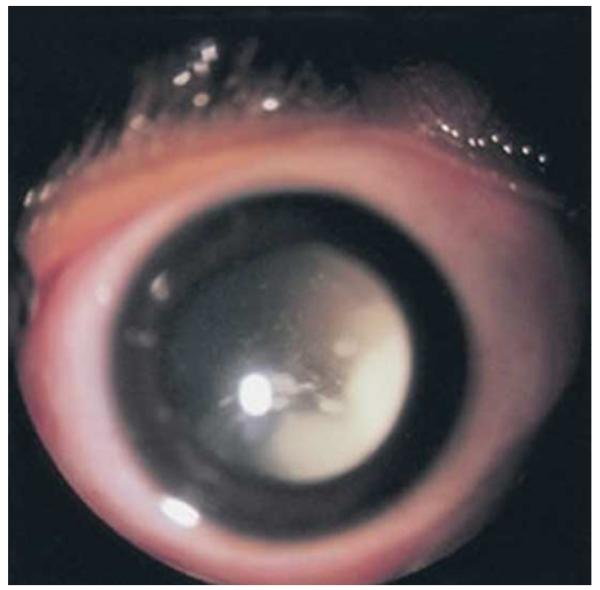
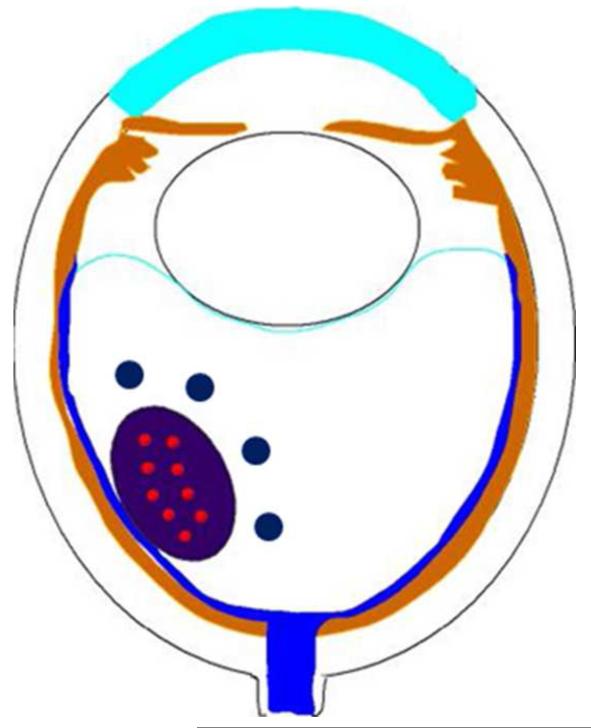
Figure 1.
Endophytic retinoblastoma. Left. Endophytic retinoblastoma extends from the retina into the vitreous, thus resulting in a white mass associated with tumor shedding into the vitreous (i.e. seeds). Right. The primary tumor is vascularized (red) whereas the vitreous seeds (blue) are avascular.
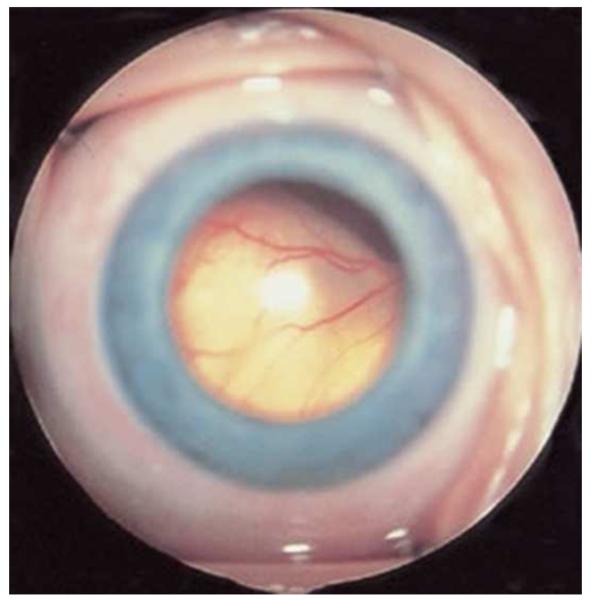
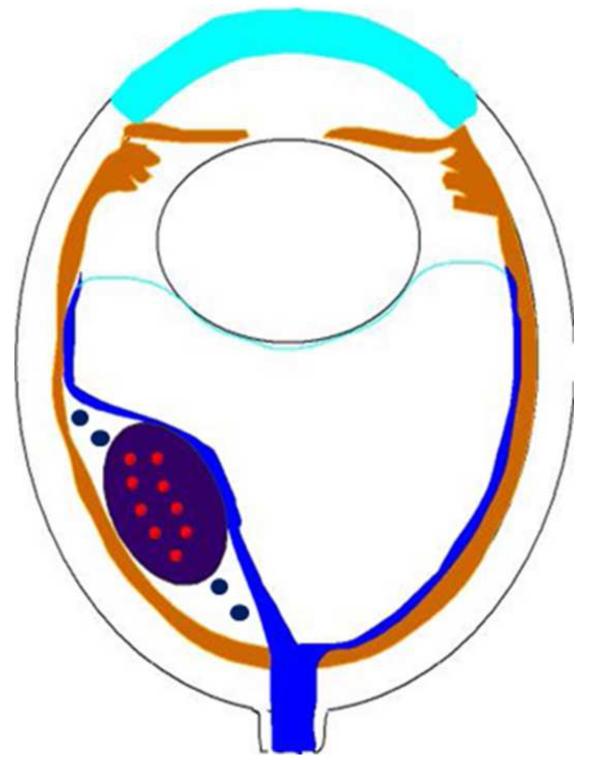
Figure 2.
Exophytic retinoblastoma. Left. Exophytic retinoblastoma extends from the retina into the subretinal space and clinically appears as a white to slightly yellow subretinal mass. Right. The primary tumor is vascularized (red) and subretinal seeds (blue) are avascular.
Figure 3.
Anterior diffuse retinoblastoma. Left. Anterior diffuse retinoblastoma results in a white pseudohypopyon. Right. The primary tumor arises as a small mass in the area of the ora serrate and seeds into the aqueous in front of the anterior hyaloid, spreads through the pupil, and accumulates in the peripheral iris.
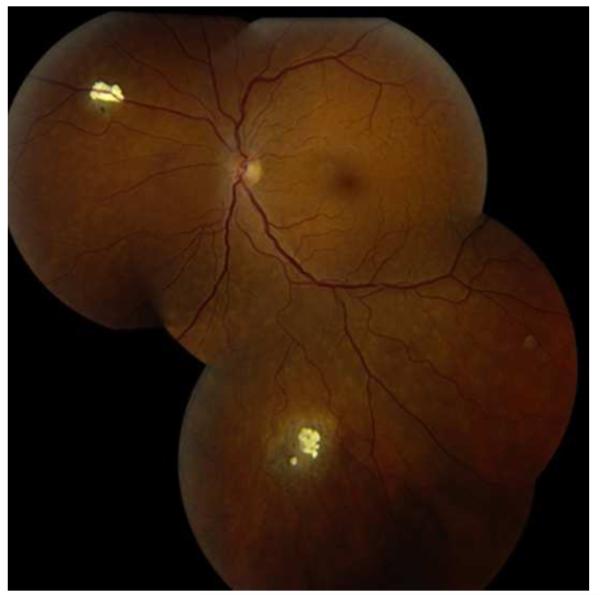
The clinical differential diagnosis of retinoblastoma includes Coats disease, persistent fetal vasculature, vitreous hemorrhage, ocular toxocariasis, familial exudative vitreoretinopathy, rhegmatogenous retinal detachment, coloboma, astrocytic hamartoma, combined hamartoma of the retina/retinal pigment epithelium and endogenous endophthalmitis27 (Table 1). The benign variant of retinoblastoma, retinocytoma, may clinically appear as regressed retinoblastoma with associated calcification (Figure 4).
Table 1.
| Differential Diagnosis for Retinoblastoma | |
|---|---|
| Coats disease | 40% |
| persistent fetal vasculature (PFV) | 28% |
| vitreous hemorrhage | 5% |
| ocular toxocariasis | 4% |
| familial exudative vitreoretinopathy (FEVR) | 3% |
| rhegmatogenous retinal detachment | 3% |
| coloboma | 3% |
| astrocytic hamartoma | 2% |
| combined hamartoma of retina/rpe | 2% |
| endogenous endophthalmitis | 2% |
approximate percentages of occurrence are shown
modified from Shields and colleagues27
Figure 4.
Retinocytoma. This patient has two calcified peripheral retinal lesion that represent retinocytoma, once confused with spontaneously regressed retinoblastoma. The patient’s daughter developed retinoblastoma. (courtesy of G. Baker Hubbard III MD)
The Reese-Ellsworth Classification of clinical stage of the disease was published in 196328 and has now been largely supplanted by the International Classification (Table 2), which was developed by a team of retinoblastoma experts in Paris in 2003 including Murphree29, Desjardins, Gallie, Shields, Shields, and others.. Retinoblastoma spreads by three pathways: anteriorly via the vitreous/subretinal space into the anterior segment; posteriorly via the optic nerve into the cranial cavity; externally via the choroid to the systemic circulation or through the sclera into the orbit.23 Prognostic risk factors for dissemination include optic nerve and/or orbital invasion30;31 and massive choroidal invasion.32-34
Table 2.
| International Classification of Retinoblastoma | |||
|---|---|---|---|
| Group | Subgroup | General | Specific Features |
| A | A | small tumor | retinoblastoma ≤ 3mm in size |
| B | B | larger tumor | retinoblstoma > 3mm in size or |
| macula | macular retinoblastoma ≤3mm to fovea | ||
| juxtapapillary | juxtapapillary retinoblastoma location ≤1.5 mm to disc | ||
| subretinal fluid | clear subretinal fluid≤3mm from margin | ||
| focal seeds | retinoblastoma with | ||
| C | C1 | subretinal seeds ≤3mm from retinoblastoma | |
| C2 | vitreous seeds ≤3mm from retinoblsatoma | ||
| C3 | both subretinal and vitreous seeds ≤3mm from | ||
| diffuse seeds | retinoblastoma with | ||
| D | D1 | subretinal seeds >3mm from retinoblastoma | |
| D2 | vitreous seeds >3mm from retinoblastoma | ||
| D3 | both subretinal and vitreous seeds >3mm from | ||
| E | E | extensive retinoblastoma |
extensive retinoblastoma occupying >50% globe or |
| neovascular glaucoma | |||
| opaque media from hemorrhage in ac, vitreous or sr space | |||
| invasion of postlaminar optic nerve, choroid, sclera, orbit, ac |
|||
mm=millimeters; ac=anterior chamber
modified from Shields and Shields22
Second primary tumors in hereditary retinoblastoma survivors represent a greater risk of death than the retinoblastoma itself.35;36 The cumulative risk for these tumors is roughly 1% per year, reaching 50% at 50 years.36 Radiotherapy, especially to the site of retinoblastoma before age 1, greatly increases the chances of a second tumor.37;38 These tumors are mainly sarcomas, in particular, leiomyosarcoma39 in the field of radiation and osteosarcoma outside of the field.
Pathology
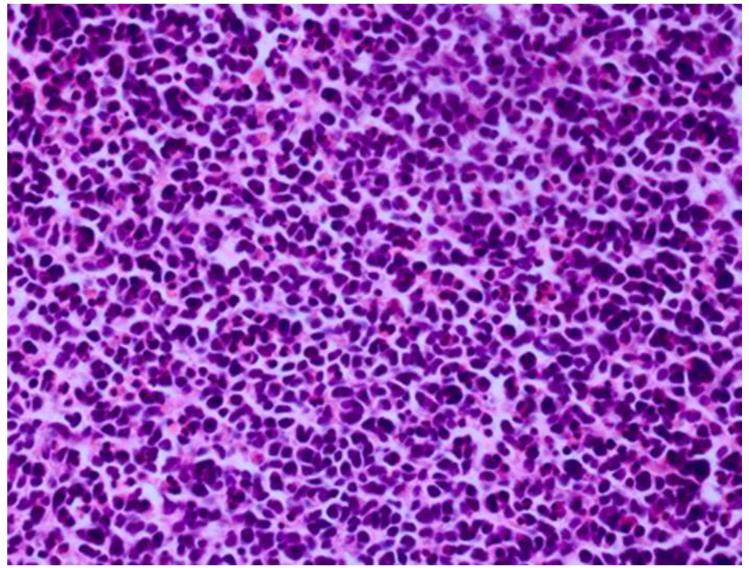
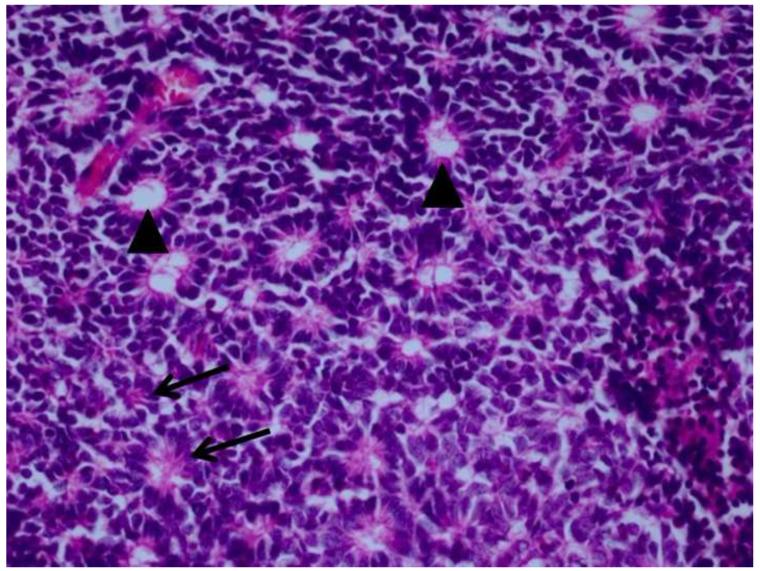
Retinoblastoma is derived from cells of neuroectodermal origin from the inner layer of the optic cup. The tumor may grow toward the vitreous and/or subretinal space forming a multilobulated white mass with associated vitreous or subretinal seeds. In these cases, the tumor grows in sleeves and cuffs around central vessels from which it derives its nutrition. When the tumor outgrows its vascular supply 90-110μm away from the central vascular channel, it undergoes ischemic necrosis and may contain areas of dystrophic calcification, thus yielding a grossly visible chalky or cottage cheese appearance.40;41 The tumor may also grow diffusely within the retina and derive its nutrition from the retinal vasculature. Vitreous and subretinal seeds of retinoblastoma are avascular and contain areas of necrosis in the center of the seed away from the vitreous or subretinal fluid. Cytologically, retinoblastoma is made up of small, round, blue cells. Retinoblastoma may exhibit differentiation which becomes less evident with age.42-44 Signs of differentiation, going from least to most differentiated, include Homer Wright or Flexner-Wintersteiner rosettes and fleurettes (Figure 5).40 Fleurettes are particularly abundant in retinocytoma, which also contain areas of calcification. The two most important histologic prognostic factors for distant metastases are optic nerve invasion beyond the lamina cribrosa and “massive” choroidal invasion measuring 3mm or greater in diameter in which the tumor comes in contact with the sclera.40;45 The approximate survival rates for children with retinoblastoma extending into the prelaminar, laminar, post-laminar optic nerve and surgical margin of resection are >90%, 85%, 60% and 35%, respectively.46-48 The overall survival rate when there is massive choroidal invasion is approximately 70%.49
Figure 5.
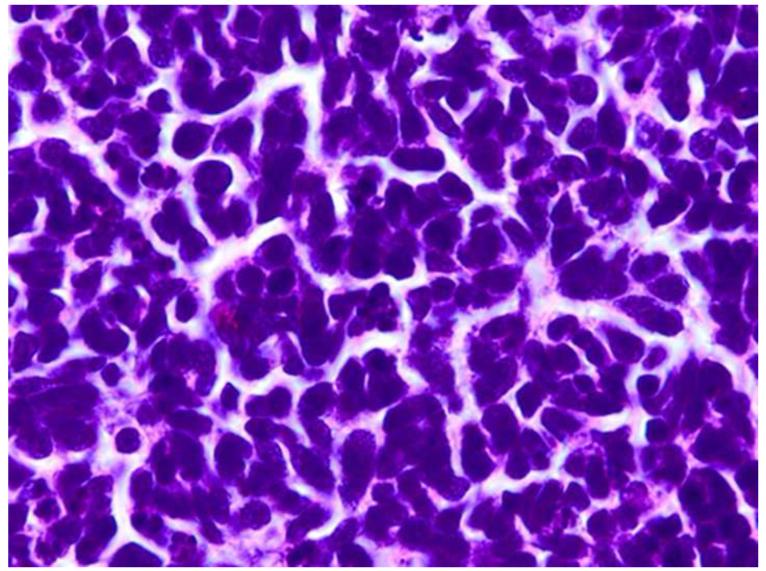
Retinoblastoma pathology. Upper left. Retinoblasotmas have a gross white, fleshy to cottage-cheese appearance. Upper right. Retinoblastoma is a small round blue cell tumor, forming sheets of cells. Lower left. Retinoblastoma may exhibit rosette formation, including Homer Wright rosettes with neuropil in the lumen (arrows), or Flexner-Wintersteiner rosettes with a clear lumen (arrowheads). Lower right. Retinoblasotma may contain well differentiated areas, exhibiting cells with small nuclei, rather abundant cytoplasm, and fleurettes with photoreceptor outer segments in the lumen. (upper right, lower left and lower right, hematoxylin and eosin, 25X)
The International Retinoblastoma Staging Working Group (IRSWG) has provided guidelines for handling eyes enucleated for retinoblastoma, including preparation of the pupil-optic nerve section and breadloafing the calottes to evaluate for choroidal invasion.45 It is estimated that approximately 20% of eyes enucleated with retinoblastoma in the United States will exhibit high risk features.50;51 Clinical predictors of high risk histologic features include older patient age, time from diagnosis to enucleation, hyphema, pseudohypopyon, staphyloma, orbital cellulitis and elevated intraocular pressure.52;53 Histologic examination of enucleated eyes after chemoreduction therapy for retinoblastoma may show Type 1 (cottage cheese), Type 2 (fish flesh), Type 3 (combination of Types 1 and 2) or Type 4 (complete) regression, or histologically intact, presumably viable tumor cells.54-56 The Type 2 regression pattern may result in retinocytoma-like clinical appearance; the tumor may have the same histological features. It is not surprising that regressed tumors have the same histological appearance of retinocytoma, since well differentiated tumors are presumably relatively resistant to chemotherapy as cells are not cycling.56 Tumors treated by intraarterial chemotherapy (IAC) exhibit similar regression patterns.57 Treatment failures are due to persistence of vitreous seeds, subretinal seeds, intraretinal tumor or tumor that is unresponsive to chemotherapy.58
Molecular Biology
The information in this section represents our current understanding of the molecular mechanisms and pathways involved in development, extraocular extension and metastasis of retinoblastoma based on evidence available at this time; some of this information will likely prove to be incorrect or an oversimplification of these mechanisms. The understanding of these mechanisms will change as experimental investigations will further clarify these molecular pathways in the future.
Genetics and Molecular Mechanisms
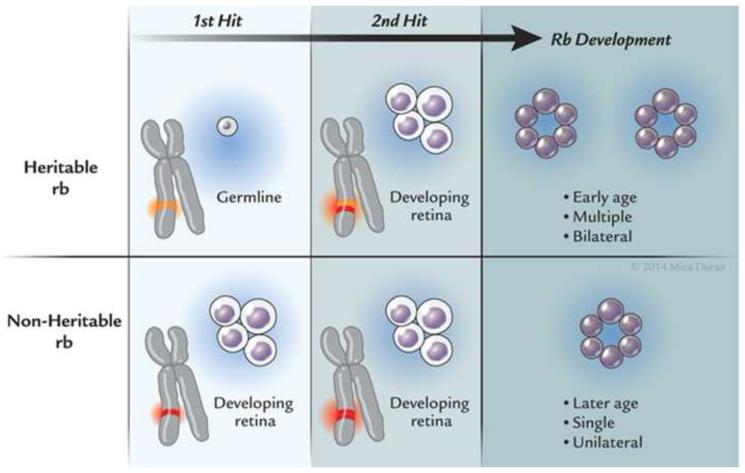
The most far reaching advances in retinoblastoma since Dunphy’s lecture2 have been in understanding the genetics and molecular origins of the tumor and mechanisms of progression. These fields of endeavor have provided major breakthroughs in deciphering the pathogenesis of cancer. It had long been known that retinoblastoma could be sporadic or heritable59 when Knudson at the MD Anderson Cancer Center developed his now celebrated “two-hit” hypothesis.60 Knudson had graphed the percent of undiagnosed cases versus age at diagnosis and found two distinct curves, one linear and the other exponential.60 The linear curve corresponded to patients who had bilateral tumors and theoretically inherited one copy of a mutated gene (germline mutation) and as their retina developed, acquired the second mutation (second “hit”). The exponential curve corresponded to children who had unilateral tumors and acquired both mutations in their developing retina. The heritable variety of retinoblastoma may be explained by the inheritance of a germline mutation in combination with an acquired mutation in the developing retina, whereas the acquired variety of retinoblastoma develops after mutations in both alleles of the retinoblastoma gene arise in the developing retina (Figure 6). Individuals who harbored a germline mutation in all cells were predisposed to develop a variety of other malignancies.61 As the field progressed and laboratory techniques improved, investigators were able to localize the retinoblastoma gene (RB1) to chromosome 13q14.2.62 In 1984, Dryja’s laboratory cloned the RB1 gene63, a landmark achievement as this was the first human tumor suppressor gene cloned. The gene was subsequently sequenced .64 The RB1 gene was found to be large (approximately 200kb) and had 27 dispersed exons.65 Two structurally related retinoblastoma-like genes (RBL1, RBL2) have since been identified. RBL1 and RBL2 are located at chromosomes 20q11.2 and 16q12.2, respectively.66;67 Investigators have recently found that retinoblastoma may arise after MYC mutation in the absence of RB mutations.68 These tumors appear to be diagnosed at an earlier age than those with RB mutations. It became evident that although mutations of both alleles of the RB1 gene were necessary in most cases, they were not sufficient for phenotypic expression .69;70 Dimaris and colleagues found that loss of both copies of RB1 may lead to retinoma (retinocytoma) and progressive genomic instability in retinoma then leads to retinoblastoma.71 Gallie’s laboratory found that additional mutational events (“three hits”) were required for progression to a fully a malignant tumor .72
Figure 6.
Retinoblastoma inheritance, Heritable retinoblastoma requires two mutations: one that is inherited in the patient’s germline and the other in the developing retina. These tumors are usually bilateral, multiple, and occur at an early age. Non-heritable (acquired) retinoblastoma also requires two mutations, although both of these mutations occur in the developing retina. These tumors are usually single, unilateral, and occur at a later age.
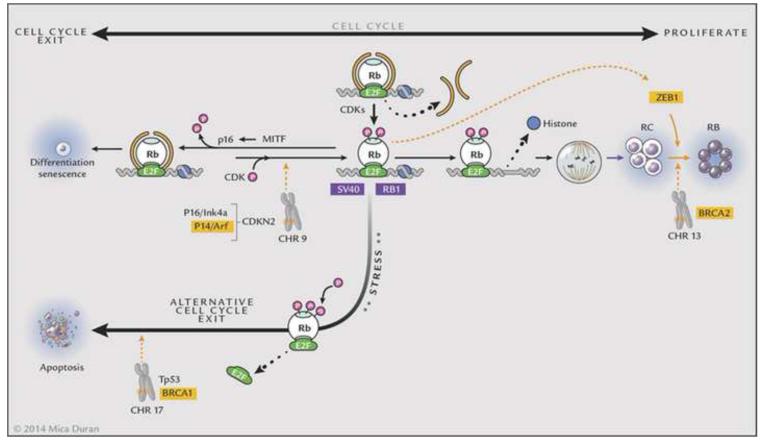
The function of RB has been described by Harbour.73 The protein (Rb), composed of 928 amino acids, is a nuclear phosphoprotein that contains an N-terminal region, a central pocket domain with A and B boxes, and a C terminus.74 The pocket is formed by interactions of the A and B boxes and tumor forming mutations disrupt this pocket.75;76 Rb binds to the chromatin remodeling proteins HDAC and BRG1 via the LxCxE binding site and to E2F transcription factors via its E2F binding site.73;77-79 Rb is multifunctional: it suppresses transcription via histone deacetylases (HDACs) and BRG; it also regulates the cell cycle via E2F. Phosphorylated Rb binds directly to E2F and blocks the E2F transactivation domain.80;81 When Rb is dephosphorylated via p16, chromatin remodeling proteins (HDAC, BRG1), which bind to Rb, alter chromatin structure and prevent access to transcription thus leading to exit from the cell cycle.82 When Rb is phosphorylated, HDAC and BRG1 are released, thus allowing progression through the cell cycle by de-repression of cyclins due to sequential Rb phosphorylation involving cyclins and cyclin dependent kinases (CDKs). Under stress and excess proliferation, phosphorylation of Ser567 changes the tertiary structure of Rb, releasing E2Fs and leading to apoptosis. Thus, Rb plays a central role in the fate of a cell either through exit of the cell cycle or apoptosis.73 Mutations in Rb and/or inhibition of Rb by oncoproteins produced by tumor causing viruses (SV40, adenovirus, HPV) abrogate Rb’s ability to mediate exit from the cell cycle or apoptosis and lead to uncontrolled cell division, a hallmark of cancer. Perturbations in genes such as BRCA2 on chromosome 13, CDKN2 on chromosome 9, and TP53/BRCA1 on chromosome 17 co-operate with alterations in Rb by promoting tumor progression.83 Breast cancer related genes BRCA1 and BRCA2 are involved with DNA damage-response pathways and in addition to viral oncoproteins may directly alter the function of Rb.83 Loss of p16/INK or p16/INK promoter hypermethylation may alter cell cycle exit. Additionally, alterations in the phosphorylated retinoblastoma protein (pRb) phosphorylation/inactivation by CDK4/cyclin D1 dysregulates Rb’s ability to promote cell cycle exit and TP53 loss facilitates cell survival.83 These other events, or “third hits”, appear to be necessary for human retinoblastoma to progress. RB1/pRb inactivation results in loss of its ability to regulate exit from the cell cycle and up-regulates ZEB protein, a transcriptional repressor of E-cadherin, a determinant of epithelial to mesenchymal transformation which allows for transmigration and invasion, two other hallmarks of cancer.83 The biologic events that lead to retinoblastoma formation are summarized in Figure 7.
Figure 7.
Rb in progression to retinoblastoma. 1. Retinoblastoma (Rb) recruits proteins such as HDAC and BRG1 to E2F sites resulting in chromatin structure alterations and prevention of access to transcriptional machinery. 2. Phosphorylation by CDKs release HDAC and BRG1, thus releasing histone from DNA, allowing access to transcriptional machinery and resulting in cell division. Mutations in both alleles of Rb result in uncontrolled proliferation/retinocytoma formation, and further events including P16Ink4a, P14/Arf, or BRCA2 mutations, SV40 interactions with Rb, or Zeb1 activation by inactivated pRb allow progression to retinoblastoma. 3. The cell cycle may be exited under normal conditions when Rb is hypophosphorylated via MITF induction of p16, thus allowing HDCAC and BRG1 to rebind to Rb via E2F sites and suppress transcription. 4. Alternative cell cycle exit occurs under stress or hyperpoliferation; Rb is hyperphosphorylated, releases E2f and undergoes Tp53 mediated apoptosis, which may be suppressed by BRCA1 mutations. Thus, Rb mutations initiate the process and retinoblastoma results through progressive mutational events involving chromosome 9, 13 and 17.
Molecular Pathology of Retinoblastoma
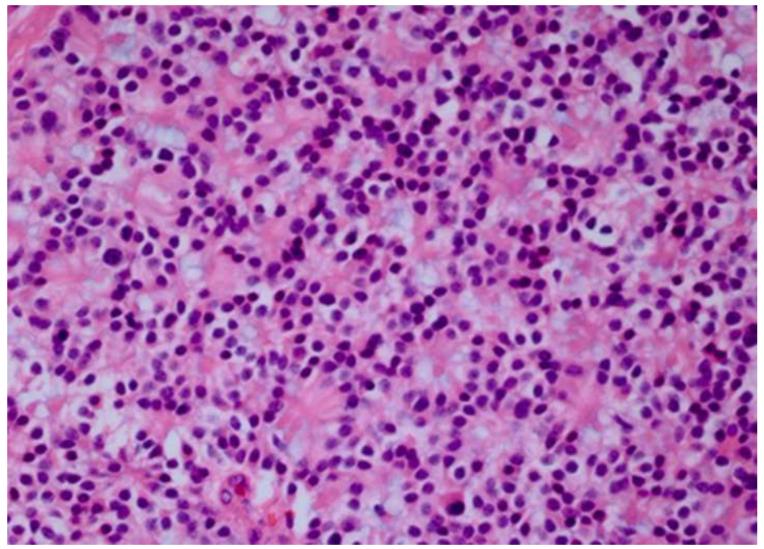
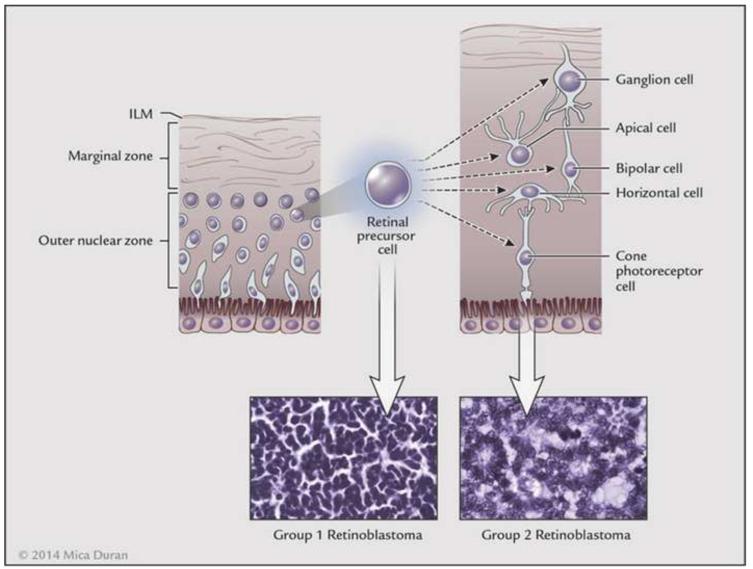
Several studies have attempted to define the cellular origin of retinoblastoma. One study suggested that human retinoblastoma exhibits properties of a cone precursor cell.84 Other investigations have implicated multiple cell types, including horizontal, amacrine, Müller glial precursors, and photoreceptors, thus suggesting the existence of a progenitor cell or retinal precursor cell (RPC) that differentiates to multiple cell types.85-88 Gene expression profiling (GEP) of mRNA and comparative genomic hybridization (CGH) of DNA were utilized recently to evaluate 21 retinoblastomas.89 Results of this initial study showed different patterns of retinal gene expression that when analyzed by unsupervised hierarchical clustering, followed by principle component analysis to categorize the retinoblastomas into three groups.89 Group 1 retinoblastomas expressed genes found in early retinal development, thus suggesting a RPC origin. Group 2 retinoblastomas expressed genes found after cone cell development, consistent with a cone cell origin. Group 3 retinoblastomas expressed both rod and cone genes, similar to normal retinal samples. When compared with histology and cytogenetics, Group 1 tumors had increased frequencies of post-laminar optic nerve invasion and choroidal invasion compared with the other groups. Group 2 tumors exhibited an increased frequency of photoreceptor differentiation based on the extent of Flexner-Wintersteiner rosettes.89 Cytogenetic alterations most frequently associated with adverse clinical features (gain of 1q or 6p and loss of 16q) were more frequent in Group 1 than the other groups.89 This implies that retinoblastoma may be stratified by gene expression profile with regards to clinical behavior and outcome. This classification system is based on cellular origin - RPCs or cone precursor cells (Figure 8). It is hypothesized that retinoblastomas that arise from RPCs exhibit a higher anaplasia grade than those that arise from cone precursor cells. Our laboratory has recently studied the association of tumor anaplasia (an apparent manifestation of the gene expression profile) and the development of metastases and survival among 125 patients who underwent enucleation for retinoblastoma (Figure 9). We found that there was a significant correlation between grade of anaplasia and survival (Figure 10). Further retinoblastoma GEP studies are needed to validate the initial study.89
Figure 8.
Retinoblastoma cell of origin. Gene expression profiling (GEP) of retinoblastoma has resulted in two general phenotypes supporting a primitive retinal precursor cell (RPC) origin of retinoblastoma. This cell type is present in the outer nuclear zone of the developing retina. If mutational events occur early, before further differentiation of the RPC, group 1 retinoblastoma occurs; if the mutational event occurs later, group 2 retinoblastoma occurs. Group 1 retinoblastoma expresses RPC genes and group 2 retinoblastoma expresses cone genes. Group 1 retinoblastoma has a more primitive phenotype and group 2 retinoblastoma may exhibit photoreceptor differentiation, including rosette and fleurette formation.
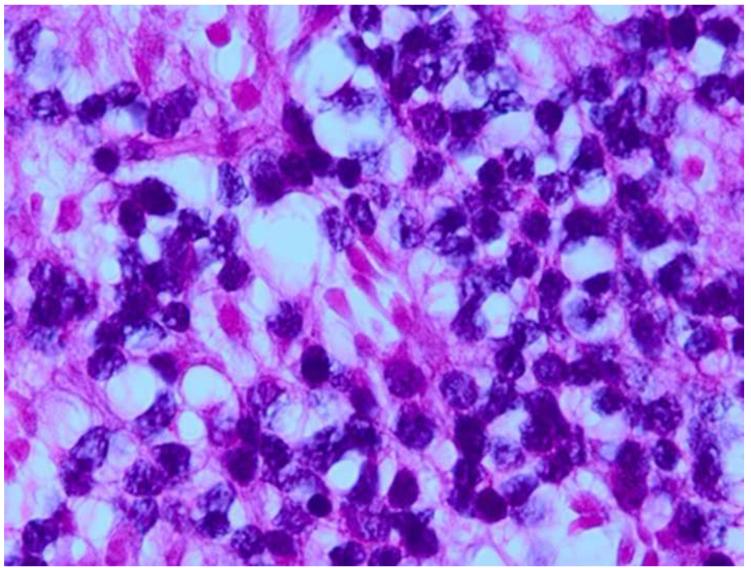
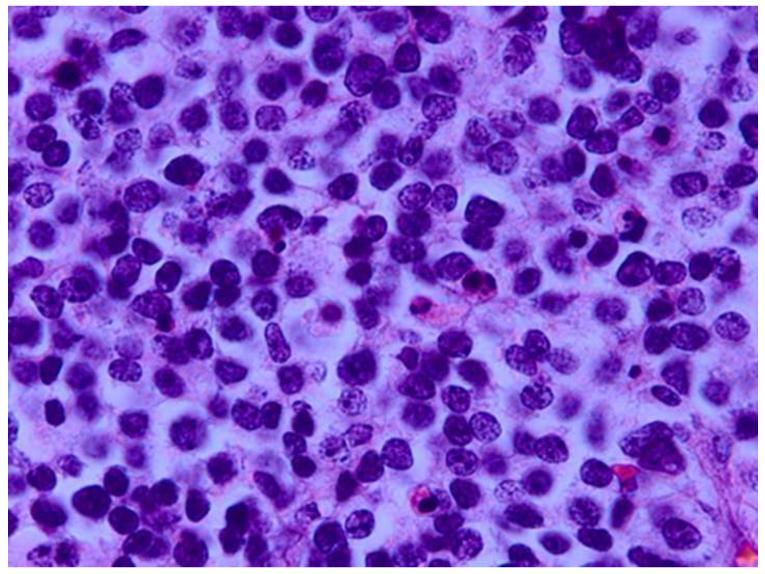
Figure 9.
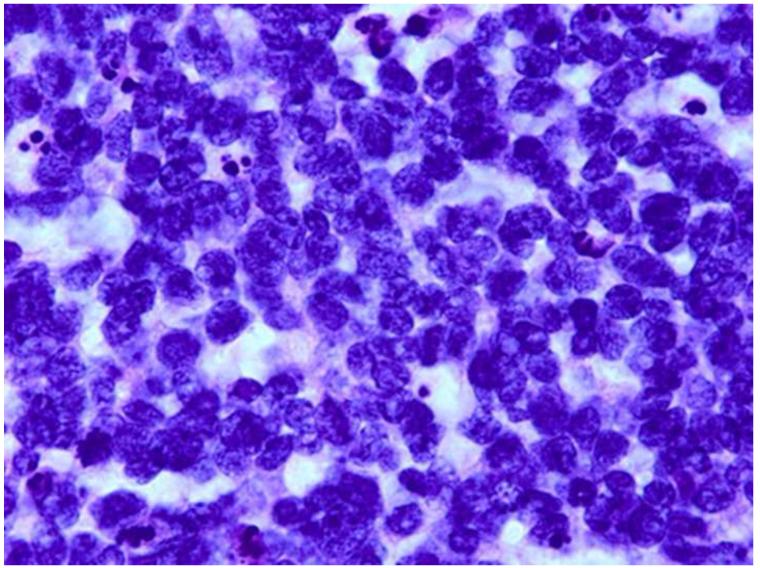
Grading anaplasia in retinoblastoma anaplasia. Upper left. No anaplasia (retinoctyoma) exhibits small, compact nuceli, abundant eosinophilic cytoplasm, and photoreceptor differentiation. Upper right. Mild anaplasia is characterized by tumor cells with round, hyperchromatic nuclei, increased nuclear to cytoplasmic ratios, and a moderate amount of eosinophilic cytoplasm. Lower left. Moderate anaplasia is characterized by cells with larger nuclei, high nuclear to cytoplasmic ratios, and scanty eosinophilic cytoplasm. Lower right. Severe anaplasia is characterized by cells with larger, hyperchromatic nuclei, nuclear molding/angulation, and virtually no cytoplasm. (hematoxylin and eosin, 100X)
Figure 10.
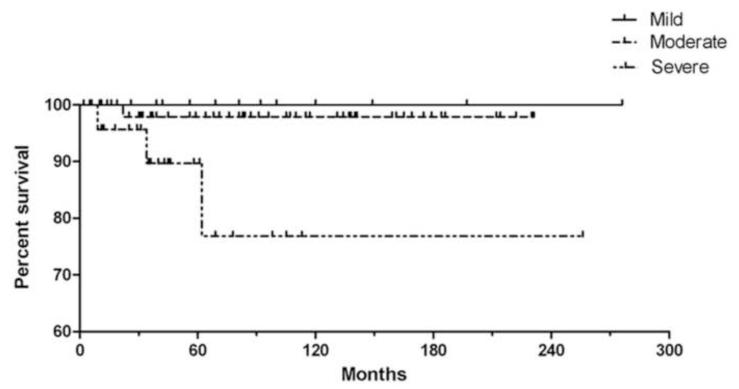
Retinoblastoma survival based on anaplasia grade. This Kaplan-Meir plot no deaths or metastases associated with mildly anaplastic tumors, an approximate 97% 10 year survival/no metastases in patients with moderately anaplastic tumors, and an approximate 75% 10 year survival/no metastases in patients with severely anaplastic tumors (P<0.01).
Targeted Therapy
Chemoreduction and Consolidation
The other major advances in retinoblastoma since Dunphy’s lecture are related to targeted therapy. The goal of treatment of retinoblastoma is to ensure elimination of the tumor while minimizing collateral injury to other tissues. Without diminishing the chances of cure, preservation of the eye and vision is an important priority. The cure rate of enucleation alone in unilateral (non-heritable) cases without extraocular disease is 85% to 90%.32;90 Adjuvant chemotherapy is utilized for eyes with optic nerve invasion posterior to the lamina cribrosa or massive choroidal invasion.91 These treatments include VDC (vincristine, cyclophosphamide, doxorubicin or idorubicin), VCE (vincristine, carboplatin, etoposide) (Figure 11) or hybrid regimens.90;92 A conservative, eye salvaging approach is recommended for unilateral cases with early stage disease, especially in young children who may have heritable retinoblastoma and later develop a tumor in the contralateral eye.90;92 Patients with bilateral (heritable) retinoblastoma should undergo chemoreduction with a combination of vincristine, carboplatin and etoposide.93-97 For early stage tumors (International Classification Group B), a two agent regimen including vincristine and carboplatin is effective.94-96 For later stage tumors with vitreous seeds (Groups C and D) a three agent regimen is utilized. Consolidation may be used alone in Group A tumors or in combination with chemoreduction in Groups C-D. Consolidation includes cryotherapy for small anterior tumors, thermotherapy/laser photocoagulation for posterior tumors and brachytherapy for larger tumors.92 The reasons for treatment failures are vitreous seeds of tumor, subretinal seeds of tumor, intraretinal tumor and tumor resistance.58 The first three types of failure are due to an inability of drug reach tumor (e.g. diffusion); the fourth type of failure is mainly due to well differentiated, non-cycling tumor cells that are resistant to treatment modalities dependent on cell division.
Figure 11.
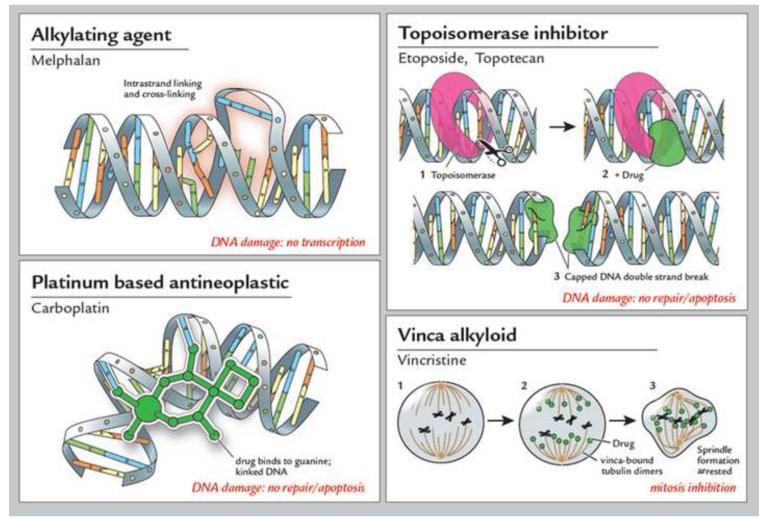
Chemotherapeutic agents used in treating retinoblastoma. In general, there are four classes of chemotherapeutic agents used to treat retinoblastoma. Alkylating agents, such as melphalan, cause DNA intrastrand linking and cross-linking, resulting in damaged DNA that is not transcribed. Topoisomerase inhibitors, such as etoposide and topotecan, work by capping free double strands of DNA caused topoisomerase cleavage. The capped, damaged DNA is unable to be repaired and is degraded via apoptosis. Platinum based antineoplastic agents, such as carboplatin, bind DNA bases, thus resulting in kinked DNA; this damaged DNA is then degraded via apoptosis. Vinca alkyloids, such as vincristine, bind tubulin dimers, thus blocking mitotic spindle formation and inhibiting mitosis. Typically, combinations of chemotherapeutic agents with different mechanisms of action are used to treat retinoblastoma via systemic chemoreduction, such as Vincristine, Etopopside, and Carboplatin (VEC) therapy, which is given over 5 to 6 cycles.
Periocular injections of carboplatin have been used for the treatment of vitreous seeds. This approach has largely been abandoned due to lack of success98 and toxicity to tissues of the visual pathway such as the optic nerve.99 Some variants of Groups C and D eyes with retinoblastoma appear to respond better to chemoreduction than other forms. For instance, posterior central tumors without subretinal seeds respond better than peripheral tumors.100 Extraocular retinoblastoma may occur locally, regionally or distantly.92 Local, isolated orbital retinoblastoma comprises 60% to 70% of recurrent/metastatic retinoblastoma with CNS involvement and distant metastases making up the remainder of these cases.101 Treatment of orbital disease at the time of diagnosis includes radiation and chemotherapy, usually with vincristine, cyclophosphamide, doxorubicin, platinum- and epipodophyllotoxin based regiments.102 This is followed by enucleation and consolidation with orbital irradiation.92 A similar treatment is recommended for patients with retinoblastoma at the margin of optic nerve resection. Preauricular and cervical lymph nodes should be evaluated and irradiated if retinoblastoma is present.90 Treatment for patients with intracranial dissemination includes platinum-based chemotherapy, CNS-directed therapy and possibly irradiation to the CNS and spinal axis.92 For patients with distant metastasis, high dose chemotherapy and autologous stem cell rescue have been utilized.92
Intra-arterial Chemotherapy (IAC)
Intra-arterial chemotherapy (IAC) was developed in Japan by Kaneko as an eye-preserving therapy for patients who had retinoblastoma with vitreous seeds.103-105 In the United States, IAC was initially used byAbramson.106-109 IAC has been usually employed used for Group D and Group E retinoblastomas and involves trans-femoral catheterization with a balloon catheter which is advanced under fluoroscopy into the origin of the ophthalmic artery. Once secured, melphalan, topotecan and/or carboplatin are infused.108 This procedure is often repeated several times over a period of weeks to months. Gobin has reported an ocular event free survival at two years of approximately 70% for all eyes, 80% for eyes without prior treatment, and 60% for eyes that had previous treatment failures.109 IAC for retinoblastoma is now performed in a number of centers in the United States110-114 and around the world.115-123 Complications include eyelid erythema/edema, emboli to the retina/choroid with occlusive vasculopathy, vitreous hemorrhage and cerebral vasoconstriction.111;113;124;125 Some argue that systemic chemoreduction is protective against retinoblastoma metastases and/or third tumors (pinealblastoma) whereas localized IAC does not provide that benefit.126 Another controversy is that IAC may mask histologic high risk features in eyes with extensive retinoblastoma that eventually undergo enucleation.127 Additionally, IAC is performed in major cancer centers by skilled interventional radiologists/neurosurgeons with technique and skill levels that can vary. Nonetheless, impressive globe salvaging results have been reported in some advanced group retinoblastoma eyes. IAC currently appears to be a reasonable option for select patients.
Intravitreal Injections
Although for many years ophthalmic surgeons were strongly advised to avoid needle passes through the eye wall into the vitreous in children with retinoblastoma for fear of tumor seeding128, various investigators have attempted intravitreal injections of chemotherapeutic agents beginning in the early 1960s.129-131 Ghassemi and Shields reported using intravitreal injections of melphalan132 and combined melphalan/topotecan133 to treat vitreous seeds of retinoblastoma. Munier of Switzerland reported a technique of intra-vitreal injection of melphalan for refractory or recurrent retinoblastoma tumor seeds using a 32 gauge needle on a tuberculin syringe and freezing the needle with a cryoprobe as it is withdrawn from the eye to prevent tumor seeding along the needle track.134;135 Using this technique, he observed no tumor seeding in 135 cases. Transient vitreous hemorrhage occurred in only 3 patients.134 Suzuki and Kaneko in Japan reported successful injections in 237 eyes of 227 patients with only one case of extra-ocular tumor spread.136 Novel injection techniques are being developed to deliver chemotherapeutic agents locally to retinoblastoma. As these techniques are perfected, it is anticipated that they will be used more widely.
Emerging Technologies
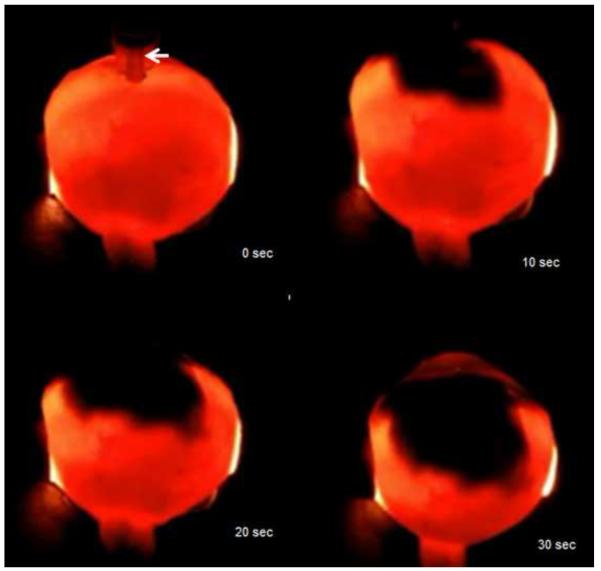
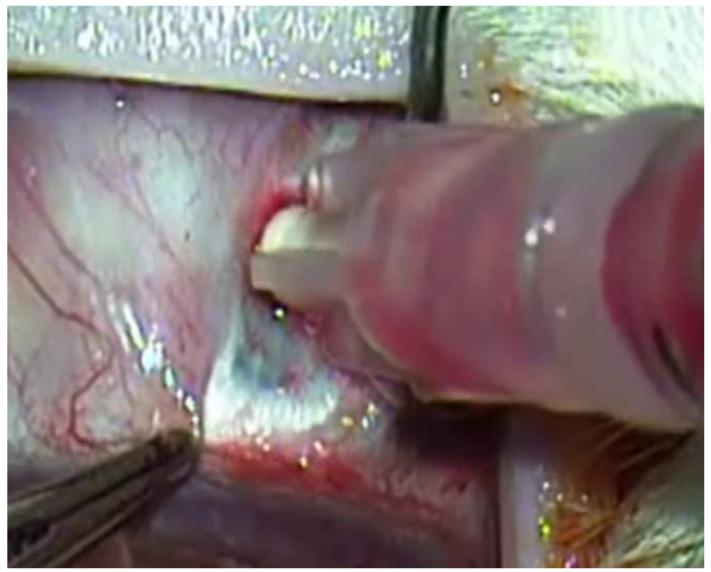
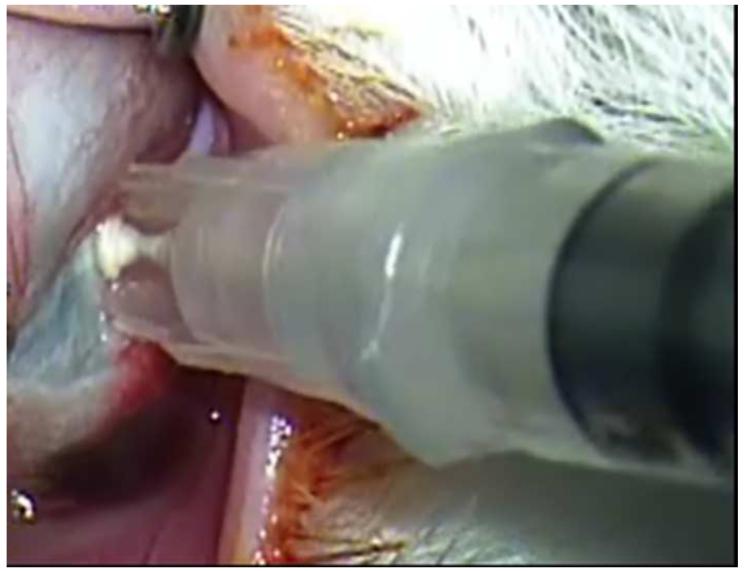
Developments in targeted killing of retinoblastoma using gene therapy and nanotechnology have paralleled progress in the field of intravitreous injection. Subramanian and colleagues in Houston have used intravitreal injections of an adenovirus vector containing herpes simplex thymidine kinase gene in one eye of eight patients with bilateral retinoblastoma and vitreous seeds.137 The patients were then given intravenous ganciclovir and one of the eight patients achieved sustained resolution of the vitreous seeds 38 months after therapy.137 In addition to gene therapy, sustained drug release platforms, some of which have implications for retinoblastoma therapy, are being developed for targeted intraocular drug delivery.138 One of the methods is a sustained release episcleral exoplant.139 My laboratory has been investigating periocular injection of sustained release nanoparticles containing carboplatin 140 and intravitreal topotecan in a rabbit model of retinoblastoma that develops vitreous seeds.141 We have be able to achieve therapeutic levels of intravitreal topotecan with successful elimination of the vitreous seeds in our model (Figure 12). We are also able to inject topotecan into the suprachoroidal space of our model using microneedles (Figure 13). Results are currently being evaluated. Suprachoroidal injection potentially allows a route of targeted drug delivery with minimal risk of extraocular spread of tumor, as the vitreous and subretinal spaces are not entered.
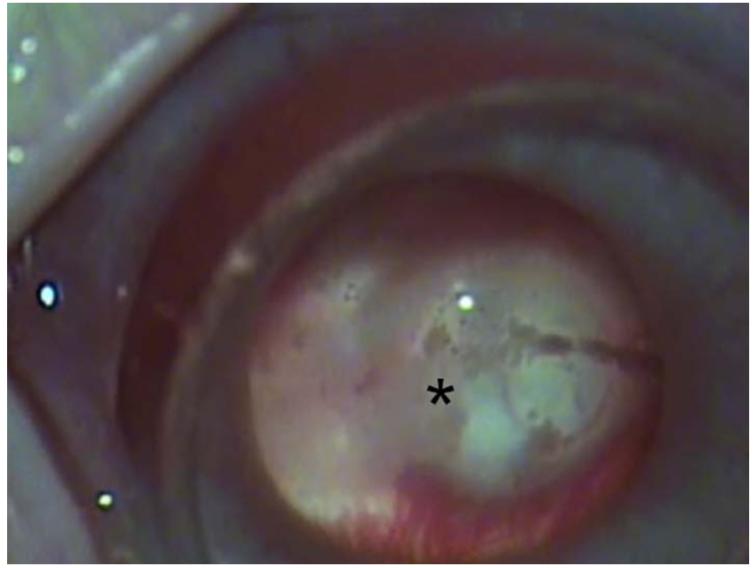
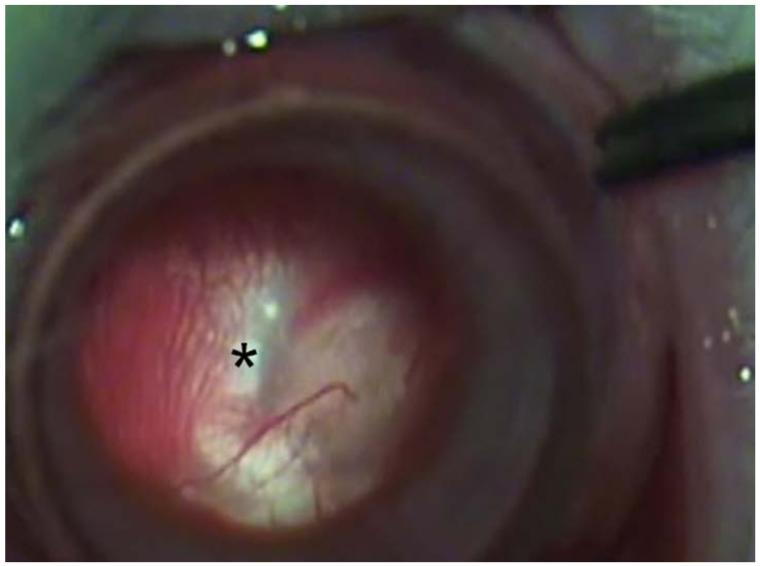
Figure 12.
Intravitreal injection of topotecan in rabbit model of retinoblastoma. Upper left. There are vitreous seeds of retinoblastoma (*) in the rabbit model. Upper right. The 32 gauge 4 mm needle (arrow) is being inserted at the pars plana. Lower left. The needle (arrow) is inserted to its hub and topotecan is injected into the vitreous. Lower right. After 3 weekly injections of 20μg of topotecan, vitreous seeds have disappeared, the retina/optic nerve appear normal and there is no spread of extraocular tumor.
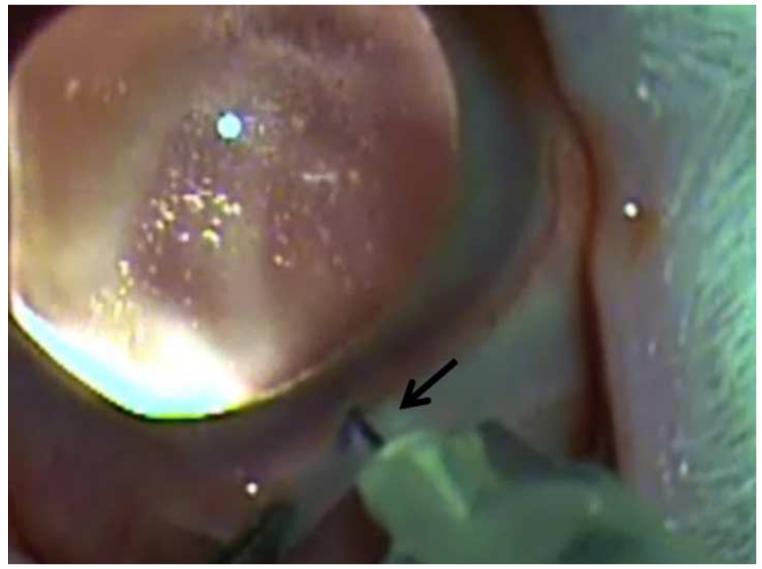
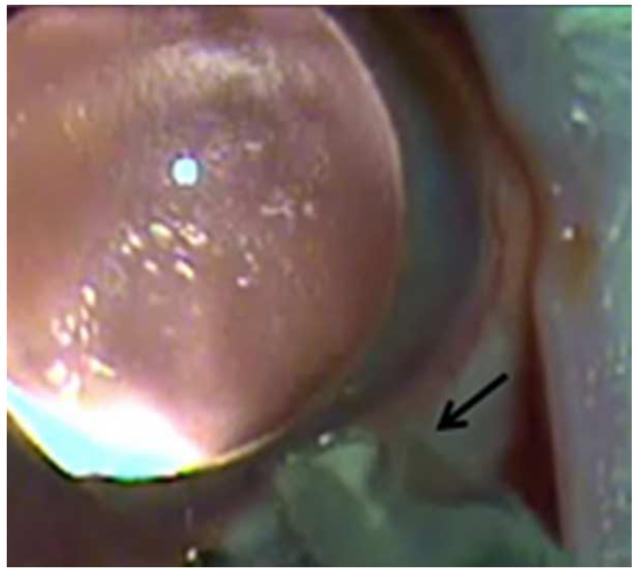
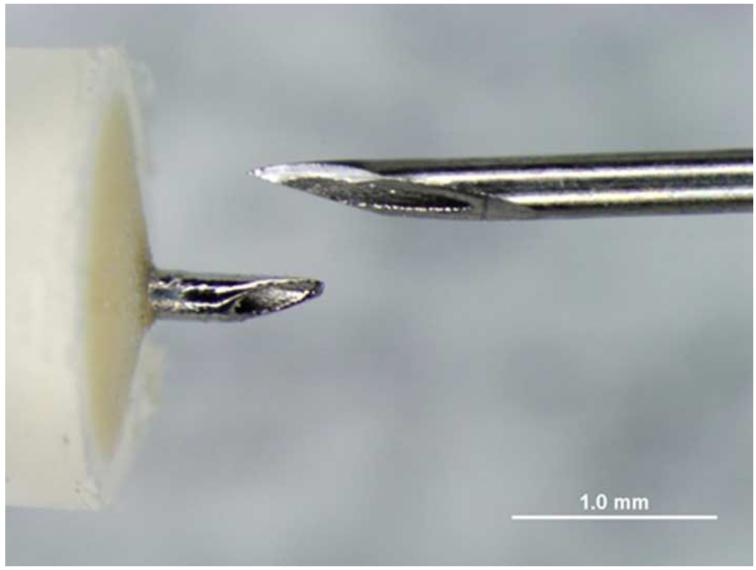
Figure 13.
Suprachoroidal injection of fluorospheres. Upper left. Comparison of microneedle (left) with standard 30 gauge needle; note that the microneedle is the same length as the bevel of the 30 gauge needle. Upper right. India ink injected over ora seratta migrates posteriorly in suprachoroidal space over 30 seconds. Lower left. 10 micron diameter pink fluorospheres in hub of 1 cc syringe with microneedle inserted into suprachoroidal space in a rabbit. Lower right. After injection, a volume of 200 microliters of flourospheres are in the suprachoroidal space and not in the hub of the needle.
Global Health
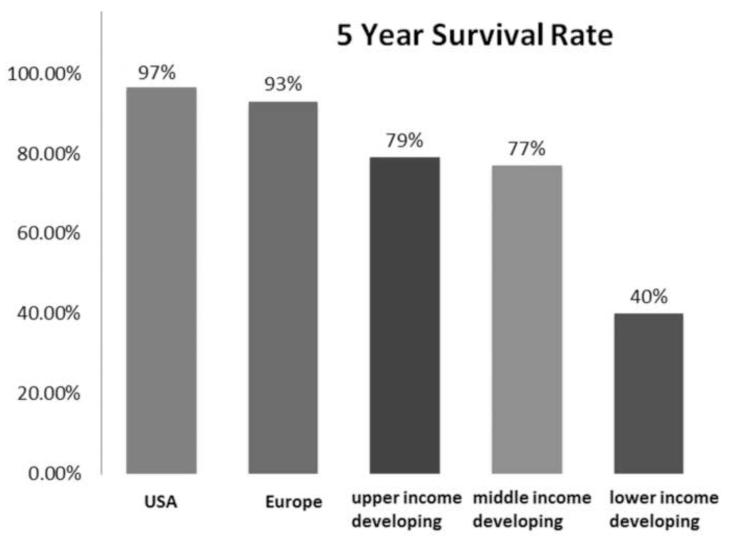
Although mortality from retinoblastoma in developed countries is approximately 3%, mortality from the primary tumor in developing countries approaches 60% in many areas.142 Over the past century, enucleation (1900s), external beam radiation (1920s), photocoagulation (1950s), cryotherapy (1970s), chemoreduction (1990s), and chemoreduction/consolidation (2000s) have sequentially improved survival in children with retinoblastoma to 97% in developed countries.143 In contrast, in developing countries survival rates range from 79% in upper middle income communities to 40% in lower income regions (Figure 14).144 Despite the great advances in molecular biology and treatment for retinoblastoma that have occurred during the past 50 years, access to care is a major barrier to successful therapy in many parts of the world. This is the great remaining challenge in retinoblastoma. Gallie conducted a World Retinoblastoma Survey through an Internet Database and found that the mean age at diagnosis and median follow-up for retinoblastoma patients were for Asia, North American and Europe were 22 months/1.2 years, 12 months/3 years and 9 months/5 years, respectively.142 This implies that access to care is a major problem in Asia, especially considering that there are about 300 new cases of retinoblastoma in North America and about 3,000 new cases in Asia each year.142 Approximately 92% of the world’s children have minimal access to care for retinoblastoma.142 Although there are relevant social, cultural and political issues, it is troubling that many children are dying from a curable disease. A group of retinoblastoma researchers and clinicians from 10 countries met in 2007 to address this issue.145 Strategies for early diagnosis in developing countries were discussed and global initiatives by non-governmental organizations were presented.145 Educational outreach, awareness campaigns, twinning with institutions in developed countries, and national programs are currently addressing access to care for retinoblastoma patients in developing countries.145 The internet and telemedicine are being used as tools to implement these initiatives. Our current challenge is not technological, but rather social: ensuring that children who live in under developed regions of the world can have access to appropriate medical care.
Figure 14.
Regional 5-year survival of children with retinoblastoma. Five-year survival rates for children with retinoblastoma vary among countries by their socioeconomic rank, ranging from nearly 97% in the United States to 40% in low income developing countries. Modified from Canturk et al 144
Summary
In summary, retinoblastoma is a remarkable success story. This cancer was virtually uniformly fatal 200 years ago and is now uniformly curable if detected early. Most children with retinoblastoma in the United States retain their eyes, and many enjoy useful vision. In the 400 years prior to Dunphy’s lecture2, progress was slow and sporadic, tied to key innovations like the ophthalmoscope, microscopic diagnosis, surgical enucleation and radiation therapy. In the 50 years since his lecture, major advances have been made at an accelerated rate, including understanding the molecular biology of the tumor and targeted, globe-sparing therapy. It is notable that these achievements were made by individual ophthalmologists, pathologists and oncologists, often in the absence of clinical trials. I wish to thank the American Journal of Ophthalmology and American Academy of Ophthalmology for giving me the great honor and privilege of delivering the LXXI Edward Jackson Memorial Lecture.
Acknowledgements
Funding support (HEG): Research to Prevent Blindness, New York, New York; Center for Pediatric Nanotechnology, Emory University/Children’s Healthcare of Atlanta/Georgia Institute of Technology, Atlanta, Georgia; National Institutes of Health National Eye Institute P30EY06360, Washington, D.C.
Financial disclosure: Patent Pending (HEG) with Clearside Biomedical, Alpharetta, Georgia
Contributions of the author: conduction of study (HEG); collection, management, analysis and interpretation of data (HEG), preparation, review and approval of manuscript (HEG)
Other acknowledgements: I wish to thank Curtis E. Margo MD, MPH, University of South Florida, Tampa, Florida for his critical review of this manuscript, and David Abramson MD, Memorial Sloan Kettering Cancer Center, New York, New York, Tero Kivelä MD, University of Helsinki, Helsinki, Finland, and Carol Shields MD, Wills Eye Hospital, Philadelphia, Pennsylvania, for providing helpful information. Mica Duran, Atlanta, Georgia provided the artwork for Figures 6, 7 and 8.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Liesegang TJ, Hoskins HD, Jensen AD. The significance of the Edward Jackson lecture. Am J Ophthalmol. 2005;139(3):530–532. doi: 10.1016/j.ajo.2004.11.049. [DOI] [PubMed] [Google Scholar]
- (2).Dunphy EB. The story of retinoblastoma. The XX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1964;58(4):539–552. [PubMed] [Google Scholar]
- (3).Albert DM. Historic review of retinoblastoma. Ophthalmology. 1987;94(6):654–662. doi: 10.1016/s0161-6420(87)33407-4. [DOI] [PubMed] [Google Scholar]
- (4).Pawius P. Observationes anatomical. 1657;16:336. Cited by Bartolini, Bartolini Hafniae. [Google Scholar]
- (5).Wardrop J. Observations on the Fungus Haematodes or Soft Cancer. George Ramsay and Co; Edinburgh: 2013. p. 1809. [Google Scholar]
- (6).Stevens A. Med & Surg Register. Collins l& Co.; New York: 1818. Case report. [Google Scholar]
- (7).Virchow R. Die krankehaften Geschwulste. Vol 2. August Hirshwald; Berlin: 1864. pp. 151–169. [Google Scholar]
- (8).Hirschberg J. Die Markschwann die Natzhaut. Hirschwald; Berlin: 1869. [Google Scholar]
- (9).Flexner S. A peculiar glioma (neuroepithelioma?) of the retina. Bull Hopkins Hosp. 1891;2:115–119. [Google Scholar]
- (10).Wintersteiner H. Eine anatomisfche und klinsche Studie. Franz Deuticke; Vienna: 1897. Das Neuroepithleioma Retinae. [Google Scholar]
- (11).Lagenbeck B. Observationes anatomico-pathologicae. Dieterichianis; Goettingae: 1836. De Retina. [Google Scholar]
- (12).Robin CH. Dictionnaire de Medecine, de Chirugie etc. 10th ed Vol 8. JB Baillere; Paris: 1815. Myelocytes. [Google Scholar]
- (13).Verhoeff FH, Jackson E. Minutes of the proceedings, 62nd annual meeting. Trans Am Ophthalmol Soc. 1926;24:38–43. [Google Scholar]
- (14).Gallie BL, Ellsworth RM, Abramson DH, Phillips RA. Retinoma: spontaneous regression of retinoblastoma or benign manifestation of the mutation? Br J Cancer. 1982;45(4):513–521. doi: 10.1038/bjc.1982.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Margo C, Hidayat A, Kopelman J, Zimmerman LE. Retinocytoma. A benign variant of retinoblastoma. Arch Ophthalmol. 1983;101(10):1519–1531. doi: 10.1001/archopht.1983.01040020521003. [DOI] [PubMed] [Google Scholar]
- (16).Bader JL, Meadows AT, Zimmerman LE, et al. Bilateral retinoblastoma with ectopic intracranial retinoblastoma: trilateral retinoblastoma. Cancer Genet Cytogenet. 1982;5(3):203–213. doi: 10.1016/0165-4608(82)90026-7. [DOI] [PubMed] [Google Scholar]
- (17).Brownstein S, de Chadarevian JP, Little JM. Trilateral retinoblastoma. Report of two cases. Arch Ophthalmol. 1984;102(2):257–262. doi: 10.1001/archopht.1984.01040030207028. [DOI] [PubMed] [Google Scholar]
- (18).Kivela T. Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol. 1999;17(6):1829–1837. doi: 10.1200/JCO.1999.17.6.1829. [DOI] [PubMed] [Google Scholar]
- (19).Marcus DM, Brooks SE, Leff G, et al. Trilateral retinoblastoma: insights into histogenesis and management. Surv Ophthalmol. 1998;43(2):59–70. doi: 10.1016/s0039-6257(98)00019-8. [DOI] [PubMed] [Google Scholar]
- (20).Tarkkanen A, Tuovinen E. Retinoblastoma in Finland 1912-1964. Acta Ophthalmol (Copenh) 1971;49(2):293–300. doi: 10.1111/j.1755-3768.1971.tb00953.x. [DOI] [PubMed] [Google Scholar]
- (21).Jijelava KP, Grossniklaus HE. Diffuse anterior retinoblastoma: A review. Saudi J Ophthalmol. 2013;27(3):135–139. doi: 10.1016/j.sjopt.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17(3):228–234. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- (23).Balmer A, Zografos L, Munier F. Diagnosis and current management of retinoblastoma. Oncogene. 2006;25(38):5341–5349. doi: 10.1038/sj.onc.1209622. [DOI] [PubMed] [Google Scholar]
- (24).Morgan G. Diffuse infiltrating retinoblastoma. Br J Ophthalmol. 1971;55:600–606. doi: 10.1136/bjo.55.9.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Girard B, Le HP, D’Hermies F, Quere MA, Rousselie F. [Diffuse infiltrating retinoblastoma] J Fr Ophtalmol. 1989;12(5):369–381. [PubMed] [Google Scholar]
- (26).Crosby MB, Hubbard GB, Gallie BL, Grossniklaus HE. Anterior diffuse retinoblastoma: mutational analysis and immunofluorescence staining. Arch Pathol Lab Med. 2009;133(8):1215–1218. doi: 10.5858/133.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shields CL, Schoenberg E, Kocher K, Shukla SY, Kaliki S, Shields JA. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases: results based on age at presentation. Ophthalmology. 2013;120(2):311–316. doi: 10.1016/j.ophtha.2012.07.067. [DOI] [PubMed] [Google Scholar]
- (28).Reese AB, Ellsworth RM. The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:164–172. [PubMed] [Google Scholar]
- (29).Murphree AL. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;8(1):41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- (30).Kopelman JE, McLean IW, Rosenberg SH. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology. 1987;94(4):371–377. doi: 10.1016/s0161-6420(87)33436-0. [DOI] [PubMed] [Google Scholar]
- (31).Messmer EP, Heinrich T, Hopping W, de SE, Havers W, Sauerwein W. Risk factors for metastases in patients with retinoblastoma. Ophthalmology. 1991;98(2):136–141. doi: 10.1016/s0161-6420(91)32325-x. [DOI] [PubMed] [Google Scholar]
- (32).Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996;77(6):1206–1213. [PubMed] [Google Scholar]
- (33).Chantada GL, de Davila MT, Fandino A, et al. Retinoblastoma with low risk for extraocular relapse. Ophthalmic Genet. 1999;20(3):133–140. doi: 10.1076/opge.20.3.133.2277. [DOI] [PubMed] [Google Scholar]
- (34).Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Ophthalmol. 1993;77(9):544–548. doi: 10.1136/bjo.77.9.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96(5):357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- (36).Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105(4):573–579. doi: 10.1016/S0161-6420(98)94006-4. [DOI] [PubMed] [Google Scholar]
- (37).Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- (38).Abramson DH, Melson MR, Dunkel IJ, Frank CM. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001;108(10):1868–1876. doi: 10.1016/s0161-6420(01)00713-8. [DOI] [PubMed] [Google Scholar]
- (39).Kleinerman RA, Tucker MA, Abramson DH, Seddon JM, Tarone RE, Fraumeni JF., Jr. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99(1):24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- (40).Eagle RC., Jr. The pathology of ocular cancer. Eye (Lond) 2013;27(2):128–136. doi: 10.1038/eye.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Burnier MN, McLean IW, Zimmerman LE, Rosenberg SH. Retinoblastoma. The relationship of proliferating cells to blood vessels. Invest Ophthalmol Vis Sci. 1990;31(10):2037–2040. [PubMed] [Google Scholar]
- (42).Ts’o MO, Zimmerman LE, Fine BS. The nature of retinoblastoma. I. Photoreceptor differentiation: a clinical and histopathologic study. Am J Ophthalmol. 1970;69(3):339–349. doi: 10.1016/0002-9394(70)92263-4. [DOI] [PubMed] [Google Scholar]
- (43).Ts’o MO, Fine BS, Zimmerman LE. The Flexner-Wintersteiner rosettes in retinoblastoma. Arch Pathol. 1969;88(6):664–671. [PubMed] [Google Scholar]
- (44).Ts’o MO, Fine BS, Zimmerman LE. The nature of retinoblastoma. II. Photoreceptor differentiation: an electron microscopic study. Am J Ophthalmol. 1970;69(3):350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- (45).Sastre X, Chantada GL, Doz F, et al. Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med. 2009;133(8):1199–1202. doi: 10.5858/133.8.1199. [DOI] [PubMed] [Google Scholar]
- (46).Chantada GL, Casco F, Fandino AC, et al. Outcome of patients with retinoblastoma and postlaminar optic nerve invasion. Ophthalmology. 2007;114(11):2083–2089. doi: 10.1016/j.ophtha.2007.01.012. [DOI] [PubMed] [Google Scholar]
- (47).Stannard C, Lipper S, Sealy R, Sevel D. Retinoblastoma: correlation of invasion of the optic nerve and choroid with prognosis and metastases. Br J Ophthalmol. 1979;63(8):560–570. doi: 10.1136/bjo.63.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Magramm I, Abramson DH, Ellsworth RM. Optic nerve involvement in retinoblastoma. Ophthalmology. 1989;96(2):217–222. doi: 10.1016/s0161-6420(89)32910-1. [DOI] [PubMed] [Google Scholar]
- (49).Bosaleh A, Sampor C, Solernou V, et al. Outcome of children with retinoblastoma and isolated choroidal invasion. Arch Ophthalmol. 2012;130(6):724–729. doi: 10.1001/archophthalmol.2012.567. [DOI] [PubMed] [Google Scholar]
- (50).Kaliki S, Shields CL, Rojanaporn D, et al. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]
- (51).Eagle RC., Jr. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med. 2009;133(8):1203–1209. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- (52).Kashyap S, Meel R, Pushker N, et al. Clinical predictors of high risk histopathology in retinoblastoma. Pediatr Blood Cancer. 2012;58(3):356–361. doi: 10.1002/pbc.23239. [DOI] [PubMed] [Google Scholar]
- (53).Wilson MW, Qaddoumi I, Billups C, Haik BG, Rodriguez-Galindo C. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95(4):553–558. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- (54).Dithmar S, Aabert TM, Jr., Grossniklaus HE. Histopathologic changes in retinoblastoma after chemoreduction. Retina. 2000;20(1):33–36. doi: 10.1097/00006982-200001000-00006. [DOI] [PubMed] [Google Scholar]
- (55).Bechrakis NE, Bornfeld N, Schueler A, Coupland SE, Henze G, Foerster MH. Clinicopathologic features of retinoblastoma after primary chemoreduction. Arch Ophthalmol. 1998;116(7):887–893. doi: 10.1001/archopht.116.7.887. [DOI] [PubMed] [Google Scholar]
- (56).Demirci H, Eagle RC, Jr., Shields CL, Shields JA. Histopathologic findings in eyes with retinoblastoma treated only with chemoreduction. Arch Ophthalmol. 2003;121(8):1125–1131. doi: 10.1001/archopht.121.8.1125. [DOI] [PubMed] [Google Scholar]
- (57).Vajzovic LM, Murray TG, ziz-Sultan MA, et al. Clinicopathologic review of enucleated eyes after intra-arterial chemotherapy with melphalan for advanced retinoblastoma. Arch Ophthalmol. 2010;128(12):1619–1623. doi: 10.1001/archophthalmol.2010.296. [DOI] [PubMed] [Google Scholar]
- (58).Hwang CK, Aaberg TM, Jr., Chevez-Barrios P, et al. Residual intraretinal retinoblastoma after chemoreduction failure. Arch Ophthalmol. 2012;130(2):246–248. doi: 10.1001/archopthalmol.2011.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Falls HF, Neel JV. Genetics of retinoblastoma. AMA Arch Ophthalmol. 1951;46(4):367–389. doi: 10.1001/archopht.1951.01700020378002. [DOI] [PubMed] [Google Scholar]
- (60).Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Monteiro AN. BRCA1: the enigma of tissue-specific tumor development. Trends Genet. 2003;19(6):312–315. doi: 10.1016/S0168-9525(03)00110-0. [DOI] [PubMed] [Google Scholar]
- (62).Lalande M, Dryja TP, Schreck RR, Shipley J, Flint A, Latt SA. Isolation of human chromosome 13-specific DNA sequences cloned from flow sorted chromosomes and potentially linked to the retinoblastoma locus. Cancer Genet Cytogenet. 1984;13(4):283–295. doi: 10.1016/0165-4608(84)90073-6. [DOI] [PubMed] [Google Scholar]
- (63).Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- (64).Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987;235(235):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- (65).Hong FD, Huang HJ, To H, et al. Structure of the human retinoblastoma gene. Proc Natl Acad Sci U S A. 1989;86(14):5502–5506. doi: 10.1073/pnas.86.14.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Claudio PP, Tonini T, Giordano A. The retinoblastoma family: twins or distant cousins? Genome Biol. 2002;3(9) doi: 10.1186/gb-2002-3-9-reviews3012. reviews3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).D’Andrilli G, Masciullo V, Bagella L, et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin Cancer Res. 2004;10(9):3098–3103. doi: 10.1158/1078-0432.ccr-03-0524. [DOI] [PubMed] [Google Scholar]
- (68).Rushlow DE, Mol BM, Kennett JY, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14(4):327–334. doi: 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- (69).Sellers WR, Kaelin WG., Jr. Role of the retinoblastoma protein in the pathogenesis of human cancer. J Clin Oncol. 1997;15(11):3301–3312. doi: 10.1200/JCO.1997.15.11.3301. [DOI] [PubMed] [Google Scholar]
- (70).Wang JY, Knudsen ES, Welch PJ. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- (71).Dimaras H, Khetan V, Halliday W, et al. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet. 2008;17(10):1363–1372. doi: 10.1093/hmg/ddn024. [DOI] [PubMed] [Google Scholar]
- (72).Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46(7):617–634. doi: 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- (73).Harbour JW. Eye cancer: unique insights into oncogenesis: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47(5):1736–1745. doi: 10.1167/iovs.05-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- (75).Harbour JW. Overview of RB gene mutations in patients with retinoblastoma. Implications for clinical genetic screening. Ophthalmology. 1998;105(8):1442–1447. doi: 10.1016/S0161-6420(98)98025-3. [DOI] [PubMed] [Google Scholar]
- (76).Horowitz JM, Park SH, Bogenmann E, et al. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Lee C, Chang JH, Lee HS, Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. 2002;16(24):3199–3212. doi: 10.1101/gad.1046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Kim HY, Cho Y. Structural similarity between the pocket region of retinoblastoma tumour suppressor and the cyclin-box. Nat Struct Biol. 1997;4(5):390–395. doi: 10.1038/nsb0597-390. [DOI] [PubMed] [Google Scholar]
- (79).Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391(6670):859–865. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- (80).Flemington EK, Speck SH, Kaelin WG., Jr. E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1993;90(15):6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13(10):6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Weintraub SJ, Prater CA, Dean DC. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358(6383):259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- (83).DiFiori R, D’Anneo A, Tesoriere G, Vento R. RB1 in cancer: different mechanisms of RB1 inactivation and alterations of pRb pathway in tumorigenesis. J Cell Physiol. 2013;228(8):1676–1687. doi: 10.1002/jcp.24329. [DOI] [PubMed] [Google Scholar]
- (84).Xu XL, Fang Y, Lee TC, et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell. 2009;137(6):1018–1031. doi: 10.1016/j.cell.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5(6):539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- (86).Pajovic S, Corson TW, Spencer C, et al. The TAg-RB murine retinoblastoma cell of origin has immunohistochemical features of differentiated Muller glia with progenitor properties. Invest Ophthalmol Vis Sci. 2011;52(10):7618–7624. doi: 10.1167/iovs.11-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Ajioka I, Martins RA, Bayazitov IT, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131(6):378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).McEvoy J, Flores-Otero J, Zhang J, et al. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell. 2011;20(2):260–275. doi: 10.1016/j.ccr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Kapatai G, Brundler MA, Jenkinson H, et al. Gene expression profiling identifies different sub-types of retinoblastoma. Br J Cancer. 2013;109(2):512–525. doi: 10.1038/bjc.2013.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Chantada G, Fandino A, Davila MT, et al. Results of a prospective study for the treatment of retinoblastoma. Cancer. 2004;100(4):834–842. doi: 10.1002/cncr.11952. [DOI] [PubMed] [Google Scholar]
- (91).Uusitalo MS, Van Quill KR, Scott IU, Matthay KK, Murray TG, O’Brien JM. Evaluation of chemoprophylaxis in patients with unilateral retinoblastoma with high-risk features on histopathologic examination. Arch Ophthalmol. 2001;119(1):41–48. [PubMed] [Google Scholar]
- (92).Rodriguez-Galindo C, Chantada GL, Haik BG, Wilson MW. Treatment of Retinoblastoma: Current Status and Future Perspectives. Curr Treat Options Neurol. 2007;9(4):294–307. doi: 10.1007/s11940-007-0015-4. [DOI] [PubMed] [Google Scholar]
- (93).Shields CL, Honavar SG, Meadows AT, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002;133(5):657–664. doi: 10.1016/s0002-9394(02)01348-x. [DOI] [PubMed] [Google Scholar]
- (94).Wilson MW, Haik BG, Liu T, Merchant TE, Rodriguez-Galindo C. Effect on ocular survival of adding early intensive focal treatments to a two-drug chemotherapy regimen in patients with retinoblastoma. Am J Ophthalmol. 2005;140(3):397–406. doi: 10.1016/j.ajo.2005.03.037. [DOI] [PubMed] [Google Scholar]
- (95).Chantada GL, Fandino AC, Raslawski EC, et al. Experience with chemoreduction and focal therapy for intraocular retinoblastoma in a developing country. Pediatr Blood Cancer. 2005;44(5):455–460. doi: 10.1002/pbc.20259. [DOI] [PubMed] [Google Scholar]
- (96).Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21(10):2019–2025. doi: 10.1200/JCO.2003.09.103. [DOI] [PubMed] [Google Scholar]
- (97).Friedman DL, Himelstein B, Shields CL, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18(1):12–17. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- (98).Abramson DH, Frank CM, Dunkel IJ. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology. 1999;106(10):1947–1950. doi: 10.1016/S0161-6420(99)90406-2. [DOI] [PubMed] [Google Scholar]
- (99).Schmack I, Hubbard GB, Kang SJ, Aaberg TM, Jr., Grossniklaus HE. Ischemic necrosis and atrophy of the optic nerve after periocular carboplatin injection for intraocular retinoblastoma. Am J Ophthalmol. 2006;142(2):310–315. doi: 10.1016/j.ajo.2006.02.044. [DOI] [PubMed] [Google Scholar]
- (100).Gombos DS, Kelly A, Coen PG, Kingston JE, Hungerford JL. Retinoblastoma treated with primary chemotherapy alone: the significance of tumour size, location, and age. Br J Ophthalmol. 2002;86(1):80–83. doi: 10.1136/bjo.86.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Doz F, Khelfaoui F, Mosseri V, et al. The role of chemotherapy in orbital involvement of retinoblastoma. The experience of a single institution with 33 patients. Cancer. 1994;74(2):722–732. doi: 10.1002/1097-0142(19940715)74:2<722::aid-cncr2820740228>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- (102).Gunduz K, Muftuoglu O, Gunalp I, Unal E, Tacyildiz N. Metastatic retinoblastoma clinical features, treatment, and prognosis. Ophthalmology. 2006;113(9):1558–1566. doi: 10.1016/j.ophtha.2006.03.039. [DOI] [PubMed] [Google Scholar]
- (103).Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33(12):601–607. [PubMed] [Google Scholar]
- (104).Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9(2):69–73. doi: 10.1007/s10147-004-0392-6. [DOI] [PubMed] [Google Scholar]
- (105).Suzuki S, Yamane T, Mohri M, Kaneko A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 2011;118(10):2081–2087. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- (106).Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115(8):1398–404. 1404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- (107).Abramson DH, Marr BP, Brodie SE, Dunkel I, Palioura S, Gobin YP. Ophthalmic artery chemosurgery for less advanced intraocular retinoblastoma: five year review. PLoS One. 2012;7(4):e34120. doi: 10.1371/journal.pone.0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery) Ophthalmology. 2010;117(8):1623–1629. doi: 10.1016/j.ophtha.2009.12.030. [DOI] [PubMed] [Google Scholar]
- (109).Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):732–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- (110).Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129(11):1399–1406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- (111).Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: report No. 2, treatment complications. Arch Ophthalmol. 2011;129(11):1407–1415. doi: 10.1001/archophthalmol.2011.151. [DOI] [PubMed] [Google Scholar]
- (112).Materin MA, Kuzmik GA, Jubinsky PT, Minja FJ, Asnes JD, Bulsara KR. Verification of supraselective drug delivery for retinoblastoma using intra-arterial gadolinium. J Neurointerv Surg. 2013;5(6):e42. doi: 10.1136/neurintsurg-2012-010508.rep. [DOI] [PubMed] [Google Scholar]
- (113).Vajzovic LM, Murray TG, Aziz-Sultan MA, et al. Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–176. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Thampi S, Hetts SW, Cooke DL, et al. Superselective intra-arterial melphalan therapy for newly diagnosed and refractory retinoblastoma: results from a single institution. Clin Ophthalmol. 2013;7:981–989. doi: 10.2147/OPTH.S43398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Schaiquevich P, Ceciliano A, Millan N, et al. Intra-arterial chemotherapy is more effective than sequential periocular and intravenous chemotherapy as salvage treatment for relapsed retinoblastoma. Pediatr Blood Cancer. 2013;60(5):766–770. doi: 10.1002/pbc.24356. [DOI] [PubMed] [Google Scholar]
- (116).Choi S, Han JW, Kim H, et al. Combined chemotherapy and intra-arterial chemotherapy of retinoblastoma. Korean J Pediatr. 2013;56(6):254–259. doi: 10.3345/kjp.2013.56.6.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Liu QL, Wang YF, Mao GS, et al. [Analysis on the safety of ophthalmic artery cannulation for intra-arterial chemotherapy in 42 patients with intraocular stage retinoblastoma] Zhonghua Er Ke Za Zhi. 2012;50(10):793–797. [PubMed] [Google Scholar]
- (118).Saglam M, Sarici A, Anagnostakou V, et al. An alternative technique of the superselective catheterization of the ophthalmic artery for intra-arterial chemotherapy of the retinoblastoma: retrograde approach through the posterior communicating artery to the ophthalmic artery. Neuroradiology. 2014 doi: 10.1007/s00234-014-1388-1. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- (119).Bracco S, Leonini S, De Francesco S, et al. Intra-arterial chemotherapy with melphalan for intraocular retinoblastoma. Br J Ophthalmol. 2013;97(9):1219–1221. doi: 10.1136/bjophthalmol-2013-303267. [DOI] [PubMed] [Google Scholar]
- (120).Fallaha N, Dubois J, Carret AS, Callejo SA, Hamel P, Superstein R. Real-time ophthalmoscopic findings of intraophthalmic artery chemotherapy in retinoblastoma. Arch Ophthalmol. 2012;130(8):1075–1077. doi: 10.1001/archophthalmol.2012.180. [DOI] [PubMed] [Google Scholar]
- (121).Trinavarat A, Chiewvit P, Buaboonnam J, Sanpakit K, Atchaneeyasakul LO. Selective ophthalmic arterial infusion of chemotherapeutic drugs for recurrent retinoblastoma. J Pediatr Hematol Oncol. 2012;34(6):e218–e221. doi: 10.1097/MPH.0b013e318253f09e. [DOI] [PubMed] [Google Scholar]
- (122).Venturi C, Bracco S, Cerase A, et al. Superselective ophthalmic artery infusion of melphalan for intraocular retinoblastoma: preliminary results from 140 treatments. Acta Ophthalmol. 2013;91(4):335–342. doi: 10.1111/j.1755-3768.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- (123).Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology. 2012;119(3):611–616. doi: 10.1016/j.ophtha.2011.08.045. [DOI] [PubMed] [Google Scholar]
- (124).Abruzzo T, Patino M, Leach J, Rahme R, Geller J. Cerebral vasoconstriction triggered by sympathomimetic drugs during intra-atrerial chemotherapy. Pediatr Neurol. 2013;48(2):139–142. doi: 10.1016/j.pediatrneurol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- (125).Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31(3):566–573. doi: 10.1097/IAE.0b013e318203c101. [DOI] [PubMed] [Google Scholar]
- (126).Shields CL, Kaliki S, Rojanaporn D, Al-Dahmash S, Bianciotto CG, Shields JA. Intravenous and intra-arterial chemotherapy for retinoblastoma: what have we learned? Curr Opin Ophthalmol. 2012;23(3):202–209. doi: 10.1097/ICU.0b013e3283524130. [DOI] [PubMed] [Google Scholar]
- (127).Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29(7):845–851. doi: 10.1200/JCO.2010.32.5332. [DOI] [PubMed] [Google Scholar]
- (128).Karcioglu ZA, Gordon RA, Karcioglu GL. Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology. 1985;92(12):1763–1767. doi: 10.1016/s0161-6420(85)34105-2. [DOI] [PubMed] [Google Scholar]
- (129).Kivela T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118(8):1689, 1689–6. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- (130).Seregard S, Kock E, af TE. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995;79(2):194–195. doi: 10.1136/bjo.79.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569–576. doi: 10.1111/j.1755-3768.1961.tb00269.x. [DOI] [PubMed] [Google Scholar]
- (132).Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined Intravitreal Melphalan and Topotecan for Refractory or Recurrent Vitreous Seeding From Retinoblastoma. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.414. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- (133).Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- (134).Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96(8):1084–1087. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- (135).Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- (136).Seregard S, Singh AD. Retinoblastoma: direct chemotherapeutic drug delivery into the vitreous cavity. Br J Ophthalmol. 2012;96(4):473–474. doi: 10.1136/bjophthalmol-2012-301528. [DOI] [PubMed] [Google Scholar]
- (137).Subramanian N, Navaneethakrishnan S, Biswas J, Kanwar RK, Kanwar JR, Krishnakumar S. RNAi Mediated Tiam1 Gene Knockdown Inhibits Invasion of Retinoblastoma. PLoS One. 2013;8(8):e70422. doi: 10.1371/journal.pone.0070422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Edelhauser HF, Rowe-Rendleman CL, Robinson MR, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51(11):5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci. 2006;47(10):4532–4539. doi: 10.1167/iovs.06-0030. [DOI] [PubMed] [Google Scholar]
- (140).Kang SJ, Durairaj C, Kompella UB, O’Brien JM, Grossniklaus HE. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol. 2009;127:1043–1047. doi: 10.1001/archophthalmol.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Kang SJ, Yang H, Grossniklaus HE. Rabbit model of retinoblastoma. J Biomed Biotechnol. 2009:2561. doi: 10.1155/2011/394730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Gallie BL, Zhao J, Vandezande K, White A, Chan HS. Global issues and opportunities for optimized retinoblastoma care. Pediatr Blood Cancer. 2007;49(7 Suppl):1083–1090. doi: 10.1002/pbc.21350. [DOI] [PubMed] [Google Scholar]
- (143).Abramson DH. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2005;46(8):2683–2691. doi: 10.1167/iovs.04-1462. [DOI] [PubMed] [Google Scholar]
- (144).Canturk S, Qaddoumi I, Khetan V, et al. Survival of retinoblastoma in less-developed countries impact of socioeconomic and health-related indicators. Br J Ophthalmol. 2010;94(11):1432–1436. doi: 10.1136/bjo.2009.168062. [DOI] [PubMed] [Google Scholar]
- (145).Rodriguez-Galindo C, Wilson MW, Chantada G, et al. Retinoblastoma: one world, one vision. Pediatrics. 2008;122(3):e763–e770. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]