Abstract
Although, we often seek social feedback from others to help us make decisions, little is known about how social feedback affects decisions under risk, particularly from a close peer. We conducted two experiments using an established framing task to probe how decision making is modulated by social feedback valence (positive, negative) and the level of closeness with feedback provider (friend, confederate). Participants faced mathematically equivalent decisions framed as either an opportunity to keep (gain frame) or lose (loss frame) part of an initial endowment. Periodically, participants were provided with positive (e.g., “Nice!”) or negative (e.g., “Lame!”) feedback about their choices. Such feedback was provided by either a confederate (Experiment 1), or a gender-matched close friend (Experiment 2). As expected, the framing effect was observed in both experiments. Critically, an individual’s susceptibility to the framing effect was modulated by the valence of the social feedback, but only when the feedback provider was a close friend. This effect was reflected in the activation patterns of ventromedial prefrontal cortex and posterior cingulate cortex, regions involved in complex decision making. Taken together, these results highlight social closeness as an important factor in understanding the impact of social feedback on neural mechanisms of decision making.
Keywords: framing effect, social feedback, decision making, ventromedial prefrontal cortex
INTRODUCTION
We often seek validation and advice from others when making decisions. From trivial to life changing choices – which dress to buy or whether to relocate for a job – we consistently rely upon input from others, or social feedback (SFB). Social feedback can be expressed in many forms such as advice (Engelmann et al., 2009, 2012), judgment (Izuma et al., 2008), or even social ranking and comparison (Bault et al., 2008, 2011). While often constructive, SFB can also be maladaptive by increasing the salience of risky options and the tendency to make irrational choices (Steinberg 2007; Guyer et al. 2012). In fact, the mere presence of another person, a peer in particular, can affect how a reward is perceived (Fareri et al., 2012; Fareri & Delgado, 2013) and increase adolescent risky behavior (Chein et al. 2011) and impulsivity (O’Brien et al. 2011). Although researchers have begun to probe how SFB is processed in the human brain (e.g., Izuma et al., 2008; Somerville et al. 2010), largely focusing on the role of the ventral striatum (VS) and the ventromedial prefrontal cortex (vmPFC), little is known about how the behavioral and neural correlates of decision making are affected when social approval or disapproval is conveyed.
We conducted two experiments investigating whether SFB from another person, either a stranger or a close friend, modulates a) an established phenomenon of framing effect observed in a well-known paradigm (adapted from De Martino et al., 2006) and b) neural regions involved in feedback processing and decision making (Clithero and Rangel, 2013; Delgado, 2007; O’Doherty, 2003; Haber & Knutson, 2010). We chose the framing effect – a cognitive bias task that exposes irrational decision making process based on how a choice is presented instead of its actual value (Tversky & Kahneman, 1974; Tversky & Kahneman, 1981) – to further probe the well-characterized behavioral patterns elicited by this task (e.g. De Martino et al. 2006; Porcelli & Delgado 2009). Our hypothesis was that SFB, even if unrelated to task performance, would exert an influence over decision making in particular contexts, such as when the feedback provider was a close friend. More specifically, we hypothesized that closeness would potentiate irrational behavioral tendencies (framing effect) based on the valence of the SFB. In line with these behavioral results, we expected that the presence of a close friend would also alter neural mechanisms of decision making (vmPFC; Clithero and Rangel, 2013) that have previously shown to be susceptible to the framing effect (DeMartino et al., 2006).
In the first experiment, a confederate, unknown to the participant, conveyed SFB about task performance. In the second experiment, SFB was provided by a close friend and thus was individually tailored. In both experiments, participants faced decisions framed as either an opportunity to win or lose money (Gain and Loss frame trials respectively). Periodically, a gender-matched confederate (Experiment 1) or close friend (Experiment 2) provided positive or negative SFB about the choices participants made. We found that the level of closeness participants have with SFB providers (confederate vs. friend) modulated the effects of SFB valence on participants’ susceptibility to the framing effect. Further, we observed changes in the neural circuitry of feedback processing and value-based decision making, namely the ventral striatum (VS), ventromedial prefrontal cortex (vmPFC) and ventral posterior cingulate cortex (vPCC), as a function of the closeness between participant and feedback giver as well as SFB valence.
METHODS
Participants
Experiment 1
Thirty-three healthy right-handed individuals from Rutgers University – Newark responded to campus advertisements. One participant was excluded from final data analysis because they always chose either the safe or gamble option (resulting in empty cells for analyses). Thus, the final sample included in reported analyses consisted of 32 participants (16 female, mean age = 21.2 ± 3.7). Participants were told their compensation comprised of an hourly rate of $25 and a task performance bonus which yielded a final payoff of $65. All participants gave informed consent in accordance with policies of the institutional review boards of Rutgers University and the University of Medicine and Dentistry of New Jersey.
Experiment 2
Thirty-one healthy right-handed individuals from Rutgers University – Newark responded to campus advertisements. Four participants were excluded from final data analysis because they always chose either the safe or gamble option (resulting in empty cells for analyses). Thus, the final sample consisted of 27 participants (14 female, mean age = 20.5 ± 3.5). All participants gave informed consent and were compensated as in Experiment 1.
Paradigm and procedure
Experiment 1
The framing paradigm (Figure 1) was adapted from De Martino and colleagues (2006) using E-prime 2.0 (Psychology Software Tools, Sharpsburg, PA). Each trial began with an initial endowment (e.g., Receive $50) presented for 2000 ms. Participants then made a binary choice between a safe option associated with a fixed proportion of the endowment, or a gamble option associated with a probability of keeping or losing the entire endowment. Participants responded with their index and middle fingers of their right hand using a MRI-compatible keypad. The experimental procedure consisted of an introduction where participants met the confederate who would be providing SFB followed by a scanner session (2 runs of 96 trials each) during which participants received SFB from the confederate. Each experimental run was broken down into 32 presentations each of Gain, Loss and Catch trials (16 gain, 16 loss) pseudorandomly ordered.
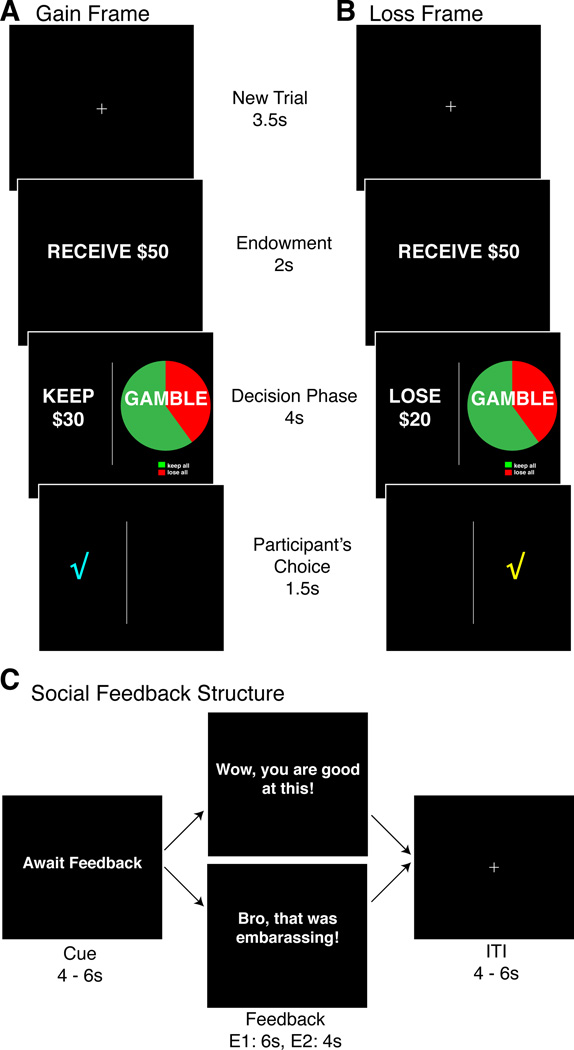
Figure 1. Experimental Task.
This figure illustrates the three main parts of the experimental designed employed in both Experiment 1 and 2. The core task consisted of the same events per trials. Panels A and B present trial structure in Gain and Loss frame respectively showed one at a time. After a fixation cross (3.5s) indicating the beginning of a new trial, participants were presented with a monetary endowment (e.g., Receive $50; Endowment, 2s) before choosing between safe and gamble options presented at a decision phase (4s). Participants were aware that they could not keep this amount and instead they were to use it to decide between two binary options: the safe option was framed such that the participant could keep (Gain frame, Panel A) or lose (Loss Frame, Panel B) a fixed proportion of the endowment. After each choice made, participants saw a confirmation screen indicating their choice (Participant’s choice, 1.5s). Gain frame trials were intermixed pseudo-randomly with Loss frame trials. No monetary outcomes were provided during the experiment. Panel C illustrates the structure according to which social feedback was delivered every few trials (Experiment 1: every 8 trials; Experiment 2: every 2 or 4 trials randomized). In both experiments, the delivery of SFB was preceded with a cue screen (‘Await Feedback’) jittered for 4–6sec. Afterwards either positive or Negative SFB screen was presented for a fixed amount of time (Experiment 1: 6s; Experiment 2: 4s). The trial was ended with a fixation cross jittered for 4–6 seconds to allow for the BOLD to return to equilibrium before a new decision phase will start. The expected value of the gamble and safe options were equivalent.
Four different endowments were offered ($25/$50/$75/$100) in individual trials presented either in the gain or loss frame. The safe option was presented as an amount of money to be retained/lost from the endowment with certainty. For example, in gain frame trials a safe option might involve keeping $30 of the initial $50 endowment. In contrast, on loss frame trials the safe option might involve losing $20 of the initial $50 endowment (Figure 1). The gamble option was the same between gain and loss frame trials. Gamble options were depicted by a pie chart reflecting four distinct probabilities (20, 40, 60, and 80%) of either keeping (green portion) or losing (red portion) the entire endowment. All experimental factors (endowment, probability of winning/losing, number of trials per session, SFB valence) were fully balanced within each experimental run. The expected outcomes of both options within a trial were mathematically equivalent. The only time this was not the case was when participants were presented with catch trials (as in De Martino et al., 2006). There were 32 catch trials per run that served as a manipulation check (to ensure participant’s attention) and were not included in the main analysis. These trials consisted of decisions associated with a clearly dominant choice (e.g., a choice between a 95% gamble to keep all of the endowment versus a safe option to keep half of the endowment).
Participants were introduced to a gender-matched confederate from whom they would receive SFB during the scanner session. Participants were informed that the confederate would observe their choices from outside the scanner. Upon viewing the participants’ responses, the confederate would periodically offer SFB about the set of choices participants had just made. Prior to the scan, participants performed practice rounds while receiving occasional SFB from the confederate seating next to them.
Participants were told that the confederate would choose between 8 keyboard buttons to select specific SFB to present to the participant. Participants received these 8 randomly selected SFB from the confederate (4 positive, 4 negative) each repeated 3 times across the entire experiment (total 24 SFB). SFB was delivered via text projected on a screen in the MRI between ‘mini-blocks’ (see below) of the task. Unbeknownst to participants, SFB valence and time of presentation was predetermined to ensure a controlled and balanced representation across the experiment.
Each functional run in the scanner session contained 13 mini-blocks of 8 trials each. After every 8 trials (or, one mini-block) a SFB item was presented for 6000 ms (Fig. 1C). Thus, each SFB item was presented based on the preceding mini-block, and we examined the impact of such feedback on the decisions in the following mini-block. The first mini-block was not preceded by SFB and was therefore discarded from behavioral and imaging analyses. Importantly, inclusion of intermittent social feedback also increased design efficiency by introducing additional jittered fixation time, thereby reducing collinearity between key variables of interest.
Experiment 2
Procedures were similar to previously described Experiment 1 except for one important variable – SFB provider (friend) – and a few other differences. The main distinction was the inclusion of a personal, close friend of the same gender (neither a romantic partner nor a family member) as a SFB provider, rather than a confederate (see Fareri et al., 2012, 2013). A day before the fMRI session, participants and their friends completed a manipulation check of closeness – the Inclusion of Other in Self scale (IOS) (Aron et al. 1992) consisting of 7 pairs of circles marked self and other respectively and varying in the level of overlap – and provided examples of 5 positive and 5 negative comments they would normally offer to each other when engaging in shared activities (e.g. while playing video games, basketball or driving a car). Eight individually tailored SFB were then used during the fMRI session (e.g., “What were you thinking?”) for a total of 32 SFB across two functional runs.
The framing task consisted of four functional runs (2 involving SFB), each with 48 trials broken down into 24 presentations of Gain and Loss trials pseudorandomly ordered. There were a few modifications from the task described in Experiment 1. First, participants were presented with two monetary endowments ($50 or $100), rather than four. Second, no catch trials were included to maximize the amount of trials for analyses. Third, SFB was provided in only two runs, as the other two runs were performed in isolation (i.e., without SFB influence). Finally, each functional run was composed of 17 mini-blocks of 2 or 4 trials each (rather than every 8 trials in Experiment 1) given the removal of the catch trials. After every mini-block a SFB item was presented for 4000 ms and its influence on the following mini-block was assessed.
fMRI data acquisition
Experiment 1
A 3T Siemens Allegra head-only scanner and standard head coil were used for structural and functional data acquisition at the University Heights Center for Advanced Imaging. Anatomical images were acquired using a T1-weighted protocol (256 × 256 matrix, 176 1-mm sagittal slices). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2000 ms, TE = 20 ms, FOV = 192 cm, flip angle = 80°, bandwidth = 2604 Hz/px, echo spacing = 0.29 ms). Thirty-five contiguous oblique-axial slices (3 × 3 × 3 mm voxels) parallel to the AC-PC line were obtained.
Experiment 2
A Siemens 3T Magnetom Trio whole-body scanner was used for data acquisition at Rutgers University Brain Imaging Center (RUBIC). Anatomical images were acquired using a T1-weighted protocol (256 × 256 matrix, 176 1-mm sagittal slices). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2000 ms, TE = 30 ms, FOV = 192 cm, flip angle = 90°, bandwidth = 2232 Hz/px, echo spacing = 0.51 ms). Thirty-two contiguous oblique-axial slices (3 × 3 × 3 mm voxels) parallel to the AC-PC line were obtained. BrainVoyager QX (v2.3, Brain Innovation) was used to preprocess and analyze neuroimaging data as in Experiment 1.
fMRI data analysis
Experiments 1 & 2
Neuroimaging analyses were conducted using BrainVoyager (Brain Innovation, Maastricht, The Netherlands). Preprocessing involved motion correction (six parameter, three-dimensional) applied to the data to correct for movement, and slice time correction using cubic spline interpolation to temporally align data. Further, spatial smoothing was performed using a three-dimensional Gaussian filter (4-mm FWHM), with voxel-wise linear detrending and temporal high-pass filtering. Structural and functional data were then normalized to standard Talairach stereotaxic space (Talairach and Tournoux, 1988).
Our general linear model examined brain regions exhibiting activation consistent with a framing effect. To examine this neural framing effect for both positive and negative social feedback, the model included 10 primary regressors of interest. We used two regressors to model the receipt of positive and negative feedback (Experiment 1 duration: 6s; Experiment 2 duration: 4s). Activation corresponding to the decision phase (duration: 6s) for trials following these feedback periods was modeled using four regressors for positive and negative feedback, yielding a total of eight decision-phase regressors. These regressors included safe and gamble choices for both loss and gain frames. In Experiment 2, we used an identical model, but also included four additional regressors of no interest to account for the decision-phase period during no feedback runs. All regressors of interest were convolved with the canonical hemodynamic response function. Activation associated with the framing effect was quantified using an interaction contrast: [(Gain_safe + Loss_gamble) − (Gain_gamble + Loss_safe)]; this contrast was computed separately for trials following positive or negative feedback. Nuisance regressors were included to account for head motion, catch trials and missed trials. We limited our neuroimaging inferences to regions (5mm spheres) implicated in value-based decision making (Clithero et al, 2013): ventromedial prefrontal cortex (vmPFC) (MNI coordinates xyz= −2 40 −4), ventral striatum (MNI coordinates xyz= 10 14 −4), and ventral posterior cingulate cortex (vPCC) (MNI coordinates xyz= −8 −56 20). Notably, prior work has suggested that these regions are modulated by social context (e.g. Fareri et al, 2012) and may contribute to computing social variables (e.g. Behrens et al., 2008).
Behavioral analysis
Behavioral data were analyzed using IBM SPSS Statistics 20 and MATLAB (Mathworks Inc.). Participants’ choices on each trial were classified as risky (choosing the gamble option) or safe (choosing the safe option) independent of endowment and gamble probability. Choices were perfectly proportional such that an increase in the proportion of risky choices corresponded to an equivalent decrease in safe choices and vice versa. Therefore, all behavioral analyses were conducted on proportions of risky choices. A framing effect magnitude was calculated for each SFB type (positive and negative) separately. A difference score was calculated between proportions of gamble options chosen in loss as compared to gain frame trials (Loss – Gain). Thus, the smaller the difference, the less affected a participant was by the decision’s frame (i.e., risk-taking levels would be similar in the gain and loss frames if difference scores were closer to zero).
A final consideration was exploration of the role of social closeness in decision making. This was informed by previous work suggesting participants’ sensitivity to the level of social closeness modulates participants’ perception of monetary decision making (e.g., Fareri et al. 2012). Although we did not collect IOS data in Experiment 1, we hypothesized that unacquainted dyads (cf. Experiment 1) would exhibit lower IOS scores compared to friendship dyads (cf. Experiment 2). To test this hypothesis – and validate our social closeness manipulation between Experiment 1 and Experiment 2 – we recruited 16 pairs of subjects (18 females; age range = 18:41, median = 20), all of whom indicated a lack of acquaintanceship. Of these 16 pairs, 8 were gender matched; however, as matched-gender pairs did not significantly differ from unmatched-gender pairs (t(30) = −0.71, p = 0.48), we combined matched- and unmatched-gender pairs in our primary test. Consistent with our hypothesis, we found that unacquainted dyads (mean IOS = 1.76) exhibited significantly lower IOS scores relative to friendship dyads (mean IOS = 5.26) collected in Experiment 2 (t(61) = −10.16, p < 0.0001).
BEHAVIORAL RESULTS
Framing effect is observed across experiments
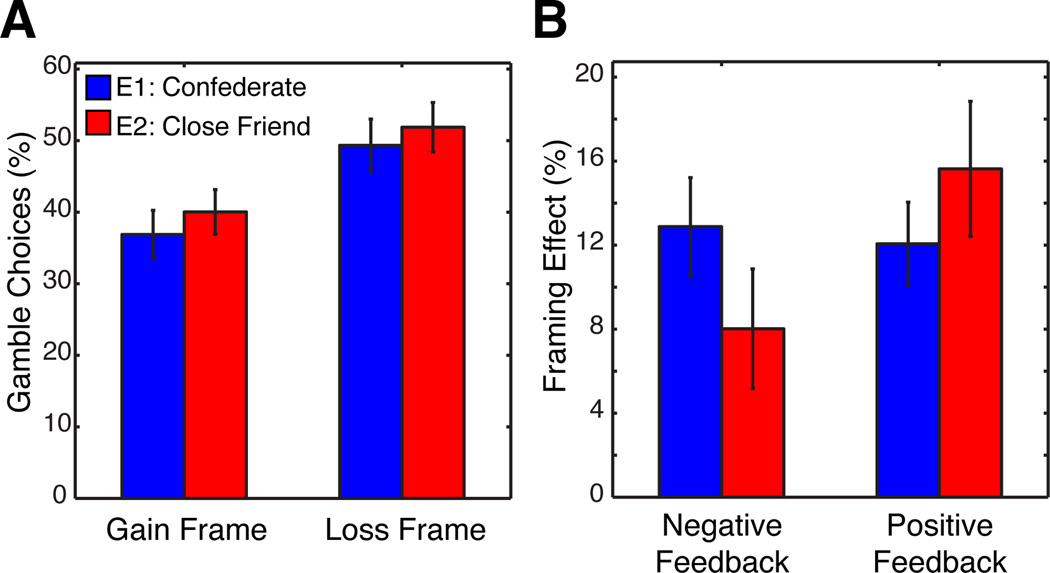
We examined the overall framing effect in each Experiment with two separate t-tests comparing amount of risk taken (% gambled) when decisions were framed as Loss compared to Gains (Fig. 2A). As expected, participants showed a susceptibility to the framing of decisions in both Experiment 1 (Loss = 49.34% (+/− 3.65%), Gain = 36.88% (+/− 3.39%); t(31) = 6.48, p < 0.001) and Experiment 2 (Loss = 51.85% (+/− 3.46%), Gain = 40.00% (+/− 3.11%); t(26) = 4.63, p < 0.001), in that they chose the gamble option significantly more often for Loss than Gain trials. All subsequent analyses focus on investigating the changes caused by SFB valence and the level of social closeness with the provider of such input on decision making.
Figure 2. Social closeness modulates the effects of SFB on the magnitude of framing effect.
Two panels A and B illustrate behavioral interaction between participants’ choices and contextual factors. (A) The percentage of choosing gamble over safe options (y-axis) in either Gain of Loss frames (x-axis) in Experiment 1 (confederate, blue bar) and 2 (close friend, red bar) indicated participants’ susceptibility to the way a choice was presented -- i.e., the framing effect. (B) Each bar represents the magnitude of the framing effect, calculated as a difference in choosing a gamble options between Loss and Gain frames (y-axis), for both positive and negative social feedback (SFB) (x-axis). Our results indicated that the effect of feedback valence was exacerbated for Experiment 2 (red) relative to Experiment 1 (blue).
Social closeness modulates the effects of SFB on irrational behavior
We next focused on the influence of SFB valence on the magnitude of the framing effect. We conducted a 2 (Experiment: 1,2) × 2 (SFB valence: Positive, Negative) mixed factorial ANOVA using the magnitude of framing effect per SFB type as the dependent variable and Experiment as a between subject factor. Of particular interest was a significant interaction observed between the change in the magnitude of framing effect after SFB valence as a function of Experiment (F(1,57) = 5.2, p < .05; Fig. 2B). Participants’ susceptibility to framing is differentially affected by the valence of the SFB, but mainly in Experiment 2 when the provider is a close friend (Fig., 2B). More specifically, the influence of SFB valence on the framing effect magnitude is larger in Experiment 2 (M = 7.61%; SE = 3.29%) compared to Experiment 1 (M = 0.81%; SE = 1.98%), hinting that positive SFB from a friend tends to exacerbate the framing effect while negative feedback from a friend is more likely to attenuate it. This observation supports prior findings that the mere presence of a friend can influence decision making (Steinberg, 2007) by suggesting that the valence of SFB from a friend can influence irrational behavioral tendencies as expressed in the framing effect.
One potential interpretation is that participants valued feedback from their friend more because of how helpful it is perceived. We asked participants to provide subjective ratings regarding the extent to which they viewed social feedback as helpful. We observed no differences between Experiments 1 and 2 (t(57) = 0.59, p = .56), suggesting the social closeness, rather than factors such as the perceived utility of feedback, provides a better explanation for the behavioral differences across experiments.
fMRI RESULTS
Social feedback elicits responses in the ventral striatum
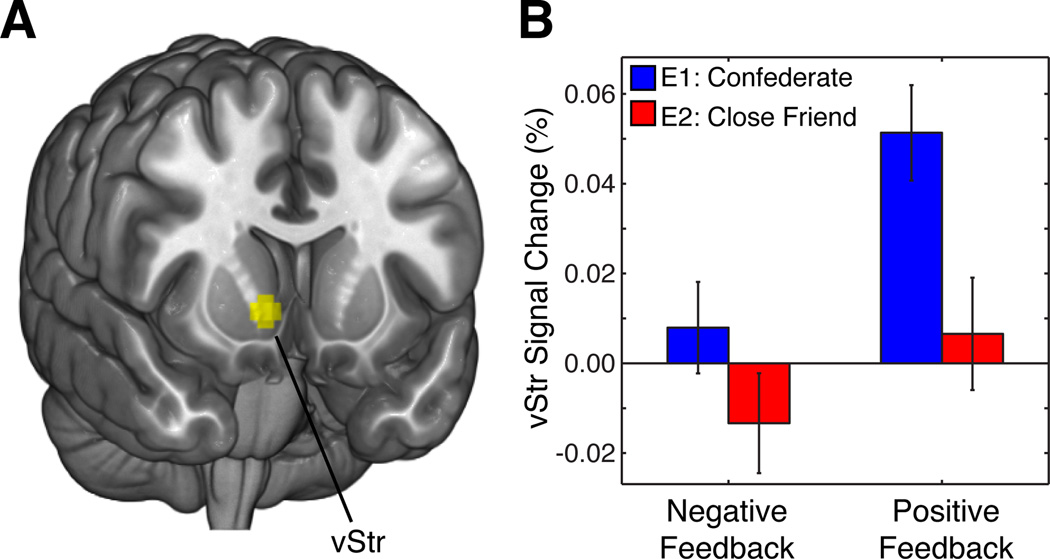
The human striatum has been known to respond to various types of outcomes, from monetary rewards (Delgado et al., 2000) to social judgments (Izuma et al., 2008), often showing a differential response between positive and negative outcomes. We investigated if a) positive and negative social feedback would yield differential responses in the striatum in both experiments and b) if this valence effect would be modulated by the level of closeness of the feedback provider. A 2 (feedback valence: Positive, negative) by 2 (Experiment: 1, 2) mixed factorial ANOVA was performed on a ventral striatum ROI (MNI coordinates xyz= 10 14 −4). Consistent with previous observations, we observed a main effect of feedback valence (F(1,57) = 16.05, p < .001, see Figure 3) where ventral striatum responses were greater for positive compared to negative SFB irrespective of Experiment. Two one-tailed t-tests showed this effect was present in both Experiment 1 (t(31) = 3.75, p < .001) and Experiment 2 (t(26) = 1.92, p = .033). No interaction between Experiment and SFB valence was observed (F(1,57) = 2.22, p = .15).
Figure 3. Ventral striatum encodes feedback valence in both experiments.
A) Ventral striatum [xyz= 10 14 −4] ROI was drawn based on functional meta-analysis (Clithero & Rangel, 2013). B) Our results indicated that feedback valence modulated ventral striatum responses in both Experiment 1 (blue) and Experiment 2 (red).
Regions implicated in value-based decisions are modulated by social closeness
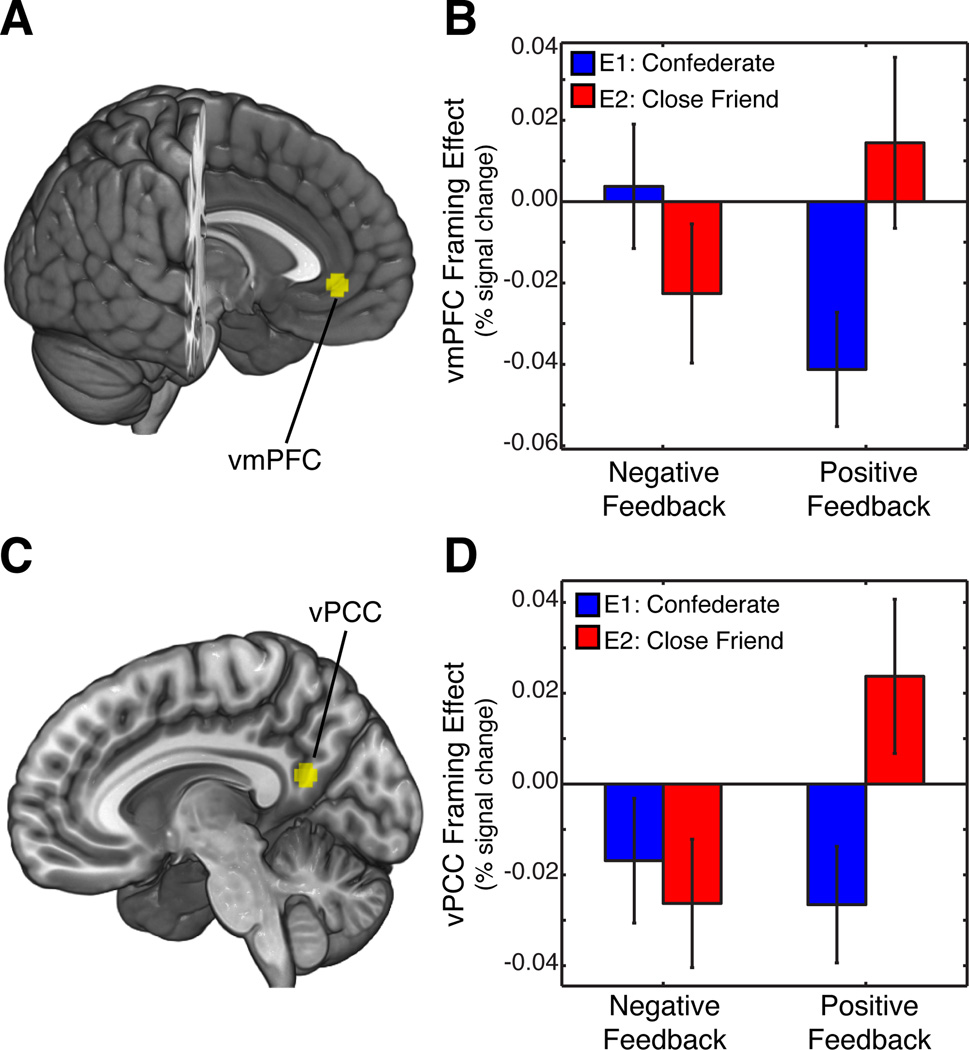
In meta-analyses of value-based decision-making, the vmPFC and vPCC are often identified as key neural structures (e.g., Clithero & Rangel, 2013), potentially playing a role in social and emotional aspects of valuation (e.g. Brosch and Sander 2013). We investigated how neural signals reflecting the susceptibility to the framing effect in these two core decision-making regions were modulated by the valence of a prior SFB and its provider (confederate or friend). Specifically, we calculated the magnitude of the framing effect by computing an interaction contrast [(Gain_safe + Loss_gamble) − (Gain_gamble + Loss_safe)] for both positive and negative SFB in each Experiment. This feedback-related framing effect measure was used in a mixed 2 (feedback-related framing effect: Positive/Negative) × Experiment (1,2) ANOVA for each ROIs separately (Fig. 4). We observed a significant interaction between the feedback-related framing effect measure and Experiment type in vmPFC (F(1,57) = 5.8, p < .05) and a trend for an interaction in vPCC (F(1,57) = 3.8, p = .06).
Figure 4. Social closeness modulates activation associated with the framing effect.
A) Ventromedial prefrontal cortex (vmPFC; [xyz= −2 40 −4]) and C) ventral Posterior cingulate cortex (vPCC; [xyz= −8 −56 20]) ROIs were drawn based on functional meta-analysis (Clithero & Rangel, 2013) indicating these two regions in value-based decision making. Panel B and D depict the neural framing effect computed as an interaction contrast [(Gain_safe + Loss_gamble) − (Gain_gamble + Loss_safe)] for each condition and experiment. Our results indicated social closeness (i.e., Experiment 1 vs. Experiment 2) modulated vmPFC and vPCC responses to the framing manipulation.
DISCUSSION
The current study investigated whether feedback from a close friend influences a well-established susceptibility to the way a choice is presented -- the framing effect. In two experiments, we employed a framing effect paradigm (DeMartino et al., 2006) and introduced intermittent feedback from another person in order to test whether a prior relationship with the feedback provider (close friend or stranger) would alter established behavioral patterns elicited by the framing effect. The presence of a framing effect – being risky when a decision is framed as a loss or conservative when a decision is framed as a gain – was apparent in both experiments, regardless of who provided feedback. Critically, the magnitude of individual susceptibility to the framing effect was sensitive to the feedback valence, positive or negative, but only from a close friend. Our behavioral findings are consistent with the idea that the presence of a personal social context (i.e., SFB from a friend) can elicit adaptations in decision making (Steinberg 2007). We extend these findings to show this adaptation even with established behavioral tendencies, suggesting that participants potentially weigh social evaluation more heavily than the frame of a given choice if such input comes from a trusted source (i.e., close friend). A similar pattern of results was observed in structures involved in decision making, social value and self-referential processes (i.e., vPCC and vmPFC; e.g., Clitheros & Rangel, 2013), suggesting a potential mechanism through which SFB from a close friend can influence decisions.
The social and interactive environment in which we function often influences our decision making process (Ariely & Norton, 2008; Kenrick et al. 2009), but the advent of investigations into the neural processes underlying social influences on decision-making is still in its infancy (e.g., Bhanji & Delgado, 2014), and only recently have investigations began to test how explicit input from others can influence neural signals associated with feedback based adaptations on decision-making (e.g., Biele et al 2011). One common finding across these studies is the role of the vmPFC in processing SFB or advice from others (e.g., Biele et al., 2011; Engelmann et al., 2012; Somerville et al., 2010).
Given the involvement of the vmPFC in complex decision making (Rangel et al. 2007; Hare et al., 2009, 2010; Zaki et al., 2011; Wright et al., 2012) particularly in value-based decision making (Clithero & Rangel 2013), including decisions framed as gains or losses (De Martino et al., 2006), we chose the vmPFC as a key region of exploration. Interestingly, one key difference between our study where intermittent SFB was provided and prior investigations where expert advice was given (e.g., Engelmann et al., 2012), was that there was no expectation to follow the feedback (unlike the advice). That is, participants were free to infer what valence of SFB might ensue based on their choices and either keep or shift their strategy to adjust behavior accordingly to the received feedback and the value they attached to the feedback provider. Our findings suggest that decision-related vmPFC activity is modulated by social feedback. Specifically, we observed a differential pattern of activation in vmPFC based on whether the decision followed feedback of different valence and who the feedback giver was, suggesting that the vmPFC may play an important role in integrating social value of feedback as a function of social closeness to inform decisions (e.g. Hampton et al. 2006; Gläscher et al 2009).
The other region of interest we selected, the vPCC, has been linked with both social and value-based decision-making (Clithero & Rangel, 2013). In this study, we observed that activation in ventral portions of posterior cingulate cortex were associated with the framing effect and modulated by social closeness. Although prior work has linked PCC to social cognition (Saxe, 2006), other studies have suggested that PCC may encode signals related to cognitive control (Hayden et al., 2010). We speculate that these disparate accounts can be reconciled by the observation that the PCC is key cortical hub within the default-mode network (Hayden et al., 2009, Buckner et al., 2008). Indeed, recent research has indicated that PCC carries out multiple functions depending on task demands (Utevsky et al., 2014; Leech et al., 2013). One speculative idea regarding the involvement of both vmPFC and vPCC during our experiments is that an increased control over the irrational tendency to choose according to decision presentation rather than its value may be associated with increased self-referential processes (Leech et al. 2011; Anticevic et al. 2012; Nakao et al. 2012).
In social context, unambiguous SFB (positive or negative) carries an affective value that can be processed by similar neural mechanisms that process positive and negative affective outcomes (Delgado, 2007). This is consistent with reports suggesting that feedback from another person conveying positive information about one’s reputation relies on partially overlapping neural reward circuits (Izuma et al., 2008), and that outcome processing in corticostriatal circuits is modulated by social context (Rignoni et al., 2010; Mobbs et al. 2006; Fareri et al., 2012). Interestingly, we did not observe a significant difference in striatum responses to SFB valence between Experiment 1 and 2, although an interaction approached significance, thus it is difficult to comment on differences with respect to closeness and striatum processing of feedback in this particular experiment.
In conclusion, our results suggest that although people are susceptible to the manner in which choice is presented to us (the framing effect), this propensity can be further modulated with social relationships (social closeness). The current study has limitations based on design changes between our two Experiments that were necessary to account for contextual differences (listed in the Methods). These include the between subjects comparison, the number and frequency of experienced feedback and the presence of catch trials (Experiment 1) or no feedback trials (Experiment 2) all of which could influence the main results presented. Importantly, however, the framing effect was observed in both experiments and the modulation of this behavioral result was apparently mostly when the social context was driven by closeness. Future studies may benefit from including both a confederate and a close friend in the same paradigm (Fareri et al. 2012) to directly test the impact of social closeness in the same individual. Nonetheless, our results highlight the power and diversity of social influence on decision making, potentially pointing to the mechanisms that help shape our interpersonal choices.
ACKNOWLEDGMENTS
We would like to thank Benedetto De Martino for his advice on design and his helpful comments on data interpretation. We also thank Victoria K. Lee, Michael A. Niznikiewicz, Ana Rigney and Meredith Johnson for their help in conducting the experiment. Additionally, we would like to thank Avi Mendelsohn for his valuable suggestions regarding data analysis. This research was supported by funding from the National Institutes of Mental Health to M.R.D. (R01MH084081).
REFERENCES
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Science. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariely D, Norton MI. How actions create--not just reveal--preferences. Trends in Cognitive Science. 2008;12(1):13–16. doi: 10.1016/j.tics.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Smollan D. Inclusion of the other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology. 1992;63:596–612. [Google Scholar]
- Bault N, Coricelli G, Rustichini A. Interdependent utilities: how social ranking affects choice behavior. PLoS One. 2008;3:e3477. doi: 10.1371/journal.pone.0003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bault N, Joffily M, Rustichini A, Coricelli G. Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proceedings of the National Academy of Sciences. 2011;108:16044–16049. doi: 10.1073/pnas.1100892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanji JP, Delgado MR. The social brain and reward: social information processing in the human striatum. Wiley Interdisciplinary Review Cognitive Science. 2014;5(1):61–73. doi: 10.1002/wcs.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biele G, Rieskamp J, Krugel LK, Heekeren HR. The neural basis of following advice. PLoS Biology. 2011;9:e1001089. doi: 10.1371/journal.pbio.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch T, Sander D. Neurocognitive mechanisms underlying value-based decision-making: from core values to economic value. Frontiers in Human Neuroscience. 2013;7:398. doi: 10.3389/fnhum.2013.00398. 2013 Jul 24; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience. 2013:1–14. doi: 10.1093/scan/nst106. 2013 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Monica Capra C, Noussair C, Berns GS. Expert financial advice neurobiologically "Offloads" financial decision-making under risk. PLoS One. 2009;4:e4957. doi: 10.1371/journal.pone.0004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Moore S, Capra MC, Berns GS. Differential neurobiological effects of expert advice on risky choice in adolescents and adults. Social Cognitive and Affective Neuroscience. 2012;7:557–567. doi: 10.1093/scan/nss050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. The Journal of Neuroscience. 2012;32:9045–9052. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR. Differential reward responses during competition against in and out-of-network others. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst006. 2013 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Hampton AN, O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19(2):483–495. doi: 10.1093/cercor/bhn098. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proceedings of the National Academy of Science. 2009;106(14):5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Frontiers in Human Neuroscience. 2010;4:223. doi: 10.3389/fnhum.2010.00223. Published online 2010 December 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. The Journal of Neuroscience. 2006;26(32):8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. The Journal of Neuroscience. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kenrick DT, Griskevicius V, Sundie JM, Li NP, Li YJ, Neuberg SL. Deep rationality: the evolutionary economics of decision making. Social Cognition. 2009;27(5):764–785. doi: 10.1521/soco.2009.27.5.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. The Journal of Neuroscience. 2013;32(1):215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of Neuroscience. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Weiskopf N, Lau HC, Featherstone E, Dolan RJ, Frith CD. The Kuleshov Effect: the influence of contextual framing on emotional attributions. Social Cognitive and Affective Neuroscience. 2006;1:95–106. doi: 10.1093/scan/nsl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Ohira H, Northoff G. Distinction between externally vs. internally guided decision-making: operational differences, meta-analytical comparisons and their theoretical implications. Frontiers in Neuroscience. 2012 Mar 5;6:31. doi: 10.3389/fnins.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L, Albert D, Chein J, Steinberg L. Adolescents prefer more immediate rewards when in the presence of their peers. Journal of Research on Adolescence. 2011;21(4):747–753. [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. The Journal of Neuroscience. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AJ, Delgado MR. Acute stress modulates risk taking in financial decision making. Psychological Science. 2009;20(3):278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for experimenting the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoni D, Polezzi D, Rumiati R, Guarino R, Sartori G. When people matter more than money: an ERPs experiment. Brain Research Bulletin. 2010;81:445–452. doi: 10.1016/j.brainresbull.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20(12):3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Current Directions in Psychological Science. Vol. 16. New York: McGraw-Hill; 2007. Risk-taking in adolescence: New perspectives from brain and behavioral science; pp. 55–59. (Reprinted in K. Cauley & G. Pannozzo (Eds.) (2009). Annual Editions: Educational Psychology. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: an approach to medical cerebral imaging. New York: Thieme Medical Publisher; 1988. [Google Scholar]
- Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Utevsky AV1, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. The Journal of Neuroscience. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ND, Symmonds M, Hodgson K, Fitzgerald TH, Crawford B, Dolan RJ. Approach-avoidance processes contribute to dissociable impacts of risk and loss on choice. The Journal of Neuroscience. 2012;32:7009–7020. doi: 10.1523/JNEUROSCI.0049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychological Science. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]