Abstract
Objectives. We assessed the protocols and system processes for colorectal cancer (CRC) screening at federally qualified health centers (FQHCs) in 4 midwestern states.
Methods. We identified 49 FQHCs in 4 states. In January 2013, we mailed their medical directors a 49-item questionnaire about policies on CRC screening, use of electronic medical records, types of CRC screening recommended, clinic tracking systems, referrals for colonoscopy, and barriers to providing CRC.
Results. Forty-four questionnaires (90%) were returned. Thirty-three of the respondents (75%) estimated the proportion of their patients up-to-date with CRC screening, with a mean of 35%. One major barrier to screening was inability to provide colonoscopy for patients with a positive fecal occult blood test (59%). The correlation of system strategies and estimated percentage of patients up-to-date with CRC screening was 0.43 (P = .01).
Conclusions. CRC system strategies were associated with higher CRC screening rates. Implementing system strategies for CRC screening takes time and effort and is important to maintain, to help prevent, or to cure many cases of CRC, the second leading cause of cancer in the United States.
Federally qualified health centers (FQHCs) attempt to provide comprehensive, quality primary health care services to medically underserved communities and vulnerable populations. Approximately 1198 centers receive operating grants from the Public Health Service Act and thus qualify for reimbursement from Medicare and Medicaid.1 FQHCs served 21 million patients in 2012, of whom 36% were uninsured and 92% were living below the 200% poverty level.1 One of the services provided by FQHCs is colorectal cancer (CRC) screening through stool testing for occult blood.2 This service is covered under the Medicare FQHC benefit for persons aged 65 years and older and for those who qualify for the Medicaid program.3
CRC is the second leading cause of cancer deaths in the United States.4 Only 63% of US adults report being up-to-date with CRC screening.5 CRC is a disease that is largely preventable; colonoscopy, through detection of early tumors and removal of precancerous polyps, could prevent 65% of CRC cases.6,7 Several national organizations have guidelines for CRC screening.8,9 National guidelines promote any of several tests for CRC screening: tests that pick up occult bleeding and endoscopic tests that visualize all or part of the colon.8–10
Clinical tests to directly visualize colorectal cancer and precancerous polyps are colonoscopy, flexible sigmoidoscopy, double-contrast barium enema, and computed tomographic colonography (virtual colonoscopy). Fecal occult blood tests (FOBTs), which detect blood in the stool that is not visible and that indicates possible cancer, are the guaiac-based test and the fecal immunochemical test (FIT). FOBTs are recommended annually, and colonoscopy is recommended every 10 years, if no polyps are found.8–10 FOBTs are much less expensive than colonoscopy and are often preferred by patients. In many safety net settings, FOBTs are the initial option for patients, because of the prohibitive cost and limited availability of colonoscopy.11,12
Through an infrastructure grant to enhance community-based cancer control in Iowa, we visited 4 FQHCs in Iowa and learned that FOBTs were available for use, but were for the most part not given to patients to avoid having to arrange and pay for a follow-up colonoscopy if FOBT results were positive. One FQHC director explained that the annual budget included a fund for extra tests that might be needed for any medical reason, and once these funds were exhausted, no more funding was available in that year. Thus, CRC screening was not a top priority, because of many other competing health care needs.
To enhance CRC screening, system strategies are appropriate. A system strategy is a group of interrelated items that are part of a plan of action to accomplish a specific goal, such as improving CRC screening. Many different system strategies have been identified for improving CRC screening, such as physician recommendation,13,14 mailed patient reminders,15–17 and electronic medical record (EMR) physician reminders.18,19
Patients at greatest risk for not receiving CRC screening are racial and ethnic minorities, Asians and Hispanics, and individuals who lack a usual source of health care or health insurance.20 Underuse of CRC screening is frequently associated with socioeconomic disadvantage21 and is associated with higher late-stage CRC rates.22 Because many of our nation’s most disadvantaged individuals make use of FQHCs, we assessed the protocols and system processes in place for CRC screening at FQHCs in 4 midwestern states and estimated rates of CRC screening in these FQHCs.
METHODS
We searched the Health Resources and Services Administration Data Warehouse to identify all 50 FQHCs in the 4 states in the American Cancer Society’s Midwest Division; 13 in Iowa, 15 in Minnesota, 6 in South Dakota, and 16 in Wisconsin. In accord with FQHC requirements, the 50 centers received funding under Section 330 of the Public Health Services Act. To personalize the cover letter, we called each facility and asked for the names of the current medical director and chief administrative officer. During the initial telephone calls, we determined that 1 FQHC was a clinic specializing in tuberculosis treatment and eliminated it from the study, leaving 49 FQHCs in the study sample.
Instruments
We developed a 2-page health center questionnaire regarding colorectal cancer screening, which the University of Iowa Department of Family Medicine’s faculty physician researchers reviewed. We used components of this questionnaire in previous CRC screening studies.16,23
The instrument had 49 questions: 6 items asked about the patient and staff demographics (number of practice providers by type, number of patients aged ≥ 50 years, number of patient visits/month for all providers, and patients' gender and race/ethnicity), and 20 items addressed clinic policy on CRC screening, use of EMRs, routine staff protocol related to CRC screening, types of CRC screening tests recommended, clinic tracking systems and their use in CRC screening, and whether referrals were given for colonoscopy (yes, no, or unsure). In addition, we asked the respondent to estimate the percentage of patients who were up-to-date with CRC screening and to identify the types of CRC tests used from the following list: FOBT, FIT, flexible sigmoidoscopy, and colonoscopy. On a list of 8 barriers to providing CRC screening, the respondent could check items that were barriers and write in additional barriers. Open-ended questions concerned the brand of EMRs used, the system used to track CRC screening, how colonoscopies were paid for, where the colonoscopies were performed, and when the clinic referred patients for a colonoscopy.
For FQHCs that used FITs, we also developed a 1-page follow-up questionnaire, which was critiqued by the Department of Family Medicine faculty physician researchers. This 15-item questionnaire addressed type of FOBT used (guaiac or immunochemical), brand of stool test used, number of stool samples requested, development of the tests, and instructions for return of the stool sample once collected.
Mailings
Mailings to each FQHC followed a modified Dillman approach.24 We sent a full packet of material to the medical directors that included a cover letter (which provided the informed consent elements: the purpose of the study, how and why they were chosen, and explanation that participation involved completing and returning the enclosed questionnaire, that unless they opted out they would receive a second mailing followed by a telephone call, and that results would be presented in aggregated form so that an individual could not be identified), the 2-page questionnaire, a $2 bill, and a postage-paid return envelope. The letter encouraged recipients to forward the questionnaire to a knowledgeable staff member if they were unable to answer questions completely. We asked recipients who were not interested in participating in the study to return their blank questionnaire in the return envelope.
Three weeks after the first mailing, we sent nonresponders an identical full packet of materials minus the $2. If, 2 weeks after the duplicate mailing, no questionnaire was returned, we called the FQHC medical director up to 4 times over 4 weeks. If, after these attempts, the medical director could not be reached, we contacted another knowledgeable staff person, such as a nursing or clinic manager, and if that person agreed, we completed the questionnaire over the phone; otherwise, we left a message, which followed a standardized script, encouraging return of the completed questionnaire. If no questionnaire had been completed after the fourth telephone call attempt and 2 medical director mailings, we sent a third and final duplicate mailing to the chief administrative officer of the FQHC. Participants who completed the questionnaire were compensated $20 for their time.
If respondents indicated that their center used FIT for CRC screening, we sent them the 1-page questionnaire, along with a cover letter and a postage-paid return envelope. If we did not receive this questionnaire after 3 weeks, we called the person who completed the initial questionnaire, up to 4 times over 4 weeks, in an attempt to answer the questions over the phone or otherwise encourage the questionnaire’s return. We provided no additional compensation for the return of this questionnaire.
Data Analysis
We double entered and verified all questionnaires. Multiple sections of both questionnaires followed a pattern of asking a broad question, then following up with specific questions, which were to be answered if the response to the previous question was yes. Some responded to these questions even though they provided an answer of no or unsure for the broad question, so we considered their specific question replies invalid and did not use them. For the 3 questions that prompted respondents to “check all that apply” (CRC screening tests offered to persons aged ≥ 50 years and to persons aged < 50 years and barriers to CRC screening), if a respondent did not check any available response, we considered the answer to be no. However, we do not know whether respondents skipped these questions or truly meant no to be their response, because we did not provide a not sure option. In light of the low number of missing responses, it is unlikely they biased results.
We compared a 35-item subset of questions from the primary questionnaire across FQHC state location (Iowa, Minnesota, South Dakota, or Wisconsin). We applied Fisher exact tests to the 34 questions with categorical responses. We combined the options of no and unsure to simplify interpretation of the Fisher exact tests. We conducted Kruskal–Wallis 1-way analysis of variance on the single question with a continuous response (estimated percentage of patients with up-to-date CRC screening). We used Kruskal–Wallis in lieu of standard (i.e., parametric) 1-way analysis of variance because the conditions of normality and homogeneous variance across states could not be verified with confidence, suggesting that a nonparametric method was appropriate.
We derived a CRC-screening system strategy score from 9 questions from the primary questionnaire. These questions asked whether the clinic had a policy for CRC screening, the policy was in writing, the clinic currently used EMRs, the staff routinely asked the patient whether CRC screening had been completed, the clinic had personnel other than health care providers involved in CRC screening, the clinic tracked return of FOBTs, the clinic implemented reminder telephone calls for nonreturn of FOBTs, the clinic implemented reminder letters for nonreturn of FOBTs, and a system was in place to offer FOBT annually. The scores for the CRC-screening system strategy could range from zero to 9, with yes answers given 1 point. We gave responses of no or unsure and missing responses zero points. We compared the scores across states with Kruskal–Wallis 1-way analysis of variance.
We calculated a Pearson correlation coefficient for the CRC-screening system strategies score against the estimated percentage of patients up-to-date with CRC screening. We plotted a least squares line to emphasize the relationship and used Spearman correlations to verify results. Because we observed a significant and strong Pearson correlation between the strategy score and the estimated percentage of patients up-to-date with CRC screening, we decided that briefly describing how the questions used to create the composite strategy score correlated with the estimated percentage of patients up-to-date with CRC screening would be useful. Because we made each question dichotomous for analysis, we used point–biserial correlation to look at the strengths of linear association. To be consistent with the strategy score, where no and missing answers received zero points, we treated missing responses as a response of no.
Because our data came from nearly all FQHCs operating in these 4 states, it might have been reasonable to interpret values that were functions of the data as population parameters rather than statistics; however, to allow for inference, we treated the data as if they were a sample coming from a larger population of FQHCs. We adjusted the descriptive statistics to account for missing responses (never > 15% for a single question, unless otherwise noted), so the denominators of the percentages did not include invalid responses. We removed missing responses, because it was reasonable to assume that questionnaire takers skipped some of the questions accidentally or did not choose to answer them for reasons other than being unsure; therefore, assuming that they meant no would be inappropriate.
We used SAS version 9.2 for Windows (SAS Institute Inc, Cary, NC) for analysis and R version 2.13.0 (R Project for Statistical Computing, http://www.r-project.org) to construct the scatterplot and corresponding least squares line. All tests were 2-tailed and evaluated at the 0.05 significance level.
RESULTS
Forty-four (90%) of 49 questionnaires were returned. Medical directors completed 80% of the questionnaires, nurse or clinic managers completed 13%, and chief administrative officers completed 7%. Respondents estimated that a mean of 34.9% (95% confidence interval [CI] = 27.5%, 42.4%) of their eligible patients were up-to-date with CRC screening (range = 2%–80%, with no significant difference by state, P = .7). For this question, 11 respondents (25%) did not provide any answer. We made no attempt to impute these values and performed the analysis for this question with the valid responses (n = 33).
The mean number of physicians at the FQHCs was 6 and of nurses, 7 (Table 1). The average number of patients aged 50 years and older served by the FQHCs was 6500, with an average of 2173 visits per month for all patients. Fifty-eight percent of patients were female; 62% were White, 21% were Hispanic, 17% were African American, 4% were American Indian, and 6% were Asian/Pacific Islander. Missing data for patient demographics ranged from 23% to 45%.
TABLE 1—
Number of Employees at Federally Qualified Health Centers in 4 Midwestern States by Position Category: 2012
| Position | Total, No. | Per Center, Mean (SD) |
| Physicians | 40 | 5.6 (6.9) |
| Nurse practitioners | 40 | 2.9 (2.2) |
| Physician assistants | 35 | 2.2 (2.5) |
| Nurse assistants | 34 | 6.9 (7.3) |
| Nursesa | 36 | 6.7 (8.4) |
Registered and licensed practical nurses.
Thirty-four respondents (79%) reported using EMRs, 23 (68%) of those with EMRs could query to determine which patients were due for CRC screening, and 19 (56%) could query to determine which patients are up-to-date with CRC screening. Seventeen (55%) of those with EMRs reported being able to perform both queries. No state’s FQHCs all used the same type of EMR. SuccessEHS (Greenway Health, Birmingham, AL) was used by 10 clinics (29%), Centricity (GE Healthcare, Waukesha, WI) by 9 clinics (26%), Epic (Epic Systems Corporation, Verona, WI) by 4 clinics (12%), and NextGen (NextGen Healthcare Information Systems, Horsham, PA) by 3 clinics (9%).
Screening
Twenty-seven respondents (61%) reported having a policy on CRC screening, and 14 reported that it was in writing. In 26 clinics (59%), various personnel helped with CRC screening. During clinic visits, personnel in 27 clinics (66%) asked patients whether they had an immediate family member with CRC or polyps, 25 (61%) routinely asked patients whether they had completed CRC screening, 18 (44%) asked whether a distant relative had CRC or polyps, and 10 (25%) asked whether patients had ulcerative colitis or Crohn’s disease.
The following CRC screening tests were recommended to patients aged 50 years or older: FOBT by 34 clinics (77%), colonoscopy by 28 (64%), FIT by 18 (41%), and flexible sigmoidoscopy by 5 (11%). Twenty clinics (54%) reported that they had the capability in their system to offer an FOBT annually.
For patients younger than 50 years who had risk factors for CRC, 38 clinics (86%) had a system in place to offer testing; 26 of these clinics (68%) offered colonoscopy, 25 (66%) offered FOBT, 14 (37%) offered FIT, and 4 (11%) offered flexible sigmoidoscopy (figures sum to more than 100% because some clinics offered more than 1 test).
Occult Blood Testing
Forty-one of the clinics (93%) provided FOBTs or FITs to patients aged 50 years or older. Of these clinics, 26 (63%) tracked return of their FOBTs and FITs, 12 (34%) called the patient if the FOBT was not returned, and 9 (26%) sent a reminder letter. Five clinics (14%) both called the patient and sent a reminder letter. Of those that tracked return of their FOBTs and FITs from patients aged 50 years or older, 15 (60%) used the EMR, 6 (24%) tracked on paper, 2 (8%) had registered nurses review the record and call the patients, 2 (8%) had laboratory personnel call for return, 1 (4%) used a tickler file, and 1 (4%) used an Excel file for tracking. Two clinics used 2 methods for tracking returns.
When a FOBT or FIT was positive, 38 of the 39 clinics (97%) that supplied these tests to patients aged 50 years or older reported that they referred the patient for a colonoscopy. Of the 30 respondents who indicated how colonoscopies were paid for, 19 (63%) said insurance; 9 (30%) said the patient; 5 (17%) said charity; 4 (13%) said the state, such as the Iowa Get Screened or the Get Screened South Dakota program; 4 (13%) said Medicaid or Medicare; and 2 (7%) said the hospital, through a free care application. Fourteen (40%) stated that the main reason for a colonoscopy referral was having a positive FOBT, 13 (37%) for having symptoms, 10 (29%) for screening, and 5 (14%) for having risk factors. One respondent wrote that the way to get covered for a referral was “if insured or we can beg someone to do it.” Another respondent wrote that referral was for “any positive FOBT, any patient with insurance older than 50, or with risk factors.”
In the initial questionnaire, 18 respondents reported using FITs and were sent the follow-up FIT questionnaire. Respondents returned 16 questionnaires (89%); we excluded 1 from our analysis because the results indicated that the respondent did not understand the difference between guaiac tests and FITs and therefore provided invalid answers. Twelve of the 15 reported that they had used 1 of 5 FITs for FOBT: Hemoccult ICT (Beckman Coulter), Hemosure iFOB (Hemosure), QuickView iFOB (Quidel), Accutest (Jant), or OC-Light (Polymedco).
In response to the question about the number of samples collected from patients, 8 respondents reported 1, 1 said 2, and 3 collected 3. Nine respondents reported that they tested their FITs in-house, with a Clinical Laboratory Improvement Amendments waiver. Three others reported that the tests were not tested at their clinic and were tested at either a local hospital or the manufacturer’s headquarters.
Eight clinics had patients return the samples by mail, and 12 provided instructions about how soon to return the sample. Instructions varied: some told patients to return the test on the day of collection, others to return it immediately by mail or in person, or within 3 or 7 days.
Barriers and System Strategies
Respondents reported that the main barriers to providing CRC screening were inability to provide colonoscopy for patients with a positive FOBT or FIT (26 of all respondents, or 59%) and inability to provide colonoscopy for patients with colon symptoms (24, or 55%; Table 2). Other important barriers reported by 18 clinics (41%) were lack of time and no tracking system. Thirteen of the respondents (30%) wrote in their own additional barriers to CRC screening. Other barriers listed were no annual exam (i.e., patients only saw the doctor when they were sick), lack of supply of FOBTs, limited patient knowledge, lack of patient acceptance, lack of insurance, and inability of the EMR to track CRC screening.
TABLE 2—
System Strategies for and Barriers to Colorectal Cancer Screening in Federally Qualified Health Centers in 4 Midwestern States: 2012
| All States (n = 44) |
Iowa (n = 12) |
Minnesota (n = 12) |
South Dakota (n = 6) |
Wisconsin (n = 14) |
||||||
| Strategy/Barrier | No. (%) or % ±SD | Valid Responses, No. | No. (%) or % ±SD | Valid Responses, No. | No. (%) or % ±SD | Valid Responses, No. | No. (%) or % ±SD | Valid Responses, No. | No. (%) or % ±SD | Valid Responses, No. |
| Policy for CRC screening in place | 27* (61) | 44 | 5 (42) | 12 | 11 (92) | 12 | 4 (67) | 6 | 7 (50) | 14 |
| Written policy | 14 (52) | 27 | 2 (40) | 5 | 7 (64) | 11 | 3 (75) | 4 | 2 (29) | 7 |
| EMRs used | 34 (79) | 43 | 6 (50) | 12 | 11 (92) | 12 | 5 (100) | 5 | 12 (86) | 14 |
| EMR can query patients due for CRC screening | 23 (68) | 34 | 5 (83) | 6 | 8 (73) | 11 | 3 (60) | 5 | 7 (58) | 12 |
| EMR can query whether patients are up-to-date for CRC screening | 19 (61) | 31 | 5 (83) | 6 | 7 (70) | 10 | 2 (50) | 4 | 5 (45) | 11 |
| Items routinely checked during patient visits | ||||||||||
| CRC screening completed | 25 (61) | 41 | 8 (73) | 11 | 5 (45) | 11 | 4 (67) | 6 | 8 (62) | 13 |
| History of ulcerative colitis or Crohn’s disease | 10 (25) | 40 | 3 (30) | 10 | 3 (27) | 11 | 2 (33) | 6 | 2 (15) | 13 |
| Immediate family with CRC or polyps | 27 (66) | 41 | 9 (82) | 11 | 7 (64) | 11 | 5 (83) | 6 | 6 (46) | 13 |
| Distant relative with CRC or polyps | 18 (44) | 41 | 7 (64) | 11 | 4 (36) | 11 | 3 (50) | 6 | 4 (31) | 13 |
| Clinic personnel help to encourage screening | 26 (59) | 44 | 9 (75) | 12 | 7 (58) | 12 | 2 (33) | 6 | 8 (57) | 14 |
| CRC screening offered to patients aged ≥ 50 y | ||||||||||
| FOBT | 34 (77) | 44 | 11 (92) | 12 | 10 (83) | 12 | 5 (83) | 6 | 8 (57) | 14 |
| FIT | 18* (41) | 44 | 1 (8) | 12 | 5 (42) | 12 | 4 (67) | 6 | 8 (57) | 14 |
| Flexible sigmoidoscopy | 5 (11) | 44 | 2 (17) | 12 | 2 (17) | 12 | 0 (0) | 6 | 1 (7) | 14 |
| Colonoscopy | 28 (64) | 44 | 7 (58) | 12 | 9 (75) | 12 | 3 (50) | 6 | 9 (64) | 14 |
| FOBT or FIT provided to patients aged ≥ 50 y | 41 (93) | 44 | 9 (75) | 12 | 12 (100) | 12 | 6 (100) | 6 | 14 (100) | 14 |
| Test return tracked | 26 (63) | 41 | 6 (67) | 9 | 7 (58) | 12 | 5 (83) | 6 | 8 (57) | 14 |
| Patient called if test not returned | 12 (34) | 35 | 3 (38) | 8 | 5 (63) | 8 | 1 (17) | 6 | 3 (23) | 13 |
| Patient contacted by mail if test not returned | 9 (26) | 35 | 3 (38) | 8 | 3 (38) | 8 | 1 (17) | 6 | 2 (15) | 13 |
| Patient offered colonoscopy after positive test | 38 (97) | 39 | 9 (100) | 9 | 11 (100) | 11 | 5 (100) | 5 | 13 (93) | 14 |
| System in place to offer FOBT annually | 20 (54) | 37 | 3 (43) | 7 | 6 (55) | 11 | 3 (60) | 5 | 8 (57) | 14 |
| CRC screening offered to patients aged ≤ 50 y if risk factors present | 38 (86) | 44 | 10 (83) | 12 | 11 (92) | 12 | 5 (83) | 6 | 12 (86) | 14 |
| FOBT | 25* (66) | 38 | 9 (90) | 10 | 6 (55) | 11 | 5 (100) | 5 | 5 (42) | 12 |
| FIT | 14 (37) | 38 | 1 (10) | 10 | 4 (36) | 11 | 3 (60) | 5 | 6 (50) | 12 |
| Flexible sigmoidoscopy | 4 (11) | 38 | 2 (20) | 10 | 0 (0) | 11 | 1 (20) | 5 | 1 (8) | 12 |
| Colonoscopy | 26 (68) | 38 | 7 (70) | 10 | 7 (64) | 11 | 3 (60) | 5 | 9 (75) | 12 |
| Patients referred for colonoscopy | 43 (98) | 44 | 12 (100) | 12 | 12 (100) | 12 | 6 (100) | 6 | 13 (93) | 14 |
| Main barriers to providing CRC screening | ||||||||||
| Lack of time | 18 (41) | 44 | 7 (58) | 12 | 3 (25) | 12 | 2 (33) | 6 | 6 (43) | 14 |
| Lack of funding for FOBT | 7 (16) | 44 | 4 (33) | 12 | 0 (0) | 12 | 0 (0) | 6 | 3 (21) | 14 |
| Lack of time for personnel to explain test | 10 (23) | 44 | 5 (42) | 12 | 1 (8) | 12 | 0 (0) | 6 | 4 (29) | 14 |
| Language | 12* (27) | 44 | 6 (50) | 12 | 4 (33) | 12 | 2 (33) | 6 | 0 (0) | 14 |
| Lack of appropriate patient education material | 12 (27) | 44 | 4 (33) | 12 | 4 (33) | 12 | 1 (17) | 6 | 3 (21) | 14 |
| Inability to provide colonoscopy for patients with positive FOBT or FIT | 26 (59) | 44 | 9 (75) | 12 | 6 (50) | 12 | 2 (33) | 6 | 9 (64) | 14 |
| Inability to provide colonoscopy for patients with colon symptoms | 24 (55) | 44 | 7 (58) | 12 | 6 (50) | 12 | 2 (33) | 6 | 9 (64) | 14 |
| No CRC screening tracking system | 18 (41) | 44 | 7 (58) | 12 | 2 (17) | 12 | 2 (33) | 6 | 7 (50) | 14 |
| Patients with up-to-date CRC screening | 34.94 ±21.01 | 33 | 30.27 ±21.33 | 11 | 34.0 ±22.70 | 8 | 38.0 ±25.15 | 5 | 39.78 ±19.17 | 9 |
Note. CRC = colorectal cancer; EMR = electronic medical record; FIT = fecal immunochemical test; FOBT = fecal occult blood test. Denominator for all percentages is respective number of valid responses.
*P ≤ .05 (Fisher exact test).
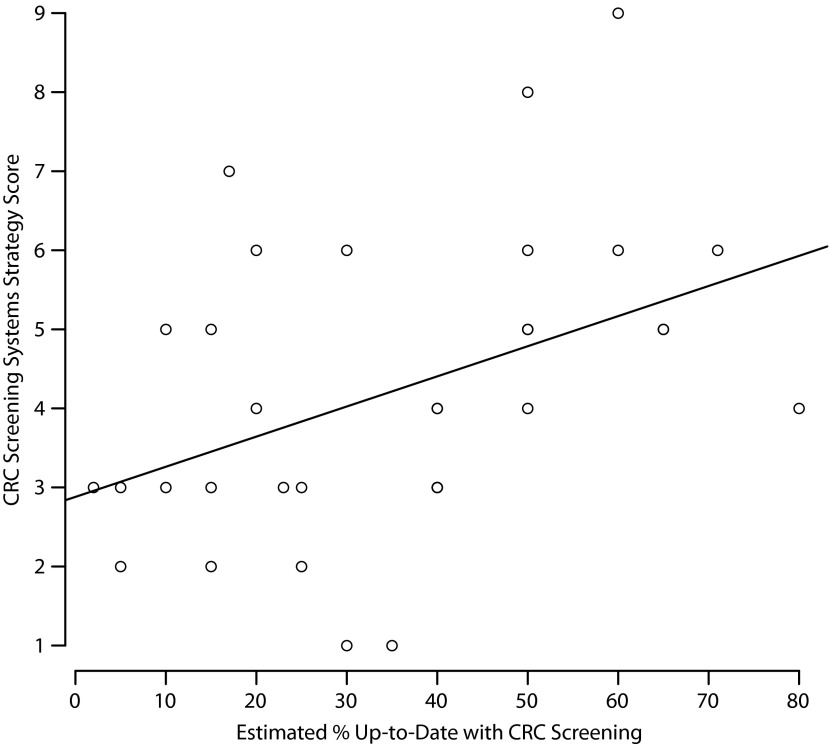
Clinics’ CRC screening system strategies scores ranged from 1 to 9, with a mean of 4.39 (SD = 2.07) and no significant difference by state (exact P = .49): Iowa had a mean of 3.75 (SD = 1.91); Minnesota, 5.17 (SD = 1.99); South Dakota, 4.67 (SD = 2.58); and Wisconsin, 4.14 (SD = 2.03). The Pearson correlation coefficient of system strategies and estimated percentage of patients up-to-date with CRC screening was 0.43 (P = .01); Figure 1 illustrates this relationship with a least squares line. We used Spearman correlation to verify results and provided a coefficient and P value similar to Pearson.
FIGURE 1—
Relationship of system strategies and estimated percentage of patients up-to-date with colorectal cancer screening at federally qualified health centers in 4 midwestern states: 2012.
Note. CRC = colorectal cancer.
Correlation coefficients for estimated percentage of patients up-to-date with CRC screening and other variables were 0.44 for reminder letters, 0.39 for routinely asking patients if they had completed a CRC screening, 0.31 for tracking return of tests, and 0.29 for clinic office staff's involvement in CRC screening. All other strategies had an absolute value lower than 0.20. The reminder letter and tracking FIT variables were highly correlated (r = 0.42).
Of the 35 comparisons across states, we found 4 statistically significant results: having a clinic policy for CRC screening (P = .04), offering FITs to patients aged 50 or older (P = .03), offering FOBTs to patients younger than 50 years with symptoms (P = .03), and language barriers as a difficulty with CRC screening (P = .01).
DISCUSSION
Estimates from the directors of the FQHCs surveyed suggested that only about 35% of their patients were up-to-date with CRC screening, substantially lower than figures for the nation as a whole. The Centers for Disease Control and Prevention reports that approximately 66% of adults aged 50 to 75 years are up-to-date with CRC screening.25 Data from the National Health Interview Survey indicate that 55% of adults in this age group report being up-to-date with screening.26 In a 2009 study comparing CRC screening in private physicians’ offices and in county health centers, a significantly higher number of patients in private physicians’ offices than in community health centers had recent CRC screening (70% vs 55%).21
Our results indicate that CRC screening is underused in FQHCs in the upper Midwest. We also found a positive association between an increasing number of system strategies for CRC screening and higher estimated CRC-screening rate. Strengths of our study were a very high response rate from the FQHCs and a complete sampling of FQHCs in the upper Midwest.
The positive correlation we observed between increasing number of system strategies and estimated percentage of patients up-to-date with CRC screening suggests that having a variety of system strategies is associated with higher screening rates.27,28 Some of the more frequently implemented system strategies in these FQHCs were office support staff encouraging CRC screening, having a clinic policy concerning CRC screening, and having a system in place to offer FOBT annually. More than one third of FQHCs did not use these strategies. The highest correlations with estimated percentage screened were using reminder letters, tracking the return of tests, involving clinic office staff in CRC screening, and routinely asking whether patients had completed CRC screening.
A previous study found that 38% of physicians in Iowa primary care offices reported that office staff helped facilitate CRC screening, but only 8% had a written policy regarding CRC screening.29 Future research could investigate system strategies in primary care clinic settings to determine whether increased CRC screening rates are similarly associated with increased system strategies. However, implementing multilevel interventions and sustaining them in routine practice is difficult.30
System strategies were not uniformly applied. In a 2007 assessment of primary care physicians’ use of system strategies for CRC screening, only 9% of 2475 respondents used both physician and patient reminders for CRC screening. Mailed and telephone reminders were most commonly used.31 We found that mailed patient education resulted in a significant increase in screening in a randomized clinical trial that used system strategies to increase screening across 16 family physicians' offices16,17,32; Sequist et al. had similar results with patient mailings to promote CRC screening.19
Although 97% of the FQHCs offered a colonoscopy after finding occult blood, 59% indicated that paying for the colonoscopy was a barrier. Other barriers were being unable to offer a colonoscopy for symptomatic patients, lack of time, and lack of a tracking system. Our findings about lack of time and lack of tracking are similar to results from another study.33
Limitations
We asked respondents to estimate the percentage of eligible patients who were up-to-date with CRC screening. Nineteen reported being able to query which patients were up-to-date with CRC screening, but we do not know whether they actually queried before answering the question. However, FQHCs administrators and medical directors generate reports to comply with guidelines for federal assistance, and reporting CRC screening is 1 of the report components. The raw data contained some specific answers, such as 17% and 71%, but for the most part respondents rounded their answer to a percentage ending in 5 or 10.
We sampled only FQHCs in the upper Midwest, so results may not be representative of the nation as a whole. Questionnaire responses were the perceptions of individuals about what was happening in their FQHC and thus were subject to the biases and inaccuracies potentially inherent in self-reported data.
We used a set of similar hypothesis tests trying to accomplish a similar goal; in this case, testing many questionnaire responses by state. At the 0.05 level (α = 0.05), out of 34 Fisher exact hypothesis tests, all with the null hypothesis of independence assumed to be true and tests assumed to be independent of each other, approximately 2 tests would be expected to incorrectly conclude dependence between state and question response, so the ability of these tests to detect true differences is questionable. In addition, we attempted to calculate odds ratios with 95% exact CIs for statistically significant results, but because of sparse data, we did not report these effect sizes and their CIs because they did not provide useful additional information. Therefore, the most valuable information provided by our questionnaire responses for comparisons by state is contained in the frequencies and percentages presented in Table 2.
Conclusions
Our results reaffirmed findings of previous studies that demonstrated that CRC-screening rates are persistently lower among low-income individuals and those with Medicaid or no health insurance.20,21,34 Only about 35% of FQHC patients were up-to-date with CRC screening, substantially fewer than in the nation as a whole. Although nearly all FQHCs reported attempting to obtain colonoscopy for patients with positive FOBTs, the high cost of colonoscopy made this difficult. Colonoscopies are out of the reach of Americans who do not have insurance or who are underinsured. The cost of colonoscopy alone, without anesthesia, varies widely in the United States, from $7471 in Austin, Texas, to $2116 in Nashville, Tennessee; the average US price is $1185.35
Implementing system strategies for preventive services is difficult and costly in any setting and especially in resource-poor FQHCs. Colorectal cancer is the second leading cause of cancer death and is preventable or curable if caught early, and we therefore need to provide funding for uninsured and underinsured patients to obtain a colonoscopy if they are found to have occult blood in their stool.
Acknowledgments
This study was funded by a grant from the National Institutes of Health (RC4 CA153493).
Human Participant Protection
The University of Iowa institutional review board approved this study. Informed consent was implied by return of a completed questionnaire.
References
- 1.2012 Program grantee comparison data. Health Resources and Services Administration, Primary Care: the Health Center Program. Available at: http://bphc.hrsa.gov/uds/datacenter.aspx?year=2012&state=IA&compare=Nat. Accessed October 22, 2013.
- 2.Forrest CB, Whelan EM. Primary care safety-net delivery sites in the United States: a comparison of community health centers, hospital outpatient departments, and physicians’ offices. JAMA. 2000;284(16):2077–2083. doi: 10.1001/jama.284.16.2077. [DOI] [PubMed] [Google Scholar]
- 3.Federally qualified health centers. Report to the Congress: Medicare and the health care delivery system. Medicare Payment Advisory Commission. Available at: http://www.medpac.gov/chapters/Jun11_Ch06.pdf. Published June, 2011. Accessed October 22, 2013.
- 4.Centers for Disease Control and Prevention. Screen for life: colorectal cancer, basic facts on screening. Publication #99-6949. Available at: http://www.cdc.gov/cancer/colorectal/pdf/Basics_FS_Eng_color.pdf. Accessed June 5, 2012.
- 5.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening among adults aged 50–75 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(26):808–812. [PubMed] [Google Scholar]
- 6.Colorectal cancer facts and figures 2011–2013. American Cancer Society. Available at: http://www.cancer.org/research/cancerfactsfigures/colorectalcancerfactsfigures/colorectal-cancer-facts-figures-2011-2013-page. Accessed March 24, 2014.
- 7.Winawer SJ, Zauber AG, Ho MN et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 9.Levin B, Lieberman DA, McFarland B et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59(1):27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 11.Wilschut JA, Habbema JD, van Leerdam ME et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011;103(23):1741–1751. doi: 10.1093/jnci/djr385. [DOI] [PubMed] [Google Scholar]
- 12.Winawer SJ, Krabshuis J, Lambert R, O’Brien M, Fried M. World Gastroenterology Organization Guidelines Committee. Cascade colorectal cancer screening guidelines: a global conceptual model. J Clin Gastroenterol. 2011;45(4):297–300. doi: 10.1097/MCG.0b013e3182098e07. [DOI] [PubMed] [Google Scholar]
- 13.Levy BT, Dawson J, Hartz AJ, James PA. Colorectal cancer testing among patients cared for by Iowa family physicians. Am J Prev Med. 2006;31(3):193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Brouse CH, Wolf RL, Basch CE. Facilitating factors for colorectal cancer screening. J Cancer Educ. 2008;23(1):26–31. doi: 10.1080/08858190701818283. [DOI] [PubMed] [Google Scholar]
- 15.Daly JM, Levy BT, Merchant ML, Wilbur J. Mailed fecal-immunochemical test for colon cancer screening. J Community Health. 2010;35(3):235–239. doi: 10.1007/s10900-010-9227-8. [DOI] [PubMed] [Google Scholar]
- 16.Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus education materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25(1):73–82. doi: 10.3122/jabfm.2012.01.110055. [DOI] [PubMed] [Google Scholar]
- 17.Levy BT, Xu Y, Daly JM, Ely JW. A randomized controlled trial to improve colon cancer screening in rural family medicine: an Iowa Research Network (IRENE) study. J Am Board Fam Med. 2013;26(5):486–497. doi: 10.3122/jabfm.2013.05.130041. [DOI] [PubMed] [Google Scholar]
- 18.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sequist TD, Franz C, Ayanian JZ. Cost-effectiveness of patient mailings to promote colorectal cancer screening. Med Care. 2010;48(6):553–557. doi: 10.1097/MLR.0b013e3181dbd8eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messina CR, Lane DS, Colson RC. Colorectal cancer screening among users of county health centers and users of private physician practices. Public Health Rep. 2009;124(4):568–578. doi: 10.1177/003335490912400414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988–2000. Cancer. 2006;107(5 suppl):1189–1195. doi: 10.1002/cncr.22016. [DOI] [PubMed] [Google Scholar]
- 23.Levy BT, Joshi M, Xu Y, Daly J, James PA. Perceptions of Iowa family physicians regarding colorectal cancer screening. Med Care. 2008;46(9 suppl 1):S103–S108. doi: 10.1097/MLR.0b013e31817c6100. [DOI] [PubMed] [Google Scholar]
- 24.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2007. [Google Scholar]
- 25.Colorectal cancer screening saves lives. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/Features/VitalSigns/CancerScreening. Accessed July 23, 2012.
- 26.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson N, Lynch C, West M, editors. Increasing Colorectal Cancer Screening in Iowa: Needs and Strategies for Improvement. Iowa City, IA: Iowa Department of Public Health and University of Iowa College of Public Health; 2006. [Google Scholar]
- 28.Scheid DC, Hamm RM, Ramakrishnan K, McCarthy LH, Mold JW Oklahoma Physicians Resource/Research Network. Improving colorectal cancer screening in family medicine: an Oklahoma Physicians Resource/Research Network (OKPRN) study. J Am Board Fam Med. 2013;26(5):498–507. doi: 10.3122/jabfm.2013.05.120230. [DOI] [PubMed] [Google Scholar]
- 29.Levy BT, Daly JM, Schmidt EJ, Xu Y. The need for office systems to improve colorectal cancer screening. J Prim Care Com Health. 2012;3(3):180–186. doi: 10.1177/2150131911423103. [DOI] [PubMed] [Google Scholar]
- 30.Yano EM, Green LW, Glanz K et al. Implementation and spread of interventions into the multilevel context of routine practice and policy: implications for the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):86–99. doi: 10.1093/jncimonographs/lgs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yabroff KR, Zapka J, Klabunde CN et al. Systems strategies to support cancer screening in U.S. primary care practice. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2471–2479. doi: 10.1158/1055-9965.EPI-11-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly JM, Xu Y, Ely JW, Levy BT. A randomized colorectal cancer screening intervention trial in the Iowa Research Network (IRENE): study recruitment methods and baseline results. J Am Board Fam Med. 2012;25(1):63–72. doi: 10.3122/jabfm.2012.01.110054. [DOI] [PubMed] [Google Scholar]
- 33.Nordin TA, Hartz AJ, Noyes R, Jr et al. Empirically identified goals for the management of unexplained symptoms. Fam Med. 2006;38(7):476–482. [PubMed] [Google Scholar]
- 34.Arnold CL, Rademaker A, Bailey SC et al. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun. 2012;17(suppl 3):252–264. doi: 10.1080/10810730.2012.713441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal E. The $2.7 trillion medical bill: colonoscopies explain why US leads the world in health expenditures. New York Times. June 2, 2013:A1. Available at: http://www.nytimes.com/2013/06/02/health/colonoscopies-explain-why-us-leads-the-world-in-health-expenditures.html?_r=0. Accessed September 1, 2013. [Google Scholar]