Abstract
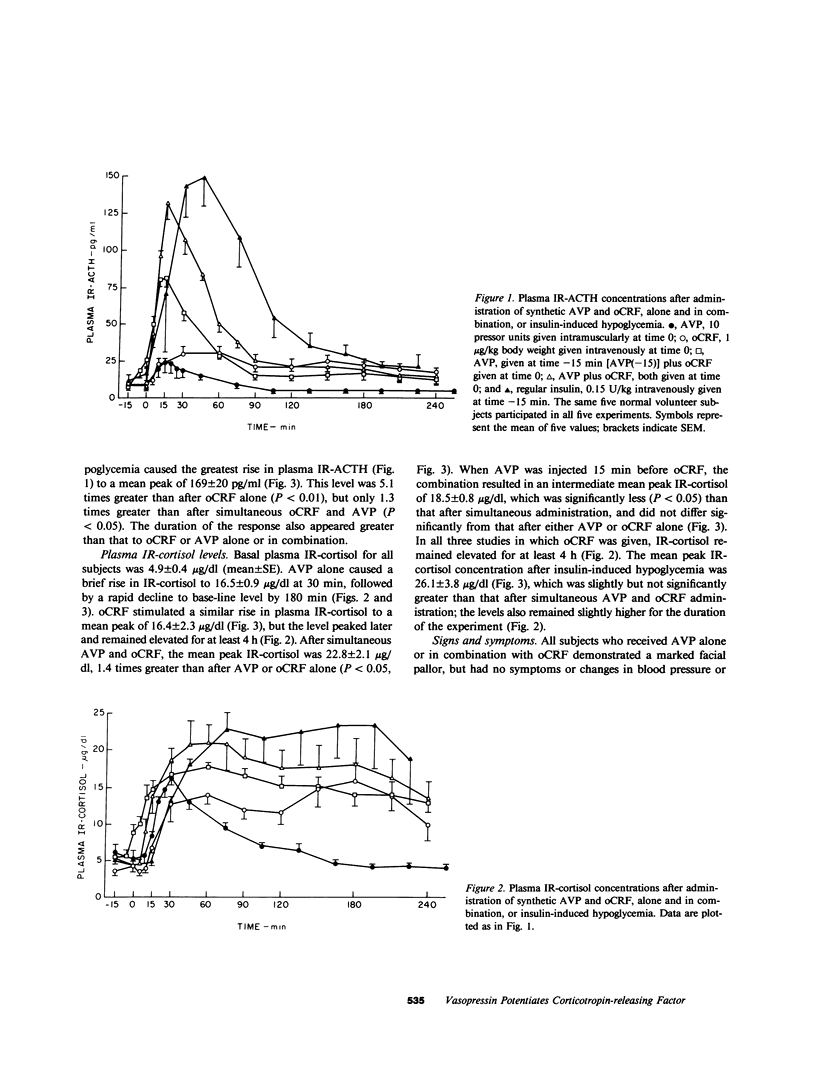
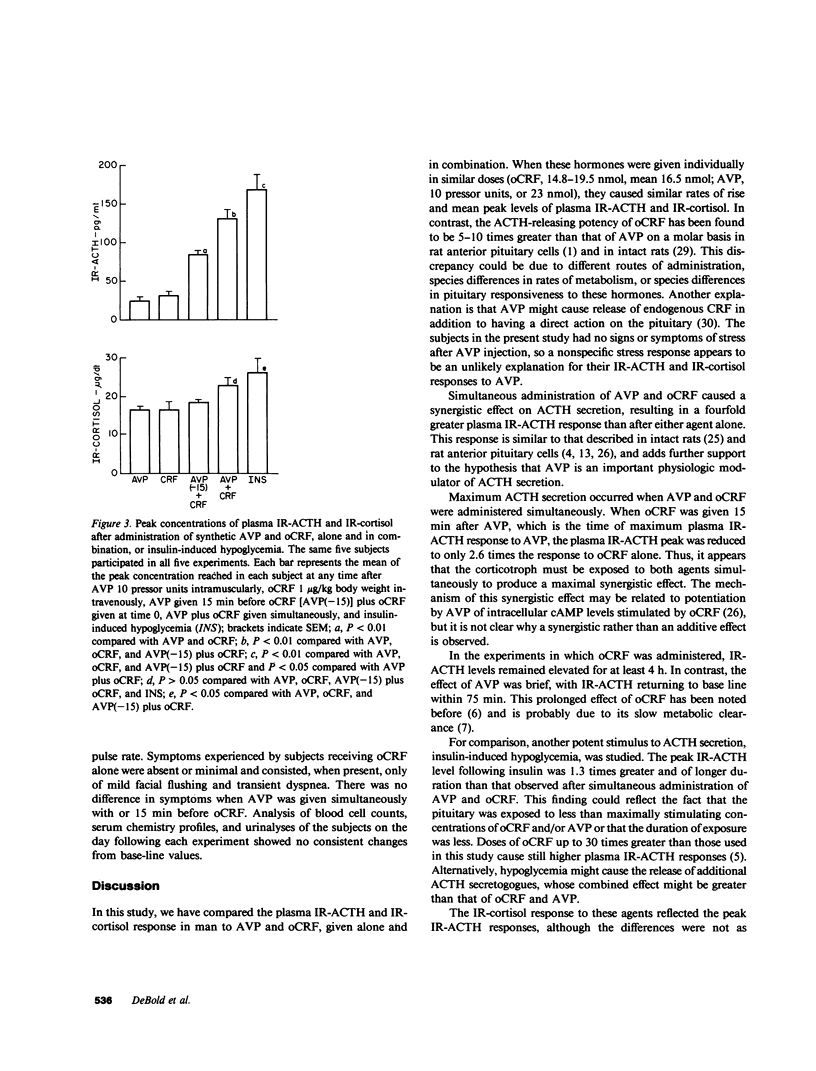
Arginine vasopressin (AVP) stimulates ACTH release in man and acts synergistically with synthetic ovine corticotropin-releasing factor (oCRF) in vitro. This study was designed to examine in man the combined effects of synthetic AVP (10 U intramuscularly) and oCRF (1 micrograms/kg intravenously) on ACTH release. Five normal male volunteers participated in five separate experiments: (a) AVP alone; (b) oCRF alone; (c) AVP followed by oCRF 15 min later; (d) simultaneous AVP and oCRF; and (e) insulin-induced hypoglycemia. Plasma immunoreactive ACTH (IR-ACTH) and IR-cortisol were measured for 4 h after injection of each hormone; basal levels for all subjects were less than or equal to 9 +/- 1.2 pg/ml and 4.9 +/- 0.4 micrograms/dl (mean +/- SE), respectively. AVP and oCRF, when given individually, caused rapid rises in IR-ACTH to similar peak levels of 25 +/- 6.6 and 33 +/- 4.6 pg/ml, respectively. AVP given 15 min before oCRF caused a 2.6-fold potentiation of the oCRF response, with a peak IR-ACTH of 85 +/- 4.6 pg/ml. AVP given at the same time as oCRF produced a fourfold potentiation of the peak IR-ACTH response to 132 +/- 11 pg/ml. These ACTH responses were far greater than those previously observed after 30-fold greater doses of oCRF alone. By way of comparison, insulin-induced hypoglycemia caused a peak IR-ACTH of 169 +/- 20 pg/ml. IR-ACTH returned to base line at 60-90 min after AVP alone, whereas the prolonged effect of oCRF was apparent whether it was given alone or in combination with AVP. The mean peak IR-cortisol responses to AVP, oCRF, and AVP given 15 min before oCRF were similar (16.5 +/- 0.9, 16.4 +/- 2.3, and 18.5 +/- 0.8 micrograms/dl, respectively), but the peak IR-cortisol responses to AVP and oCRF given simultaneously and to insulin-induced hypoglycemia were 1.5 and 1.7 times greater, respectively. IR-cortisol returned to base line within 2-3 h after AVP alone, but remained elevated for at least 4 h after oCRF alone or in combination with AVP. These results indicate that AVP acts synergistically with oCRF to release ACTH in man and suggest that AVP may play a physiologic role in modulating the ACTH response mediated by corticotropin-releasing factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham J. C. The influence of vasopressin on hypothalamic corticotrophin releasing activity in rats with inherited diabetes insipidus. J Physiol. 1981 Mar;312:9–16. doi: 10.1113/jphysiol.1981.sp013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte-Devolx B., Oliver C., Giraud P., Castanas E., Boudouresque F., Gillioz P., Millet Y. Adrenocorticotropin, and corticosterone secretion in Brattleboro rats. Endocrinology. 1982 Jun;110(6):2097–2100. doi: 10.1210/endo-110-6-2097. [DOI] [PubMed] [Google Scholar]
- DeBold C. R., DeCherney G. S., Jackson R. V., Sheldon W. R., Alexander A. N., Island D. P., Rivier J., Vale W., Orth D. N. Effect of synthetic ovine corticotropin-releasing factor: prolonged duration of action and biphasic response of plasma adrenocorticotropin and cortisol. J Clin Endocrinol Metab. 1983 Aug;57(2):294–298. doi: 10.1210/jcem-57-2-294. [DOI] [PubMed] [Google Scholar]
- Giguere V., Labrie F. Vasopressin potentiates cyclic AMP accumulation and ACTH release induced by corticotropin-releasing factor (CRF) in rat anterior pituitary cells in culture. Endocrinology. 1982 Nov;111(5):1752–1754. doi: 10.1210/endo-111-5-1752. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. J. Corticotrophin releasing activity in extracts of the stalk median eminence of Brattleboro rats. J Endocrinol. 1980 Jan;84(1):65–73. doi: 10.1677/joe.0.0840065. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. Corticotrophin releasing factor may be modulated vasopressin. Nature. 1979 Mar 29;278(5703):463–464. doi: 10.1038/278463a0. [DOI] [PubMed] [Google Scholar]
- Gillies G., Van Wimersma Greidanus T. B., Lowry P. J. Characterization of rat stalk median eminence vasopressin and its involvement in adrenocorticotropin release. Endocrinology. 1978 Aug;103(2):528–534. doi: 10.1210/endo-103-2-528. [DOI] [PubMed] [Google Scholar]
- Hedge G. A., Yates M. B., Marcus R., Yates F. E. Site of action of vasopressin in causing corticotropin release. Endocrinology. 1966 Aug;79(2):328–340. doi: 10.1210/endo-79-2-328. [DOI] [PubMed] [Google Scholar]
- MCCANN S. M. The ACTH-releasing activity of extracts of the posterior lobe of the pituitary in vivo. Endocrinology. 1957 May;60(5):664–676. doi: 10.1210/endo-60-5-664. [DOI] [PubMed] [Google Scholar]
- MCDONALD R. K., WEISE V. K. Effect of arginine-vasopressin and lysine-vasopressin on plasma 17-hydroxycorticosteroid levels in man. Proc Soc Exp Biol Med. 1956 Jul;92(3):481–483. doi: 10.3181/00379727-92-22518. [DOI] [PubMed] [Google Scholar]
- Nicholson W. E., DeCherney G. S., Jackson R. V., DeBold C. R., Uderman H., Alexander A. N., Rivier J., Vale W., Orth D. N. Plasma distribution, disappearance half-time, metabolic clearance rate, and degradation of synthetic ovine corticotropin-releasing factor in man. J Clin Endocrinol Metab. 1983 Dec;57(6):1263–1269. doi: 10.1210/jcem-57-6-1263. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Jackson R. V., DeCherney G. S., DeBold C. R., Alexander A. N., Island D. P., Rivier J., Rivier C., Spiess J., Vale W. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. J Clin Invest. 1983 Mar;71(3):587–595. doi: 10.1172/JCI110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlmutter A. F., Dokas L., Kong A., Miller R., Saffran M. Is corticotropin releasing factor modulated vasopressin? Nature. 1980 Feb 14;283(5748):697–698. doi: 10.1038/283697b0. [DOI] [PubMed] [Google Scholar]
- Pearlmutter A. F., Rapino E., Saffran M. A semi-automated in vitro assay for CRF: activities of peptides related to oxytocin and vasopressin. Neuroendocrinology. 1974;15(2):106–119. doi: 10.1159/000122299. [DOI] [PubMed] [Google Scholar]
- Portanova R., Sayers G. Isolated pituitary cells: CRF-like activity of neurohypophysial and related polypeptides. Proc Soc Exp Biol Med. 1973 Jul;143(3):661–666. doi: 10.3181/00379727-143-37386. [DOI] [PubMed] [Google Scholar]
- Rivier C., Brownstein M., Spiess J., Rivier J., Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982 Jan;110(1):272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W., Guillemin R. An in vivo corticotropin-releasing factor (CRF) assay based on plasma levels of radioimmunoassayable ACTH. Proc Soc Exp Biol Med. 1973 Mar;142(3):842–845. doi: 10.3181/00379727-142-37129. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Weber E., Barchas J. D. Immunoreactive corticotropin releasing factor (CRF) and vasopressin are colocalized in a subpopulation of the immunoreactive vasopressin cells in the paraventricular nucleus of the hypothalamus. Life Sci. 1982 Oct 18;31(16-17):1857–1860. doi: 10.1016/0024-3205(82)90228-4. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Morimoto Y., Furutani Y., Notake M., Takahashi H., Shimizu S., Horikawa S., Numa S. Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J. 1983;2(5):775–779. doi: 10.1002/j.1460-2075.1983.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Rivier C., Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Vale W. Sequence analysis of rat hypothalamic corticotropin-releasing factor with the o-phthalaldehyde strategy. Biochemistry. 1983 Aug 30;22(18):4341–4346. doi: 10.1021/bi00287a027. [DOI] [PubMed] [Google Scholar]
- Staub J. J., Jenkins J. S., Ratcliffe J. G., Landon J. Comparison of corticotrophin and corticosteroid response to lysine vasopressin, insulin, and pyrogen in man. Br Med J. 1973 Feb 3;1(5848):267–269. doi: 10.1136/bmj.1.5848.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., River C. Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed Proc. 1977 Jul;36(8):2094–2099. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Yasuda N., Greer M. A., Aizawa T. Corticotropin-releasing factor. Endocr Rev. 1982 Spring;3(2):123–140. doi: 10.1210/edrv-3-2-123. [DOI] [PubMed] [Google Scholar]
- Yasuda N., Greer M. A., Greer S. E., Panton P. Studies on the site of action of vasopressin in inducing adrenocorticotropin secretion. Endocrinology. 1978 Sep;103(3):906–911. doi: 10.1210/endo-103-3-906. [DOI] [PubMed] [Google Scholar]
- Yates F. E., Russell S. M., Dallman M. F., Hodge G. A., McCann S. M., Dhariwal A. P. Potentiation by vasopressin of corticotropin release induced by corticotropin-releasing factor. Endocrinology. 1971 Jan;88(1):3–15. doi: 10.1210/endo-88-1-3. [DOI] [PubMed] [Google Scholar]



