Abstract
Schizophrenia has been conceptualized as a disorder of both neurodevelopment and a disorder of connectivity. One important aspect of the neurodevelopmental hypothesis is that schizophrenia is no longer thought to have discrete illness time points, but rather a long trajectory of brain changes, spanning many years, across a series of stages of the disease including the prodrome, first episode, and chronic period. As the disease progresses, there is a complex relationship between age related changes and disease related changes. Therefore, neural changes, and specifically white matter based connectivity changes, in schizophrenia may be best conceptualized based on a lifespan trajectory. In this selective review, we discuss healthy changes in white matter integrity that occur with age, as well as changes that occur across illness stages. We further propose a set of models that might explain lifespan changes in white matter integrity in schizophrenia, with the conclusion that the evidence most strongly supports a pattern of disrupted maturation during adolescence, with the potential for later changes that may be a result of disease neurotoxicity, abnormal or excessive aging effects, as well as medication, cohort or other effects. Thus, when considering white matter integrity in psychosis, it is critical to consider age in addition to other contributing factors including disease specific effects. Discovery of the factors driving healthy white matter development across the lifespan and deviations from the normal developmental trajectory may provide insights relevant to the discovery of early treatment interventions.
Keywords: schizophrenia, development, prodrome, white matter, diffusion tensor imaging
1. Introduction
For many years there has been a conceptualization of schizophrenia as a disorder of disrupted connectivity (Bleuler, 1950; Friston and Frith, 1995; Weinberger et al., 1994). Schizophrenia is also a disorder with a known developmental component, based on its onset in late adolescence as well as the insidious pattern of gradual onset of symptoms. Interestingly, the late adolescent period associated with a heighted risk for disease onset is also associated with continuing development of the white matter (WM) which is the physical basis of the connections between brain regions (Karlsgodt et al., 2008a; Karlsgodt et al., 2012; Peters et al., 2014; Peters et al., 2012). With the advent of magnetic resonance imaging techniques, especially diffusion tensor imaging (DTI) (Basser and Pierpaoli, 1996; Beaulieu, 2002), in the last few decades, we have had a new ability to investigate the way that WM connections develop with age, as well as how these developmental changes might play a role in schizophrenia.
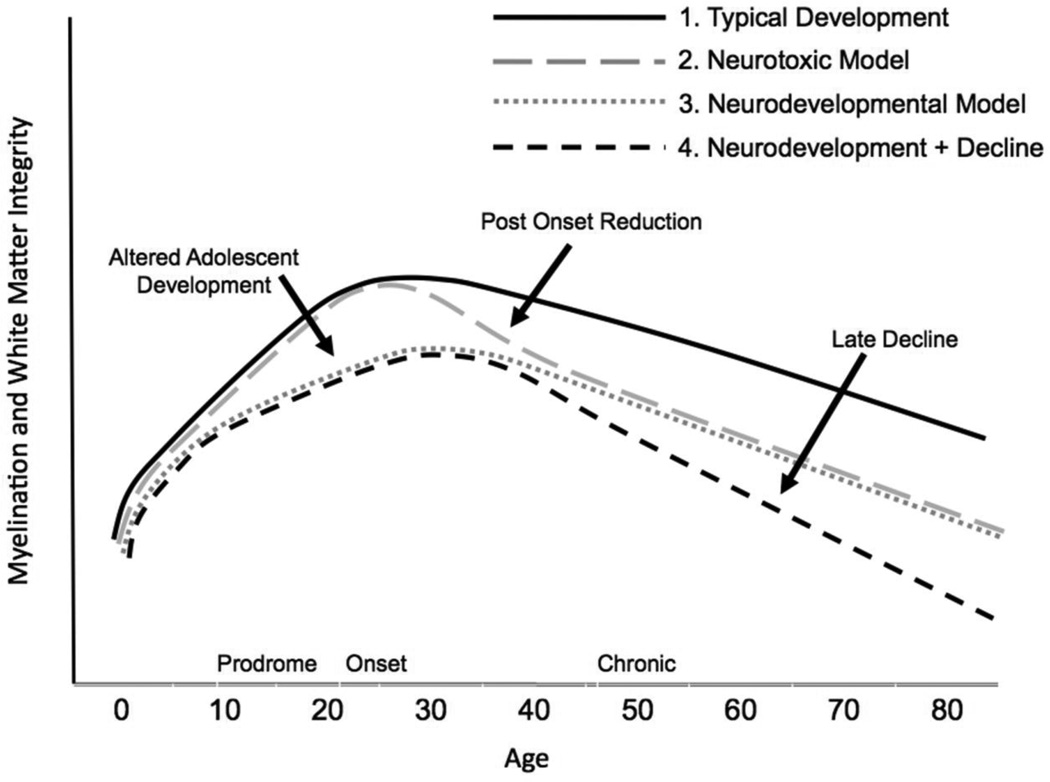
The goal of this selective review is to summarize the trajectory of WM microstructure changes not just with age but across the early stages of the illness. We first provide an introductory discussion of typical WM development, and the factors that may influence individual differences in neurodevelopmental trajectories, to establish what age related changes occur in healthy individuals. We will then break down the early phases of the illness into stages (genetic high risk, clinical high risk, and first episode), and while giving an overview of the literature on white matter during each stage, also focus on specific investigations that can elucidate the role of developmental changes. We will discuss the transition from the first episode to the chronic phase of the illness, by focusing on papers that directly compare first episode and chronic patients and/or assessed age effects across a broad age range. Finally, we will summarize this work by proposing a series of potential models for white matter development in schizophrenia, and suggesting which model currently appears to have the most supporting evidence (Figure 1). With this approach, we hope to provide an overview of the intersection of neurodevelopmental change, and progression of psychotic illnesses.
Figure 1.
Hypothesized models of developmental WM changes across the different stages of schizophrenia, compared to healthy WM changes across the lifespan. 1. Typical development: this line represents the predicted normal trajectory of white matter changes across the lifespan, with a peak in early adulthood (Peters et al., 2014; Peters et al., 2012). 2. Neurotoxic model: this line represents the trajectory according to a model in which the disease state is itself neurotoxic, resulting in white matter decline after onset. 3. Neurodevelopmental model: this model shows early alterations in the neurodevelopmental trajectory, resulting in lower white matter integrity that persists across adulthood, although the trajectory in adulthood follows a normal slope and curvature. 4. Neurodevelopmental model with later decline: this model includes both early differences in the developmental trajectory, as well as continuing decline after onset, which may be due to medication effects, disease toxicity, or an alteration in aging effects.
2. White matter development in adolescence
2.1 White matter changes across adolescence
On the microscopic level, normal WM development includes formation of axonal projections prenatally, elimination of axons postnatally (LaMantia and Rakic, 1990, 1994) and myelination from birth into adulthood. Elimination of axons starts after birth and is completed well before puberty, that is, about 3 months of age in the rhesus monkey (LaMantia and Rakic, 1990, 1994). Myelination in humans is completed for most tracts within the first year after birth, but continues during childhood, adolescence and adulthood and follows a region-specific course ((Benes et al., 1994; Paus, 1999; Sakuma et al., 1991; Yakovlev and Lecours, 1967) (Lenroot and Giedd, 2006)).In the anterior commissure, for example, increases in axon diameter and myelination appear to begin before the major phase of axon elimination and is completed long after the adult number of axons is reached (LaMantia and Rakic, 1994). An important study that examined human myelination across the life span by direct post-mortem myelin staining found that myelination of the sensory and motor roots was completed within the first few months postnatally, of the cingulum bundle within the first year postnatally, of the cerebellar peduncles, pyramidal tracts and striatum within the first few years after birth, of the great cerebral commissures and thalamic radiations before age 10, while myelination of association areas continued well into adulthood (Yakovlev and Lecours, 1967).
Brain imaging techniques have expanded our knowledge of human brain WM development through interrogation of the brain in vivo. Structural MRI (sMRI) across the lifespan has shown inverted U-curves of WM volume peaking at middle age, for example, frontal lobe WM volume at approximately age 44 years and temporal lobe WM volume at approximately age 47 years (Bartzokis et al., 2001). DTI has shown that fractional anisotropy (FA), a putative measure of neural fiber coherence, axon diameter and myelination (Basser and Pierpaoli, 1996; Beaulieu, 2002), displays age-related differences in a tractdependent manner. FA of the corticospinal tract appears to peak earliest in the early twenties, followed by the corpus callosum and then association tracts with the cingulum peaking last in the late twenties to early forties, depending on the statistical model used (Lebel et al., 2012; Peters et al., 2014). Transverse relaxation rate (R2), an MRI measure derived from the transverse relaxation time (T2) and more specific to myelin content than DTI, peaks in frontal WM at approximately age 32 (Bartzokis et al., 2012). Moreover, in this study, the DTI measure radial diffusivity was more closely related to R2 and known age-related patterns of myelination across the life span, compared to FA (Bartzokis et al., 2012), which is consistent with animal data (Song et al., 2002).
An early sMRI study showed increases in WM density from childhood into late adolescence that were most pronounced in the corticospinal tract and arcuate/superior longitudinal fasciculus (SLF) (Paus, 1999). DTI studies across adolescence found substantial increases in FA of several WM tracts, which were most consistent in the SLF (Lebel and Beaulieu, 2011; Lebel et al., 2008; Peters et al., 2012), which, in turn, is strikingly consistent with the sMRI study by Paus et al. (Paus, 1999). A DTI study that focused on sex differences in adolescence, found that females, compared to males, undergo earlier maturation of WM integrity (Asato et al., 2010). Magnetization transfer imaging, another MRI modality considered specific to myelin content, showed age-related decreases in the WM magnetization transfer ratio (MTR) in male adolescents, accompanied by increases in WM volumes (Perrin et al., 2009). In female adolescents, on the other hand, small age-related increase in WM volumes and no age-related differences in WM MTR were observed, with the exception of the frontal lobe where MTR increased ((Perrin et al., 2009).
The differences in age effects observed between MR modalities, such as the differences in peak ages between WM volume, WM MTR (Perrin et al., 2009) and WM FA (Bartzokis et al., 2001; Bartzokis et al., 2012; Lebel et al., 2012; Peters et al., 2014; Westlye et al., 2010), emphasize the critical issue of the underlying substrate of age-related WM differences observed with brain imaging. Myelination may not be the only driver of the observed age-related WM differences. For example, myelination of the cingulum bundle appears completed within the first year of life as observed with direct myelin staining (Yakovlev and Lecours, 1967), while this bundle is the last to reach peak FA values among major WM tracts (Lebel et al., 2012; Peters et al., 2014). In fact, myelination beyond the first decade of life appears to be restricted to intra-cortical myelination according to post-mortem myelin staining (Yakovlev and Lecours, 1967). Thus, it has been questioned whether the WM changes observed with sMRI and DTI may involve more increases in axon diameter than increases in myelination (Paus, 2010), or perhaps a combination of both (Baumann and Pham-Dinh, 2001). Perrin and colleagues (Perrin et al., 2009) have proposed, based on their MTR findings, that adolescent WM development may involve primarily age-related increases in axon diameter in males and increased myelination in females. However, Bartzokis et al. (Bartzokis et al., 2012) did observe increases in R2 and radial diffusivity from adolescence into the mid thirties, while they found no evidence for a sex effect, which suggests that protracted myelination occurs in both males and females into adulthood.
2.2 Genetic and molecular determinants of white matter development
Human and animal studies have provided important insights in the genetic and molecular pathways that shape WM development across adolescence. Asato et al. (Asato et al., 2010) found that WM maturation proceeded in parallel to the pubertal stages, suggesting hormonal influences on WM development. In accordance with this, MTR findings suggest that testosterone is an important driver of WM development in male adolescents and dependent on androgen receptor genotype (Perrin et al., 2008). In addition, lipid metabolism plays a critical role in myelination. Myelin consists of 70% lipids, including glycolipids, phospholipids and cholesterol, with phospholipids and cholesterol accounting for the largest proportion of membrane lipids in mammals (Baumann and Pham-Dinh, 2001; Sastry, 1985). Long-chain fatty acids are essential components of the phospholipid bilayers of all cell membranes including supporting oligodendrocytes, which form the myelin sheath of axons. Bourre et al. (Bourre et al., 1984) determined in rats that docosahexaenoic acid (DHA, an omega-3 long-chain polyunsaturated fatty acid [LC-PUFA]) constituted 5.8% of myelin and 5.1% of oligodendrocytes. Since the essential PUFAs must be acquired dietarily, the amount of intake is one of the factors that affects the rate of phospholipid synthesis, which in turn influences the quantity and quality of membrane phospholipids in the nervous system (Arvindakshan et al., 2003). If these lipids are unavailable during myelin synthesis, disruptions, which can take the form of amyelination or dysmyelination, may occur (Kobayashi et al., 1997). For instance, in rats with LC-PUFA deficiency, morphological myelin changes were found (Trapp and Bernsohn, 1978). In addition, genetic factors impact PUFA synthesis. We recently found that a human-specific haplotype of the faty acid desaturase genes, which is associated with decreased biosynthesis of omega-3 and omega-6 long-chain fatty acids, was also associated with decreased brain white matter development from childhood to adulthood (Peters et al., in press) The findings that fatty acid intake and biosynthesis may be a limiting factor for myelination has intriguing implications for disorders with disrupted WM development. In light of the importance of LC-PUFAs to myelination, it is of interest for our review, that a substantial body of literature has implicated both LC-PUFA deficiencies as well as compromised WM integrity in the pathophysiology of schizophrenia as indicated by studies in high-risk and first-episode patients (Amminger et al., 2011; Amminger et al., 2010; Hoen et al., 2013; Peters et al., 2010).
Genetic factors, in combination with environmental factors, influence many of these molecular drivers of brain WM maturation. Below (Section 3.1), we review the role of individual schizophrenia risk genes that have been studied thus far. This is a critical line of research, as studies that link specific genes to brain WM volume or microstructure through childhood and adolescence can produce further important clues to the molecular pathways influencing human brain WM development. By gaining a clearer understanding of the timing and nature of these molecular changes, we may be able to take advantage of this dynamic period and develop interventions for WM disorders that can capitalize on these ongoing changes. This approach would represent the first step towards the development of treatments specifically tailored to individuals in different neurodevelopmental stages.
2.3 White matter development and cognitive performance
WM changes across adolescence have been related to cognitive development in healthy individuals, which may be relevant to the early development of cognitive deficits in schizophrenia-like disorders (Bilder et al., 2006). For example, increases in FA of the SLF have been associated with increasing working memory performance and development of language functions across adolescence (Brauer et al., 2011; Peters et al., 2012). This same tract was correlated with working memory performance in individuals with recent-onset schizophrenia (Karlsgodt et al., 2008b), and working memory performance and FA of the SLF showed evidence of pleiotropy, or influence by shared genes (Karlsgodt et al., 2010). FA of the inferior fronto-occipital fasciculus was associated with global cognitive maturation by subserving maturation of processing speed from childhood to adulthood (Peters et al., 2014). Increases in FA of the cingulum bundle were associated with development of executive functioning and cognitive control in the transition from adolescence to adulthood (Fjell et al., 2012; Peters et al., 2014). These data suggest that WM development across adolescence is involved in the development of higher-order cognitive functions that are critical to adaptive human behavior, which may be key to the early disease process in schizophrenia (Bilder et al., 2000; Crow, 2000).
In summary, adolescence is a critical period for brain WM development, which may be characterized by increases in both myelin content and axon diameter. These increases appear most pronounced in the SLF during adolescence and in the cingulum bundle in the transition from adolescence to adulthood. These WM changes likely facilitate the development of higher-order cognitive functions, which are found compromised already in the early phase of schizophrenia-like disorders. Sex hormones and LC-PUFAs may play important roles in healthy WM development across adolescence and provide well-described targets for early intervention strategies, which have show promising results in the early stages of the disorder (Amminger et al., 2010; Berger et al., 2007). If LC-PUFA intervention does impact myelination in young individuals, then it may be a reasonable first line of intervention in subjects at high-risk for psychosis and a reasonable intervention in patients presenting with first-episode psychosis, especially in those individuals affected by dysregulated myelination.
3.White matter development in schizophrenia
Schizophrenia is a developmental disorder that emerges gradually across adolescence and early adulthood. The illness can be considered to be divided into a series of stages, which generally progress with age. McGorry has proposed a 5 stage model to characterize the onset of severe mental disorders (McGorry et al., 2006). Stage 0 consists of a non-symptomatic state in which there is increased risk for the disorder (for instance, first degree relatives before the typical age of onset). In Stage 1, mild symptoms begin to appear, usually in adolescents or young adults, however during this stage, the symptoms do not cross full diagnostic threshold; these individuals would be considered clinical high risk (CHR) and if they do progress to the full disorder this stage would be considered the prodromal phase of their illness. Stage 2 represents the first onset of a full psychotic episode and these individuals are often referred to as first episode patients. Stage 3 follows the first episode, and may include a pattern of remission and relapse. Stage 4 is the most severe, and is a persistent, debilitating illness that is associated with substantial loss of function and continued severe symptoms. There have been observations of WM changes across all five phases of schizophrenia; we will describe changes across the early stages here, in the context of the changes that are observed during healthy white matter development.
3.1 Stage 0: Genetic High-Risk
Investigations in genetic high risk individuals who carry risk genes for the disorder, but are not yet diagnosed with a psychotic disorder, can help us understand factors that contribute to disease onset. In addition, such studies also allow us to look at the effect of risk genes without the confounds of symptomatology or of medication effects. A number of studies have explored neural endophenotypes in individuals at genetic risk, including WM and connectivity changes. One approach to assessing individuals at risk is to recruit siblings, co-twins, or children of patients with schizophrenia. These individuals are thought to carry a higher than normal load of risk genes, but do not themselves have (symptoms of) the disorder. Such studies have largely shown decreases in FA and WM volume across a wide range of brain regions including the prefrontal cortex (Hao et al., 2009), the cingulate (Munoz Maniega et al., 2008), hippocampal regions (DeLisi et al., 2006; Hao et al., 2009), the parietal lobe (Gogtay et al., 2012; Hoptman et al., 2008), and uncinate fasciculus (Munoz Maniega et al., 2008). One study did find an increase in FA in relatives, which was in the arcuate fasciculus (Boos et al., 2013). Notably, given the above reviewed patterns of lifetime WM development, these decreases were observed whether the group of relatives was comprised of adolescents (DeLisi et al., 2006; Gogtay et al., 2012; Hao et al., 2009) or of primarily older subjects (Hoptman et al., 2008; Munoz Maniega et al., 2008). This indicates that risk genes confer white matter changes that are apparent by adolescence and persist into adulthood. Furthermore, looking at change across age in WM volume, Gogtay et al (Gogtay et al., 2012) found that siblings of childhood onset schizophrenia (COS) patients, showed a disrupted trajectory relative to controls, indicating that risk genes may also be implicated in alterations in normal developmental patterns.
The second approach to assessing genetic high risk subjects is to investigate individual genes thought to be associated with the disorder, either in healthy or affected individuals. One gene that has received much attention is neuregulin-1 (NRG1), which is involved in neuronal migration, axon guidance and myelination (Winterer et al., 2008), and its receptor ERBB4. Variability NRG1 has been associated with decreased FA in medial frontal regions (Winterer et al., 2008) and both NRG1 and ERBB4 have been associated with decreased FA in the anterior limb of the internal capsule (McIntosh et al., 2008; Zuliani et al., 2011). Another gene of interest, 22q11.2 (which includes the catechol-O-metyltransferase, or COMT gene), is associated with schizophrenia because 22q11.2 deletion syndrome (velo-cardio-facial syndrome or DiGeorge syndrome), is associated with high rates of schizophrenia (Murphy, 2002). WM investigations in 22q patients have revealed decreased FA in fronto-temporal (Ottet et al., 2013) and parietal (Kikinis et al., 2012) regions. Ottet et al studied adolescent patients, while Kikinis investigated patients in middle adulthood; the similarity of the direction of the finding even at different phases of development again provides evidence, like that in the family studies, that these genetically induced changes are observable at least to some extent, across different stages of the lifespan. To complement these findings, one study in healthy children aged 9 to 15 years found that the COMT gene, a major enzyme responsible for dopamine degradation in prefrontal brain, was significantly associated with frontal WM FA (Thomason et al., 2010), which suggests a role for dopamine in WM development. Further studies are needed to examine the effects of specific genes on human WM development across adolescence. In healthy adult individuals, WM FA has been associated with the schizophrenia risk genes microRNA-137 (Lett et al., 2013) and DISC1 (Sprooten et al., 2011), and schizophrenia-related oligodendrocyte gene variants (Voineskos et al., 2013). Finally, zinc finger protein 804A (ZNF804A) has been investigated as related to WM. Studies in healthy individuals have shown an effect of the risk allele on WM volume (Lencz et al., 2010) and WM microstructure as measured with FA in a study including both younger and older adults (Ikuta et al., 2014). However, other studies have had negative results (Sprooten et al., 2012; Voineskos et al., 2011) In an adult sample that included both healthy individuals and schizophrenia patients, there was an interaction such that patients with the T/T risk genotype showed greater FA deficits than controls, and than other patients, in the parietal, temporal, and cingulate cortices (Kuswanto et al., 2012). This finding is important, as it may indicate that the effects of risk genes are different in healthy individuals than in patients, where they are acting in concert with other risk factors.
3.2 Stage 1: Clinical High-Risk
Stage 1 of the illness is the prodromal phase, in which there is an insidious decline into full-blown schizophrenia over the course of a several years (Cornblatt et al., 2002; Cornblatt et al., 2003; Yung and McGorry, 1996). Individuals believed to be in this phase, those at clinical high risk (CHR), are typically identified based on a combination of factors, which can include either the presence of sub-threshold psychotic symptoms, brief psychotic symptoms (with spontaneous remission within one week), or a gradual decline in functioning together with a genetic risk (Woods et al., 2009). The strength of these studies lies in the potential to assess the same individual before, during, and after onset, which offers the opportunity to fully characterize the factors contributing to or associated with transition to psychosis. These studies have an advantage related to genetic high risk studies, in that the participants are more likely to advance to a disease state, as they are already exhibiting symptoms, although in turn, the presence of symptoms often necessitates medication, which is a confound that does not occur in genetic high risk studies. While they have the potential to be highly informative, CHR studies are methodologically challenging, as the symptoms exhibited during this phase of the illness are highly variable, and once identified, only a portion of recruited individuals will progress to a diagnosis of a psychotic disorder.
There are still relatively few DTI studies in CHR individuals. Some studies focused on baseline comparisons between CHR groups and typically developing control groups, while others track patients longitudinally in an attempt to capture conversion. Baseline comparisons have yielded findings of decreased FA in CHR subjects. A study of children aged 11–13 who were experiencing sub-threshold psychotic-like symptoms (Jacobson et al., 2010) found decreased FA in the high-risk group along the inferior fronto-occipital fasciculus within the visual cortex, along the parahippocampal cingulum and along the inferior longitudinal fasciculus. This study was the first to identify WM deficits in a non-treatment seeking sample of at-risk children and gives rise to the notion that these subtle microstructural differences in WM integrity may be apparent not only in the risk state but are identifiable at a younger age than previously suspected. Peters et al (Peters et al., 2008) found decreases in superior and middle frontal lobe regions in CHR patients with sub-threshold psychotic symptoms. Finally, Karlsgodt et al (Karlsgodt et al., 2009) found lower baseline WM FA in CHR subjects in the SLF compared to healthy controls, and Clemm von Hohenberg (Clemm von Hohenberg et al., 2013) recently showed increased MD, consistent with decreased WM integrity, in the SLF, corona radiata, and corpus callosum. However, interpretation of baseline CHR analysis is complicated by the fact that a significant portion of subjects will experience either a continuation of low level sub threshold symptoms or else a resolution of their symptoms (Woods et al., 2009). Therefore, a number of studies have focused on longer term assessments, either predicting clinical or functional outcome based on baseline data, or using longitudinal MRI paradigms to assess individuals at multiple time points. Lower baseline FA of medial temporal lobe WM was predictive of lower functional outcome at 16 months follow up (Karlsgodt et al., 2009). Additionally the CHR group failed to show the normal age-related increase in medial temporal lobe FA, consistent with the hypothesis of a disrupted developmental trajectory (Karlsgodt et al., 2009).In the first DTI study to examine conversion to psychosis Peters et al (Power et al., 2010) compared baseline FA along a priori selected WM tracts in CHR subjects who converted to psychosis and those who did not (Power et al., 2010). In this study they did not find any baseline differences between the CHR groups and the healthy subjects or between the converters and non-converters. Bloemen and colleagues (Bloemen et al., 2010) investigated whole-brain FA maps for high-risk individuals who later converted to psychosis compared to those who did not. They found reduced FA in the converters, compared to the controls, in medial frontal WM approximate to the left anterior thalamic radiation and along the inferior frontooccipital fasciculus. They also found reduced FA approximate to the SLF and inferior longitudinal fasciculus in the superior temporal WM and inferior fronto-occipital fasciculus in high-risk individuals who converted compared to those who did not. Finally, Carletti (Carletti et al., 2012) found baseline decreases relative to controls in a parietal region that likely included the SLF, the corpus callosum, and the inferior fronto-occipital fasciculus. While in this case baseline FA did not predict conversion, there was an interaction such that those who did not convert showed a normal developmental increase in FA in left frontal WM including the anterior limb of the internal capsule, corpus callosum, frontal occipital fasciculus, while converters did not show an increased and actually showed a decrease.
In summary, the use of DTI for the study of individuals at genetic and clinical risk for schizophrenia is still in a relatively early stage and thus interpretations of this complex literature are somewhat limited. Taken together, the existing data indicate that in both genetic and clinical risk groups there is evidence for decreased WM integrity that is observable not just in adulthood, but as early as adolescence. Family studies support the impact of genetics on WM, and the search for individual genes that may drive this effect have produced valuable associations with several schizophrenia risk genes. CHR studies have shown decreased FA at baseline in a broad group of at risk individuals, however the most intriguing possibility, the prediction of conversion psychosis, is an area that still has mixed findings and a limited number of studies. This may be due to the diagnostic heterogeneity of CHR samples, differences in the stage of the prodrome at which individuals are recruited, medication differences between samples and diagnostic groups, age differences in samples, and differences in the definition of the prodrome and the definition of conversion. The use of DTI for prediction is an area in need of increased investigation.
Interestingly, across genetic and clinical risk groups, there was evidence for an impact of risk factors on developmental trajectories (Addington et al., 2007; Carletti et al., 2012; Gogtay et al., 2012; Karlsgodt et al., 2009). This may indicate that it would be possible to get more traction on the issue of how clinical and genetic risk factors impact WM if there were increased attention to the interaction of these risk factors with not just group differences, but with developmental trajectories.
3.3 Stage 2: First Episode Schizophrenia
3.3.1 White matter findings in patients with a first psychotic episode
Finally, Stage 2 represents the first full blown psychotic episode, subjects assessed during approximately the first two years after the first break are often referred to as first episode or recent onset patients. The strength of studies in this population are that the patients have the full disorder, without any potential effects of chronic antipsychotic treatment (Keshavan et al., 1998). DTI studies have produced evidence for WM abnormalities in first-episode patients (Cheung et al., 2011; Douaud et al., 2009; Luck et al., 2011; Peters et al., 2010; Rathi et al., 2011; Wang et al., 2011) and chronic patients (Konrad and Winterer, 2008), though findings are less consistent in the first-episode group. A significant portion of studies have shown no differences between first-episode patients and healthy individuals (Kong et al., 2011; Mulert and Scarr, 2012; Peters et al., 2010), while whole brain voxel-based analyses tend to produce positive findings and fiber tracking or region-of-interest analyses more often indicate no abnormalities (Collinson et al., 2014; Kong et al., 2011; Mulert et al., 2012; Peters et al., 2010). One factor that might contribute to the conflicting results is variability in medication status and symptom history, even in those individuals relatively early in the illness. With increases in identification of early stage and prodromal individuals, there is also a subsequent increase in medication use, and not a great deal of data regarding how these medications may impact WM. Furthermore, medication use is often deeply confounded with disease severity, with those who have a more severe course often receiving higher doses of antipsychotics. Thus, WM differences in individuals receiving antipsychotics at higher dose and/or for a longer duration may be due either directly to the medication, or to an underlying neural difference related to their more severe course of disease. Therefore, studies in antipsychotic drug-naive first-episode patients are of particular interest, showing FA reductions not attributable to antipsychotic medication (Cheung et al., 2008; Cheung et al., 2011; Gasparotti et al., 2009; Mandl et al., 2013; Zou et al., 2008).
Data on the relationship between DTI measures and clinical variables are still limited, but it is interesting that positive correlations were found between FA and positive symptoms (Cheung et al., 2011; Mulert et al., 2012; Szeszko et al., 2008), which confirm findings in chronic patients (Hubl et al., 2004; Seok et al., 2007; Shergill et al., 2007). In addition, negative symptoms have been associated with FA of WM approximate to the uncinate fasciculus (Szeszko et al., 2008). Cognitive deficits have also been investigated in patients as related to FA. Verbal learning/memory was correlated with FA approximate to the uncinate (Szeszko et al, 2008), working memory correlated with FA of the SLF (Karlsgodt et al., 2008), IQ with fronto-parietal WM FA (Wang et al., 2013), and psychomotor and executive dysfunctioning with several WM regions including the cortico-cortical, cortico-subcortical and cortico-spinal/pontine fasciculi (Perez-Iglesias et al., 2010). Few DTI studies have provided insight in the pathophysiology of these WM abnormalities (other than genetic factors). A novel application of MR-DTI (‘free-water imaging’) showed increases in extracellular volume in first-episode patients, which may indicate neuroinflammation as a prominent process affecting WM integrity (Pasternak et al., 2012). LC-PUFA concentrations in erythrocyte membranes have shown strong positive correlations with WM FA in first-episode psychosis patients (Peters et al., 2009; Peters et al., 2013), which may suggest that LC-PUFAs deficiencies (Hoen et al., 2013) are causally related to WM deficits observed at illness onset.
3.3.2 Progressive development of white matter abnormalities after onset of the first psychotic episode
Developmental change in WM across illness stages is complex and interpretation of existing data can be difficult due to the challenges of disentangling normal developmental patterns, disease specific patterns, individual differences in symptom severity and effects of medication or chronicity. Here we propose three feasible scenarios for the timing and progression of DTI abnormalities (see Figure 1). First, it has been hypothesized that FA abnormalities after the onset of schizophrenia may result from illness-related, possibly neurotoxic effects that take place around onset of the first psychotic symptoms. In this model, early development is intact, with the WM changes occurring at onset, and continuing as the disease progresses. Secondly, FA abnormalities may represent an aberrant developmental trajectory, in which patients fail to show the normal WM increases that occur in healthy individuals during adolescence, resulting in lower WM integrity and volume that persist, but do not progress, beyond onset. Finally, the possibility of an early developmental difference does not preclude the presence of later changes due to either abnormal or accelerated aging trajectories, disease toxicity, or treatment effects. There may be a combination of early developmental changes with later life changes.
Structural MRI and DTI in adolescent-onset patients and adult-onset patients showed greater severity of WM abnormalities in the adolescent patients, which seemed to arise because adolescent patients showed delayed and altered maturation of WM compared to healthy controls (Douaud et al., 2009). Interestingly, some abnormalities specific to adolescent-onset schizophrenia were less marked or even disappeared during the longitudinal follow-up (>2 years) (Douaud et al., 2009). Similarly, sMRI in childhood–onset schizophrenia (COS) patients and healthy children showed slower local WM growth rates over a 5-year period in COS patients than healthy controls (Gogtay et al., 2012). Siblings of these COS patients aged 7 to 14 years also displayed slower WM growth rates than age-matched healthy controls in the left parietal WM, which was not detectable at older ages, implying that early WM growth deficits may normalize with age in individuals with genetic risk who do not develop the illness. It cannot yet be known whether this difference is because the unaffected and affected siblings did not share genes related to disrupted WM development during later adolescence, or whether disease related effects resulted in continued developmental changes in COS patients and not their unaffected siblings. Furthermore, genetic analyses in COS patients have shown that carriers of a neuregulin-1 risk allele had a steeper rate of decline in WM volume during adolescence, compared to patients without the risk allele (Addington et al., 2007). Studies comparing adolescent and chronic patients identified effects of age of onset, and suggest that prefrontal FA deficits develop when illness onset occurs at a later age (Kyriakopoulos et al., 2009; Schneiderman et al., 2009).
Only a few studies have directly compared first episode patients with multi-episode/ chronic patients. In these cross-sectional studies, it was found that WM deficits in first-episode patients were either absent or less severe than in the multi-episode patients (Collinson et al., 2014; Friedman et al., 2008; Kong et al., 2011). Several studies have found greater FA decline with increasing age in schizophrenia patients, compared to healthy controls, from young-adulthood to late adulthood (reviewed by Peters et al. (Peters et al., 2010)), which would be consistent with the model that includes decline after illness onset (i.e. the first psychotic episode), described above. In addition, Mitelman et al. (Mitelman et al., 2009) found that poor-outcome patients had lower baseline anisotropy but tended to show less decline than good-outcome patients, suggesting that the most significant FA decreases in poor-outcome patients occur in an earlier stage of the illness. Young-adult first-episode patients with poor response to treatment at 3 to 6-month follow-up had lower FA, compared to patients with a good response and healthy controls (Luck et al., 2011; Reis Marques et al., 2014). Lastly, duration of illness was not related to FA in two samples of adult patients with recent-onset of illness (Kanaan et al., 2009a; Kanaan et al., 2009b; Mandl et al., 2008), but a meta-analysis of first episode and multi-episode studies showed that longer duration of illness was associated with more severe WM abnormalities (Bora et al., 2011). However, these crosssectional studies are hindered by possible cohort effects, that is, differences between first-episode and multi-episode patients may not reflect disease progression but rather selection bias of poor prognosis patients, especially since multi-episode patients in these studies included patients from institutional long-term care settings.
Recent longitudinal studies in first-episode patients have provided valuable data on progressive WM changes over at least the short-term, early course of the illness. Longitudinal analysis in first-episode patients after 12 weeks showed an increase in FA in both responders and non-responders, which correlated positively with antipsychotic exposure (Reis Marques et al., 2014). A limitation of this study is that treatment was not standardized and likely comprised a mix of different antipsychotics, making it difficult to hypothesize on the specific mechanism that may account for the observed effects. However, this may also point to a reason why different first episode samples may show different degrees of WM deficits. In a double-blind, randomized trial comparing risperidone to aripiprazole, first-episode patients showed significant FA decreases over 12 week follow-up, which concurs with another 6 week follow-up study (Wang et al., 2013). Moreover, this decrease correlated with increases in serum LDL and cholesterol (Peters et al., 2014), which suggests that metabolic side-effects may compromise WM integrity. In accordance with this, a large cohort of first-episode patients showed progressive decrement in white matter volume during follow-up of on average 7.2 years, which was most evident among patients who received more antipsychotic treatment (Ho et al., 2011).
In summary, these findings in first-episode patients support an overall pattern of disrupted WM development especially if the illness onset occurs in childhood or adolescence, and more severe and extensive abnormalities in chronic patients than in first-episode patients, especially in those patients with a poorer prognosis. While we do not yet know the cause of these later changes, they may be related to abnormal or excessive aging effects, illness-related neurotoxic effects, or antipsychotic medication, but possibly also cohort effects. Thus, at this time, the evidence is consistent with the “neurodevelopment with later decline” model, described above.
4. Conclusions
Adolescence is a critical period for development of brain WM, particularly of the long association tracts which are known to be disrupted in complex psychiatric disorders such as schizophrenia. Furthermore, these WM tracts support development of complex cognitive functions, which are compromised even in the early stages of psychosis. A number of factors may impact the trajectory of healthy WM development, including genetic factors, sex hormones and LC-PUFA levels. Importantly, our growing understanding of the role of these factors in healthy development may ultimately lead to new targets for early intervention strategies. One key area of focus is on understanding how such interventions might be used in prevention of the illness. One very intriguing possibility is the eventual use of DTI measures to predict the conversion to psychosis, however, the DTI literature in individuals at genetic or clinical risk for schizophrenia is in a relatively early stage. Nevertheless, the existing evidence does support the hypothesis that in both clinical and genetic risk groups WM integrity is decreased even as early as adolescence. Moreover, in both risk groups, there is also evidence for an alteration in developmental trajectories, highlighting the possibility that interventions that can be targeted at specific developmental phases might be particularly powerful. In individuals who have experienced full onset of the disorder but are in the early stages (first-episode patients), the findings support an overall pattern of disrupted WM development which is particularly pronounced if the illness onset occurs in childhood or adolescence. Investigations using never-medicated first-episode patients have made a particularly strong contribution to our understanding of the changes that occur during this phase of the illness. Chronic patients do appear to exhibit more severe and extensive abnormalities in than first-episode patients, especially in those patients with a poorer prognosis, which may involve excessive aging effects, as well as neurotoxic effects, however these effects are very difficult to disentangle from medication or cohort effects.
We have proposed here a series of models that may describe the lifetime developmental trajectory of WM change in individuals with schizophrenia (Figure 1). These include (1) a pattern of normal early development with WM deficits arising only after disease onset presumably due to some neurotoxic effect of psychosis, (2) a pattern of disrupted development specific to adolescence with a stable trajectory in subsequent periods, and (3) a pattern of early developmental change with the addition of later decline either due to an accelerated aging process (a disruption in the late development trajectory) or due to disease chronicity and medication effects. Overall, the current evidence seems most in support of the final model, combining early and late differences. The early component of the model is supported by data showing disrupted trajectories in risk groups with the later component supported by data showing that chronic patients may have more severe WM deficits than early episode patients. This model by definition represents an average group of patients, and there are very likely to be substantial individual differences depending on genetic background, environmental factors, illness severity, and other factors, all of which should be further investigated.
In general, the investigation of disease related neural changes against the background of ongoing neurodevelopment, is a relatively new approach to understanding neural differences in patient populations. This approach may yield valuable insights, but it is in great need of further research. One potential area of research is in developmentally targeted treatments. For instance, animal and human data suggest that LC-PUFAs may play an important role in WM development. However, the results of treatment trials administrating PUFAs have been inconclusive and it is not clear what other underlying factors might affect their efficacy. For instance, it is possible they are only effective during developmental periods when myelination is occurring, which targeted studies are needed to investigate further. Relatedly, large-scale human imaging data are urgently needed to examine the genetic-molecular underpinnings of WM trajectories across adolescence. This area of work is important in part because differences in gene expression, and the downstream effects of those genes across the lifetime may have an important impact on not only imaging phenotypes but also potential treatment targets that may exist during specific developmental windows. The prediction of conversion to psychosis in individuals at high risk is an area that still has mixed findings and limited data. Increased focus should be given to the interaction of clinical and genetic-molecular risk factors with developmental WM trajectories in high risk individuals. Long-term longitudinal studies are needed to provide further insight in the WM trajectories and their determinants across the different stages of the illness, with specific attention for the effects of antipsychotic medication and prognosis. In general, intervention strategies aiming to halt or even reverse progression of WM abnormalities across all illness stages are ultimately needed. However, first we must understand the difference between the molecular and genetic bases of changes occurring in the early and late stages, as this will allow the development of new, developmentally appropriate, age-targeted intervention strategies.
Acknowledgements
We would like to acknowledge the support of the Research Division at Zucker Hillside Hospital and The Feinstein Institute for Medical Research.
Funding Body Agreements and Policies
This manuscript was supported in part by MH101506 (KHK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Peters and Karlsgodt both completed the literature review, wrote the manuscript, and have approved the final version of the manuscript.
Conflict of Interest
Dr. Peters has received compensation from ProPhase. Dr Karlsgodt does not report any conflict of interest.
References
- Addington AM, Gornick MC, Shaw P, Seal J, Gogtay N, Greenstein D, Clasen L, Coffey M, Gochman P, Long R, Rapoport JL. Neuregulin 1 (8p12) and childhood-onset schizophrenia: susceptibility haplotypes for diagnosis and brain developmental trajectories. Molecular psychiatry. 2007;12(2):195–205. doi: 10.1038/sj.mp.4001906. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez-Jimenez M, Herrman H, Jackson HJ, McGorry PD. Outcome in early-onset schizophrenia revisited: findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophrenia research. 2011;131(1–3):112–119. doi: 10.1016/j.schres.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of general psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophrenia research. 2003;62(3):195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cerebral cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Archives of general psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Heydari P, Couvrette A, Lee GJ, Kalashyan G, Freeman F, Grinstead JW, Villablanca P, Finn JP, Mintz J, Alger JR, Altshuler LL. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biological psychiatry. 2012;72(12):1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of general psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. The Journal of clinical psychiatry. 2007;68(12):1867–1875. doi: 10.4088/jcp.v68n1206. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. The American journal of psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JM. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28(2):270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or The Group of Schizophrenias. New York: International Universities Press; 1950. [Google Scholar]
- Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, Dingemans P, Linszen DH, van Amelsvoort TA. White-matter markers for psychosis in a prospective ultrahigh- risk cohort. Psychological medicine. 2010;40(8):1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- Boos HB, Mandl RC, van Haren NE, Cahn W, van Baal GC, Kahn RS, Hulshoff Pol HE. Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23(4):295–304. doi: 10.1016/j.euroneuro.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise metaanalysis and meta-regression analysis. Schizophrenia research. 2011;127(1–3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Pascal G, Durand G, Masson M, Dumont O, Piciotti M. Alterations in the fatty acid composition of rat brain cells (neurons, astrocytes, and oligodendrocytes) and of subcellular fractions (myelin and synaptosomes) induced by a diet devoid of n-3 fatty acids. J Neurochem. 1984;43(2):342–348. doi: 10.1111/j.1471-4159.1984.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Fusar Poli P, Valmaggia L, Broome MR, Bramon E, Johns L, Giampietro V, Williams SC, Barker GJ, McGuire PK. Alterations in white matter evident before the onset of psychosis. Schizophrenia bulletin. 2012;38(6):1170–1179. doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological medicine. 2008;38(6):877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Cheung V, Chiu CP, Law CW, Cheung C, Hui CL, Chan KK, Sham PC, Deng MY, Tai KS, Khong PL, McAlonan GM, Chua SE, Chen E. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychological medicine. 2011;41(8):1709–1719. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- Clemm von Hohenberg C, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton ME. White Matter Microstructure in Individuals at Clinical High Risk of Psychosis: A Whole-Brain Diffusion Tensor Imaging Study. Schizophrenia bulletin. 2013 doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson SL, Gan SC, Woon PS, Kuswanto C, Sum MY, Yang GL, Lui JM, Sitoh YY, Nowinski WL, Sim K. Corpus callosum morphology in first-episode and chronic schizophrenia: combined magnetic resonance and diffusion tensor imaging study of Chinese Singaporean patients. The British journal of psychiatry : the journal of mental science. 2014;204:55–60. doi: 10.1192/bjp.bp.113.127886. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Lencz T, Obuchowski M. The schizophrenia prodrome: treatment and high-risk perspectives. Schizophrenia research. 2002;54(1–2):177–186. doi: 10.1016/s0920-9964(01)00365-6. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophrenia bulletin. 2003;29(4):633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain research. Brain research reviews. 2000;31(2–3):118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, Branch CA, Ardekani BA. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry research. 2006;148(1):61–66. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, Connell J, Roberts N, Crow TJ, Matthews PM, Smith S, James A. Schizophrenia delays and alters maturation of the brain in adolescence. Brain : a journal of neurology. 2009;132(Pt 9):2437–2448. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, Venkatraman V, Roddey JC, Erhart M, McCabe C, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Darst BF, Schork NJ, Casey BJ, Chang L, Ernst TM, Gruen JR, Kaufmann WE, Kenet T, Frazier J, Murray SS, Sowell ER, van Zijl P, Mostofsky S, Jernigan TL, Dale AM, Pediatric Imaging N, Genetics S. Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. The American journal of psychiatry. 2008;165(8):1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B, Sacchetti E. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophrenia research. 2009;108(1–3):41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Hua X, Stidd R, Boyle CP, Lee S, Weisinger B, Chavez A, Giedd JN, Clasen L, Toga AW, Rapoport JL, Thompson PM. Delayed white matter growth trajectory in young nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of general psychiatry. 2012;69(9):875–884. doi: 10.1001/archgenpsychiatry.2011.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Yan Q, Liu H, Xu L, Xue Z, Song X, Kaneko Y, Jiang T, Liu Z, Shan B. Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophrenia research. 2009;114(1–3):128–135. doi: 10.1016/j.schres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of general psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry research. 2013;207(1–2):1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophrenia research. 2008;106(2–3):115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of general psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Peters BD, Guha S, John M, Karlsgodt KH, Lencz T, Szeszko PR, Malhotra AK. A schizophrenia risk gene, ZNF804A, is associated with brain white matter microstructure. Schizophrenia research. 2014;155(1–3):15–20. doi: 10.1016/j.schres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M, Connolly C, O'Hanlon E, Garavan H, Cannon M. Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. NeuroImage. 2010;49(2):1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P. White matter microstructure in schizophrenia: effects of disorder, duration and medication. The British journal of psychiatry : the journal of mental science. 2009a;194(3):236–242. doi: 10.1192/bjp.bp.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Borgwardt S, McGuire PK, Craig MC, Murphy DG, Picchioni M, Shergill SS, Jones DK, Catani M. Microstructural organization of cerebellar tracts in schizophrenia. Biological psychiatry. 2009b;66(11):1067–1069. doi: 10.1016/j.biopsych.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt K, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and psychopathology. 2008a;20(4):1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K, van Erp TG, Poldrack R, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological psychiatry. 2008b;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Jacobson SC, Seal M, Fusar-Poli P. The relationship of developmental changes in white matter to the onset of psychosis. Current pharmaceutical design. 2012;18(4):422–433. doi: 10.2174/138161212799316073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Kochunov P, Winkler AM, Laird AR, Almasy L, Duggirala R, Olvera RL, Fox PT, Blangero J, Glahn DC. A multimodal assessment of the genetic control over working memory. Journal of Neuroscience. 2010;30(24):8197–8202. doi: 10.1523/JNEUROSCI.0359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Schooler NR, Sweeney JA, Haas GL, Pettegrew JW. Research and treatment strategies in first-episode psychoses. The Pittsburgh experience. The British journal of psychiatry. Supplement. 1998;172(33):60–65. [PubMed] [Google Scholar]
- Kikinis Z, Asami T, Bouix S, Finn CT, Ballinger T, Tworog-Dube E, Kucherlapati R, Kikinis R, Shenton ME, Kubicki M. Reduced fractional anisotropy and axial diffusivity in white matter in 22q11.2 deletion syndrome: a pilot study. Schizophrenia research. 2012;141(1):35–39. doi: 10.1016/j.schres.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Shinnoh N, Kondo A, Yamada T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochemical and Biophysical Research Communications. 1997;232(3):631–636. doi: 10.1006/bbrc.1997.6340. [DOI] [PubMed] [Google Scholar]
- Kong X, Ouyang X, Tao H, Liu H, Li L, Zhao J, Xue Z, Wang F, Jiang S, Shan B, Liu Z. Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. Journal of psychiatry & neuroscience : JPN. 2011;36(2):120–125. doi: 10.1503/jpn.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophrenia bulletin. 2008;34(1):72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto CN, Woon PS, Zheng XB, Qiu A, Sitoh YY, Chan YH, Liu J, Williams H, Ong WY, Sim K. Genome-wide supported psychosis risk variant in ZNF804A gene and impact on cortico-limbic WM integrity in schizophrenia. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2012;159B(3):255–262. doi: 10.1002/ajmg.b.32032. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Perez-Iglesias R, Woolley JB, Kanaan RA, Vyas NS, Barker GJ, Frangou S, McGuire PK. Effect of age at onset of schizophrenia on white matter abnormalities. British Journal of Psychiatry. 2009;195(4):346–353. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10(7):2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. J Comp Neurol. 1994;340(3):328–336. doi: 10.1002/cne.903400304. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lencz T, Szeszko PR, DeRosse P, Burdick KE, Bromet EJ, Bilder RM, Malhotra AK. A schizophrenia risk gene, ZNF804A, influences neuroanatomical and neurocognitive phenotypes. Neuropsychopharmacology. 2010;35(11):2284–2291. doi: 10.1038/npp.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and biobehavioral reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lett TA, Chakavarty MM, Felsky D, Brandl EJ, Tiwari AK, Goncalves VF, Rajji TK, Daskalakis ZJ, Meltzer HY, Lieberman JA, Lerch JP, Mulsant BH, Kennedy JL, Voineskos AN. The genome-wide supported microRNA-137 variant predicts phenotypic heterogeneity within schizophrenia. Molecular psychiatry. 2013;18(4):443–450. doi: 10.1038/mp.2013.17. [DOI] [PubMed] [Google Scholar]
- Luck D, Buchy L, Czechowska Y, Bodnar M, Pike GB, Campbell JS, Achim A, Malla A, Joober R, Lepage M. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. Journal of psychiatric research. 2011;45(3):369–377. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Mandl RC, Rais M, van Baal GC, van Haren NE, Cahn W, Kahn RS, Hulshoff Pol HE. Altered white matter connectivity in never-medicated patients with schizophrenia. Human brain mapping. 2013;34(9):2353–2365. doi: 10.1002/hbm.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Zwiers MP, van der Schaaf A, Kahn RS, Hulshoff Pol HE. Functional diffusion tensor imaging: measuring task-related fractional anisotropy changes in the human brain along white matter tracts. PloS one. 2008;3(11):e3631. doi: 10.1371/journal.pone.0003631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. The Australian and New Zealand journal of psychiatry. 2006;40(8):616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, Sussmann JE, Baig BJ, Bastin ME, Porteous D, Evans KL, Johnstone EC, Lawrie SM, Hall J. The effects of a neuregulin 1 variant on white matter density and integrity. Molecular psychiatry. 2008;13(11):1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Chu KW, Brickman AM, Shihabuddin L, Hazlett EA, Buchsbaum MS. Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophrenia research. 2009;113(2–3):241–245. doi: 10.1016/j.schres.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Whitford TJ, Alvarado J, Pelavin P, McCarley RW, Kubicki M, Salisbury DF, Shenton ME. Hearing voices: a role of interhemispheric auditory connectivity? The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2012;13(2):153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Scarr E. Editorial: New treatment strategies in schizophrenia beyond dopamine: glutamatergic neurotransmission and more. Curr Pharm Biotechnol. 2012;13(8):1474–1475. doi: 10.2174/138920112800784871. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S, Lymer GK, Bastin ME, Marjoram D, Job DE, Moorhead TW, Owens DG, Johnstone EC, McIntosh AM, Lawrie SM. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophrenia research. 2008;106(2–3):132–139. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Murphy KC. Schizophrenia and velo-cardio-facial syndrome. Lancet. 2002;359(9304):426–430. doi: 10.1016/S0140-6736(02)07604-3. [DOI] [PubMed] [Google Scholar]
- Ottet MC, Schaer M, Cammoun L, Schneider M, Debbane M, Thiran JP, Eliez S. Reduced fronto-temporal and limbic connectivity in the 22q11.2 deletion syndrome: vulnerability markers for developing schizophrenia? PloS one. 2013;8(3):e58429. doi: 10.1371/journal.pone.0058429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(48):17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and cognition. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. The American journal of psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Hervé PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. NeuroImage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? Journal of Psychiatry Research. 2010;44(15):993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, Akkerman EM, Majoie CB, van Amelsvoort T, den Heeten GJ, Linszen DH. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58(1):19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- Peters BD, Duran M, Vlieger EJ, Majoie CB, den Heeten GJ, Linszen DH, de Haan L. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81(1):61–63. doi: 10.1016/j.plefa.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, Derosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biological psychiatry. 2014;75(3):248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Machielsen MW, Hoen WP, Caan MW, Malhotra AK, Szeszko PR, Duran M, Olabarriaga SD, de Haan L. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophrenia bulletin. 2013;39(4):830–838. doi: 10.1093/schbul/sbs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophrenia bulletin. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Voineskos AN, Szeszko PR, Lett TA, Derosse P, Guha S, Karlsgodt KH, Ikuta T, Felsky D, John M, Kennedy JL, Lencz T, Malhotra AK. White matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.2818-13.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi Y, Kubicki M, Bouix S, Westin CF, Goldstein J, Seidman L, Mesholam-Gately R, McCarley RW, Shenton ME. Statistical analysis of fiber bundles using multi-tensor tractography: application to first-episode schizophrenia. Magnetic resonance imaging. 2011;29(4):507–515. doi: 10.1016/j.mri.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis Marques T, Taylor H, Chaddock C, Dell'acqua F, Handley R, Reinders AA, Mondelli V, Bonaccorso S, Diforti M, Simmons A, David AS, Murray RM, Pariante CM, Kapur S, Dazzan P. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain : a journal of neurology. 2014;137(Pt 1):172–182. doi: 10.1093/brain/awt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusionweighted MR imaging. Radiology. 1991;180(1):229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24(2):69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Canfield EL, Tang C, Aronowitz J, Paul-Odouard R, Hof PR. Age and diffusion tensor anisotropy in adolescent and adult patients with schizophrenia. NeuroImage. 2009;45(3):662–671. doi: 10.1016/j.neuroimage.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok JH, Park HJ, Chun JW, Lee SK, Cho HS, Kwon JS, Kim JJ. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry research. 2007;156(2):93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. The American journal of psychiatry. 2007;164(3):467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sprooten E, McIntosh AM, Lawrie SM, Hall J, Sussmann JE, Dahmen N, Konrad A, Bastin ME, Winterer G. An investigation of a genomewide supported psychosis variant in ZNF804A and white matter integrity in the human brain. Magnetic resonance imaging. 2012;30(10):1373–1380. doi: 10.1016/j.mri.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Sussmann JE, Moorhead TW, Whalley HC, Ffrench-Constant C, Blumberg HP, Bastin ME, Hall J, Lawrie SM, McIntosh AM. Association of white matter integrity with genetic variation in an exonic DISC1 SNP. Molecular psychiatry. 2011;16(7):685, 688–689. doi: 10.1038/mp.2011.15. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33(5):976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dougherty RF, Colich NL, Perry LM, Rykhlevskaia EI, Louro HM, Hallmayer JF, Waugh CE, Bammer R, Glover GH, Gotlib IH. COMT genotype affects prefrontal white matter pathways in children and adolescents. NeuroImage. 2010;53(3):926–934. doi: 10.1016/j.neuroimage.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Bernsohn J. Essential fatty acid deficiency and CNS myelin. Biochemical and morphological observations. Journal of the neurological sciences. 1978;37(3):249–266. doi: 10.1016/0022-510x(78)90207-1. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM, Lobaugh NJ, Shenton ME, Rajji TK, Miranda D, Pollock BG, Mulsant BH, McIntosh AR, Kennedy JL. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cerebral cortex. 2013;23(9):2044–2057. doi: 10.1093/cercor/bhs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, Lobaugh NJ, Pollock BG, Mulsant BH, Kennedy JL. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36(9):1871–1878. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, Wang Y, Jiang L, McAlonan G, Sham P, Collier DA, Gong Q, Chua SE, Li T. Fronto-parietal white matter microstructural deficits are linked to performance IQ in a first-episode schizophrenia Han Chinese sample. Psychological medicine. 2013;43(10):2047–2056. doi: 10.1017/S0033291712002905. [DOI] [PubMed] [Google Scholar]
- Wang Q, Deng W, Huang C, Li M, Ma X, Wang Y, Jiang L, Lui S, Huang X, Chua SE, Cheung C, McAlonan GM, Sham PC, Murray RM, Collier DA, Gong Q, Li T. Abnormalities in connectivity of white-matter tracts in patients with familial and non-familial schizophrenia. Psychological medicine. 2011;41(8):1691–1700. doi: 10.1017/S0033291710002412. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Aloia MS, Goldberg TE, Berman KF. The frontal lobes and schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):419–427. doi: 10.1176/jnp.6.4.419. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cerebral cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Winterer G, Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N. Association of 5' end neuregulin-1 (NRG1) gene variation with subcortical medial frontal microstructure in humans. NeuroImage. 2008;40(2):712–718. doi: 10.1016/j.neuroimage.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophrenia bulletin. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A. Regional development of the brain in early life. Blackwell Scientific Publications, Boston. 1967 [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophrenia bulletin. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC. Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Academic radiology. 2008;15(3):285–289. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Zuliani R, Moorhead TW, Bastin ME, Johnstone EC, Lawrie SM, Brambilla P, O'Donovan MC, Owen MJ, Hall J, McIntosh AM. Genetic variants in the ErbB4 gene are associated with white matter integrity. Psychiatry research. 2011;191(2):133–137. doi: 10.1016/j.pscychresns.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]