Abstract
Purpose
Heat shock protein A2 (HspA2) expression was quantitatively measured in human testis and its relationship with the spermatogenetic status and laboratory outcomes of intracytoplasmic sperm injection (ICSI) was investigated.
Methods
Testicular tissues of azoospermia men were divided into four groups according to histopahtology: normal spermatogenesiss, hypospermatogenesis, maturation arrest and Sertoli cell-only syndrome (SCOS). HspA2 immunostaining was measured by Image Pro-Plus (IPP) and laboratory outcomes were calculated. The regression analysis between HspA2 expression and Johnsen score of as well as fertilization, cleavage and high quality embryo rate was performed.
Results
HspA2 was strongly present in the cytoplasm of spermatocytes and spermatides in normal testis. However, hypospermatogenesis and maturation arrest testicular tissues demonstrated light staining and no staining for SCOS. Quantitative image analysis showed that there were significant differences among groups (P = 0.000 & P = 0.001). HspA2 exspression was founded significantly correlated spermatogenetic status (R2 = 0.726, P = 0.000) as well as fertilization rate in ICSI (R2 = 0.569, P = 0.000).
Conclusions
The fertilization rate with ICSI is associated with HspA2 expression in the testis from which sperm retrieved and the alteration of HspA2 expression has been involved in spermatogenic impairment.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0360-7) contains supplementary material, which is available to authorized users.
Keywords: Spermatogenesis, Heat shock protein A2, Immunohistochemistry, Fertilization rate, ICSI
Introduction
Heat shock protein A2 (HspA2), a testis-specific member of the 70-kDa family, is a molecular chaperone that assists in the folding, transport, and assembly of proteins in the cytoplasm, mitochondria, and endoplasmic reticulum [1, 2]. The previous studies have indicated that HspA2 mRNA is down-regulated in human testes with abnormal spermatogenesis and the protein was high in normal spermatogensis, low in spermatogensis arrest, and no stain was demonstrated in Sertoli cell-only syndrome (SCOS) testis specimens [3, 4]. But till now, neither quantitative analysis of its expression in testis of azoospermic men nor the correlation of HspA2 expression and histopathology of spermatogenesis was performed.
Indeed, HspA2 has been established as a measure of human sperm cellular maturity, function and fertility [5, 6] and possessed a good ability to predict IVF pregnancy outcome [7]. However, the lack of reliable methods to assess sperm-fertilizing potential in ICSI programme has been a longstanding problem for azoospermic patients and their physicians. Undoubtedly, it is easy to measure HspA2 activity/level for sperm populations in IVF programme. However, it will be very difficult for a single sperm measurement in ICSI programme. Thus, the possibility to indirectly evaluate sperm cellular maturity via the HspA2 expression measurements in testicular tissue was investigated.
Ever since a long time ago, immunohistochemistry (IHC) technology had been actually applied in morphology study and the immunostaining was mainly assessed by visual assessment. However, IHC has gained significance and already taken a crucial position in diagnosis and prognosis of diseases. So, new demands on the quantitative analysis, reproducibility, and accuracy of IHC put on agenda and computerized image analysis software has been engineered and applied for quantification in IHC studies. Image Pro-Plus (IPP) is one of the digital image analysis systems serving as platform to quantify IHC staining.
In the present study, the quantitative HspA2 expression was firstly determined by IPP software and then, based on 70 cases, the regression analysis between HspA2 expression and histopathology of spermatogenesis was performed. At last, the possible predictive value of HspA2 expression for laboratory outcomes of ICSI was discussed.
Materials and methods
Tissue preparation
Seventy specimens were obtained from male patients (age: 16–45 years; mean: 31.5 years) undergoing biopsies for either azoospermia or severe oligospermia. None of the patients had received hormonal therapy or chemotherapy before the collection of tissue samples and all of the patients gave informed consent for this study. Each specimen was pathologically classified into one of 4 groups: normal spermatogenesis with obstruction (n = 25, mean Johnsen score 9.61), hypospermatogenesis (n = 18, mean Johnsen score 6.63), maturation arrest (n = 20, mean Johnsen score 4.25), or Sertoli-only syndrome (n = 7, mean Johnsen score 2.19). None of the patients had any other known potential causes for their infertility, such as hormonal abnormalities or varicoceles. Histological examination was performed using standard techniques: fixed in Bouin’s solution, embedded in paraffin, stained with hematoxylin and eosin and thereafter processed for histologic studies by at least two pathologists. The Johnsen score [8] was also calculated to quantitatively analyze the spermatogenetic status of germinal epithelium.
Immunohistochemistry
The specificity of monoclonal mouse anti-HspA2 antibody (sc-100760, Santa Cruz Bio., Dallas, USA) used in study was validated by a Western blot for testicular tissues extract firstly (Supplement fig 1). Immunochemistry technique was modified from previously described protocol [9]. Briefly, all sections were cut at 5 μm, mounted and dewaxed with xylene and rehydrated by serial graded ethanol solutions, then the sections were autoclaved at 120 °C for 2.5 min in 10 mM citrate buffer (pH 6.0). After inactivation of endogeneous peroxidase activity with 0.3 % H2O2 (room temperature, 30 min), the sections were preincubated with 1 % BSA in phosphate buffered saline (PBS) for 1 h to block nonspecific reaction with anti-HspA2 antibody. Then, the sections were reacted overnight with the first antibody at a dilution of 1:1500. After washing with rinsing with PBS, the sections were incubated with HRP-labeled goat anti-mouse IgG (sc-2064, Santa Cruz Bio., Dallas, USA) at a 1:100 dilution for 1 h. After washing with PBS, the sites of HRP were visualized by DAB and H2O2 at 20 °C for 5 min. All incubations were carried out in a humidified chamber. Finally, the sections were counterstained with hematoxylin. Tissue sections incubated with either buffer, normal serum, or the secondary conjugated antibody without primary antibody were utilized as negative controls and the controls were processed as described above.
Image analysis and quantification
Quantitative analysis of HspA2 immunostaining was made by IPP (version 6.0, Media Cybernetics, Silver Spring, MD, USA). Firstly, to determine the area covered by tissue in each image, non-tissue area was subtracted from the total area of the image. Then the digital images at same pixel resolution at 200×magnification were captured by the DP 70 CCD camera (Olympus Corp., Tokyo, Japan) coupled to Zeiss IM 35 microscope (Zeiss Corp., Oberkochen, Germany). The optical density was calibrated and the area of interest was set through: hue, 0B30; saturation, 0B255; intensity, 0B255, then the image were converted to gray scale image, and the mean integrated optical density (IOD) values were counted.
ICSI programme, fertilization and cleavage verification
Altogether, 28 cases (15 normal spermatogenesiss patients, 12 hypospermatogenesis patients, 1 maturation arrest patient) successfully followed by ICSI programme using sperm by microdissection testicular sperm extraction (m-TESE). All female patients were treated with a long gonadotrophin-releasing hormone agonist (GnRH-a, Decapeptyl, Ferring, Kiel, Germany)/gonadotrophin protocol. Oocytes were retrieved 36 h after injection of human chorionic gonadotrophin (HCG, Ovidrel®, Serono, Geneva, Switzerland) administration using follicular aspiration with guidance from transvaginal sonography. The ICSI procedure was performed only for metaphase II (MII) oocytes that had extruded their first polar bodies. After all microinjections from a single case were completed, the injected oocytes were transferred to a closed culture system micro-droplets under mineral oil. Fertilization was considered normal when two pronuclei (2PN) were visualized and the extrusion of the 2nd polar corpuscle was observed after incubated for 16–18 h at 37 °C and 5.5 % CO2. Cleavage and embryo quality were assessed 48–72 h post-ICSI procedure. The embryos were classified as good quality when they exhibited 3–4 symmetric blastomeres on the second day of culture and 7–8 symmetric blastomeres on the third day of culture, in the absence of multinucleation and zona pellucida alterations, Grade I (absence of fragmentation) or II (up to 20 % of cytoplasmic fragments) of cytoplasmic fragmentation [10]. After ICSI, normal (2PN) fertilization rates, cleavage and high quality embryo rates on 48 h (day 2) and 72 h (day 3) were analyzed.
Statistical analysis
If necessary, the measurements were log10 transformed to make their distribution closer to normal distribution and evaluated the relationship between Johnsen score and the density of HspA2 immunostaining using regression analysis. The one way ANOVA was performed to examine the significance of HspA2 expression determined by IPP among the groups determined by pathologic feature and Dunnett test was used for the intergroup comparisons. All statistical analyses were performed using the SPSS software package (version 12.0, SPSS, Inc., Chicago, USA). Tests were two-sided and P < 0.05 was considered as statistically significant.
Results
Expression of HspA2 in testis samples
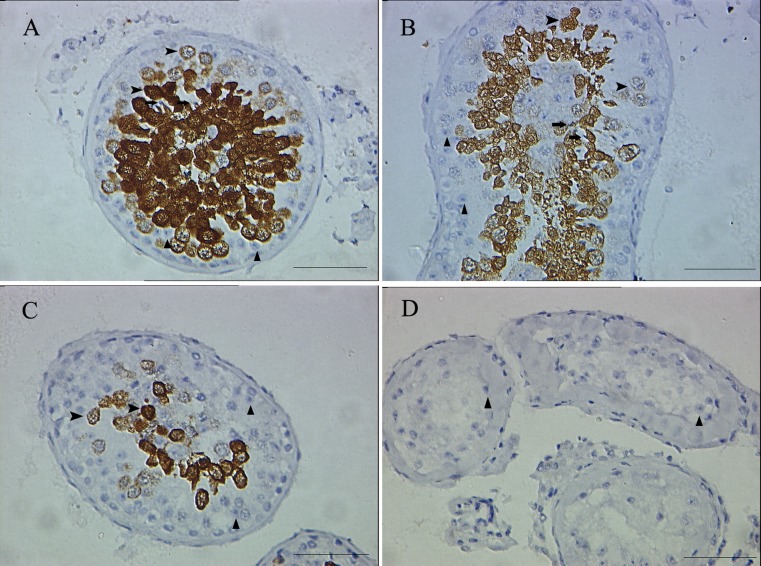
In normal spermatogenesis testis, significant HspA2 expression was observed from early meiosis spermatocytes to the postmeiotic spermatides in the adluminal compartment of the seminiferous epithelium (Fig. 1a). Weak staining was demonstrated within spermatocytes and spermatides in testis with hypospermatogenesis and maturational arrest (Fig. 1b & c). No HspA2 staining was found in Sertoli cells-only syndrome tissues (Fig. 1d). Spermatogonia as well as Leydig cells, Sertoli cells in the basal compartment of testicular tissues were not stained (Fig. 1). Similarly, negative control sections in which were labeled with the absence primary antibody failed to produce any immnostain signals in various cells.
Fig. 1.
Immunohistochemical detection of HSPA2 in normal spermatogenesis (a), hypospermatogenesis (b), maturation arrest (c) and Sertoli-only syndrome (d) testicular specimens. HSPA2 was present in the cytoplasm of spermatocytes (arrow) & spermatides (arrow head) and absent in Sertoli cell (triangle) cytoplasm. Bar = 25 μm
Quantitative expression of HspA2 relation to testis histopathology
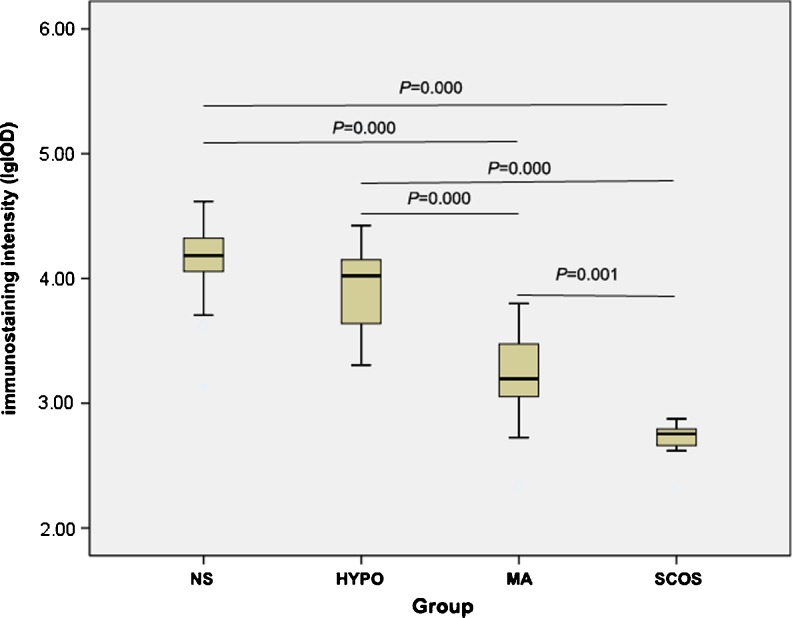
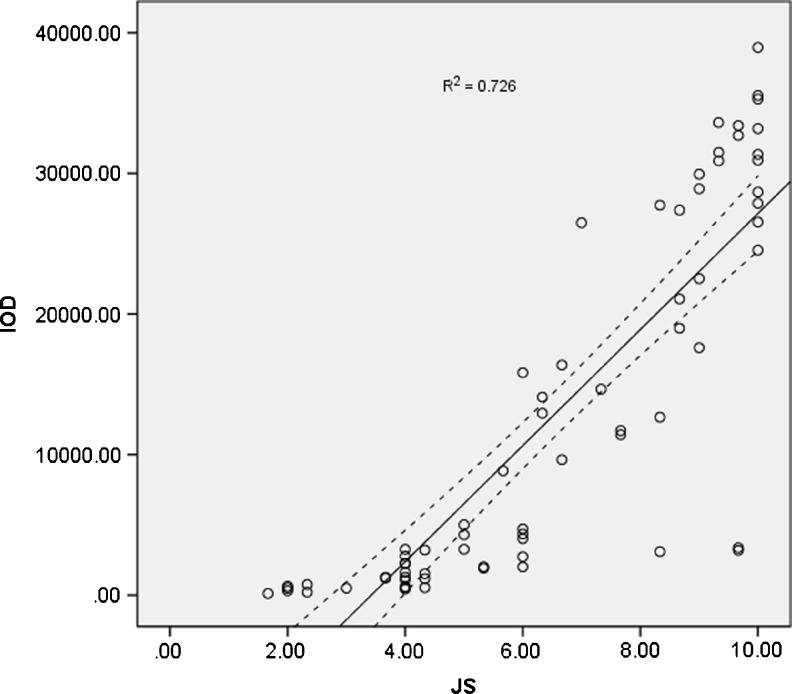
Quantitative analysis of HspA2 expression determined by IPP showed that there were significant differences between testicular tissues grouped by Johnsen score (P = 0.000 & P = 0.001) except for normal spermatogenesis and hypospermatogenesis (P = 0.441) (Fig. 2). Moreover, within seminiferous tubules, a high significant correlation between HspA2 expression and histological Johnsen score was noted (Fig. 3, R2 = 0.726, P = 0.000).
Fig. 2.
Comparison of HSPA2 immunostaining intensity (lgIOD) in relation to fertile individuals with normal spermatogenesiss (NS, n = 25), hypospermatogenesis (HYPO, n = 18), maturational arrest (MA, n = 20) and Sertoli cell-only syndrome (SCOS, n = 7). Data were analyzed by one-way ANOVA and following Dunnett test
Fig. 3.
There was a significant correlation between Johnsen score (JS) and HSPA2 immunostaining intensity (Pearson correlation coefficient, R2 = 0.726, P = 0.000). Solid line indicates the regression line for JS and immunostaining intensity, while the two dotted curves depict the 95 % confidence limits for the population regression line
Laboratory outcomes of ICSI
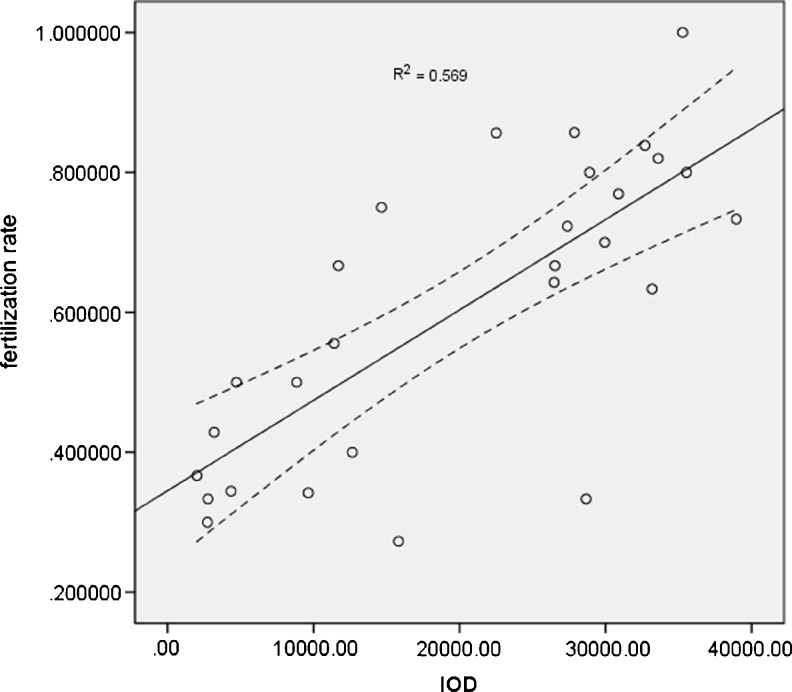
The rates of fertilization, cleavage and high quality embryo in normal spermatogenesis and hypospermatogenesis group (not including maturation arrest because of only one case) are listed in Table 1. There were no statistical differences in the rates of fertilization (P = 0.5604), embryo cleavage (P = 0.6195) and high quality embryo (P = 0.3967) between normal spermatogenesis and hypospermatogenesis group. However, a significant correlation (R2 = 0.569, P = 0.000) between HspA2 expression and fertilization rate in intracytoplasmic sperm injection (ICSI) programme was demonstrated in Fig. 4. However, no significant correlation was found between HspA2 expression and cleavage and high quality embryo rate (Supp fig 2 & fig 3).
Table 1.
Comparison of fertilization rate, cleavage and good quality embryo rate between normal spermatogenesis and hypospermatogenesis group
| Group (N) | Fertilization rate (%) | Cleavage rate (%) | Good quality embryo rate (%) |
|---|---|---|---|
| Normal spermatogenesis (N =15) | 63.1 ± 23.9 | 82.6 ± 27.3 | 78.3 ± 27.7 |
| Hypospermatogenesis (N =12) | 64.8 ± 19.9 | 78.1 ± 15.6 | 68.6 ± 29.3 |
No statistical differences were observed in the rates of fertilization, cleavage and good quality embryo between normal spermatogenesis and hypospermatogenesis groups. N: number of cases; all values are expressed as mean±SD
Fig. 4.
There was a significant correlation between HSPA2 immunostaining intensity and fertilization rate (Pearson correlation coefficient, R2 = 0.569, P = 0.000). Solid line indicates the regression line for immunostaining intensity and fertilization rate, while the two dotted curves depict the 95 % confidence limits for the population regression line
Discussion
Heat shock proteins (HSPs) are evolutionarily highly conserved molecular chaperones from prokaryotic bacteria to mammals, including humans to overcome an exogenous stress such as exposure to high temperatures [11]. Several members of HSP70 and HSP90 families play crucial roles in cellular division and development during spermatogenesis process. The 70-kDa heat shock proteins (HSP70s) are chaperones that assist in the folding, assembly and disassembly of protein complexes [12]. In mice, spermatocyte-specific hsp70-2 is a unique member of the mouse HSP70 family that is developmentally and specifically expressed in spermatogenic cells. High level of hsp70-2 expression in pachytene spermatocytes was observed during the meiotic phase of spermatogenesis. It also participates in the synaptonemal complex (SC) function during meiosis in male germ cells, and is linked to mechanisms that inhibit apoptosis, DNA repair and recombination processes [13]. In hsp70-2 −/− knockout mice model, the weight of testes of adult mice was one third of those from wild-type mice and postmeiotic germ cells lacked in testicular tissues. However, endocrine function appeared intact because seminal vesicle mass was normal and other secondary sex characteristics were not been affected. It indicates that hsp70-2 is necessary for assembly of protein complexes required for completion of meiosis. Histological examination of the mice revealed that mitotic and meiotic germ cells were present, but postmeiotic germ cells and spermatozoa were absent.
Human HspA2 gene is highly homologous the mouse hsp70-2 gene, except for only four amino acids. HspA2 additionally contains a six amino acid sequence near the carboxy terminal end not present in mice and rats [14]. The protein (previously creatine kinase M isoform) [2] as well as mRNA are expressed significantly in human testes with normal spermatogenesis, but repressed in testes with abnormal spermatogenesis [3, 4]. Moreover, the HspA2 gene expression was down-regulated in ejaculated spermatozoa from infertile men with idiopathic oligoteratozoospermia [15] and adolescents with varicocele and oligozoospermia [16]. According a more recent research, spermatozoal HspA2 expression was significantly higher in fertile compared to infertile individuals and the presence of the protein on sperm surfaces significantly increased following capacitation [17]. Actually, a growing number of researchs about HSPA2 and fecundity was reported. Some have evaluated HSPA2 in testicular tissue [3, 4, 18] while others have assessed HSPA2 expression in sperm [7, 15, 19, 16, 17]; some have assessed HSPA2 expression at RNA level [4, 16, 17] while the others at protein [3, 7, 15, 17–20]; some are based on humankind data [3, 4, 7, 15–17, 19] while other animal model [12, 13, 21, 18, 20]. The source of the analyte may acount for the consistent or nconsistent and even contradictory inference. However, till now, the acknowledged HSPA2 related physiological and pathological processes can be summarized as following: frequency of chromosomal aneuploidies, DNA repair [16, 22], cell apoptosis [13, 23], morphology and/or cytoplasmic extrusion [19], absence of histone–protamine exchange [23], zona binding sites on sperm surface [2, 24], fertilization and pregnancy following in vitro fertilisation (IVF) [25]. However, the studies aforementioned were mainly descriptive and/or semi-quantitative ones and neither quantitative analysis of HspA2 expression in testis nor the correlation of histological scores of spermatogenesis and intensity of HspA2 were performed till now.
It is generally acknowledged that computerized image analysis plays an important role in transforming qualitative perception to quantitative characteristics, descriptive results to numerical value. Among the parameters achieved through image analyzer, integrated optical density (IOD), usually used to quantify both the area and the intensity of the positive staining of immunohistochemistry, has been proved as an accurate and representative one to quantify the immunostaining [26]. In the present study, we firstly examined the expression of HspA2 protein in infertile human testes with various histopathological types by immunohistochemical technology. With normal spermatogenesis, HspA2 from early meiosis spermatocytes through the postmeiotic spermatides in the adluminal compartment of the seminiferous epithelium was observed. Also, relative weak staining within the spermatogenic cells of spermatocytes and spermatides was observed within hypospermatogenesis and spermatogensis arrest testicular tissues. However, immunohistochemical staining was negative in Sertoli cells-only syndrome tissues. Summarily, the expression pattern is in line with previous studies [3, 4]. Actually, HSPA2 is also reported to be present in the single sperm by immunoflurecence technology [27]. But in our work, the immunostain positive sperm is very diffcult to identify beacause of the thin sperm membrane with backgroud of the concentrated nucleus stained by hematoxylin. Then, the immunostaining density was translated to the more precise numerical values by an image analysis software IPP, which has been used for quantification in immunohistochemical studies [26, 28, 29]. Thereafter, IOD quantitative analysis of HspA2 expression was performed and the results revealed that there was significant differences of HspA2 stain contents in testis (P = 0.000 & P = 0.001) with different spermatogenesis status except for normal spermatogenesis and hypospermatogenesis (Fig. 2). Furthermore, a significant positive correlation between Johnsen score and expression level of HspA2 in seminiferous tubules was found (r = 0.846, P = 0.000). Johnsen score is a score system assessing spermatogenesis status in testicular tissue. The significant positive correlation between two parameters means that depression of HspA2 in human testis is associated with spermatogenic impairment. In fact, it has been demonstrated that the expression of HspA2 is biphasic pattern, first in spermatocytes related to meiosis and then at the time of terminal spermiogenesis in elongated spermatids. Accordingly, the first phase regulates progression through first meiosis while the second phase regulates late spermiogenic events such as DNA condensation [3, 30]. Actually, so much intrinsic and extrinsic factors may cause DNA [31] and RNA [32] damage, arrested protein systhesis, disruption of the cell cycle and inappropriate apoptosis, resulting in abnormal spermatogenesis. Meanwhile, the 70-kDa heat shock proteins (HSP70s) are chaperones that assist in the folding, assembly and disassembly of protein complexes [12] that continuous expression of HSPA2 has been proved to be very necessary for preventing apoptosis and completion of spermatogenesis [3] . Complete absence of HSPA2 expression of in the first phase lead to azoospermia [13] and low level HspA2 synthesis during terminal spermiogenesis may potentially prevents protein movements of cytoplasmic extrusion and plasma membrane remodeling in the elongating spermatids and leading to immature or improperly developed spermatozoa or oligozoospermia [15, 16, 22]. Moreover, depression of HSPA2 is associated with abnormal morphology and/or cytoplasmic retention too [19]. Therefore, improved or high expression of HSPA2 accounts for complete spermatogenesis (high Johnsen score) and thus maintains a certain sperm concentration capable for natural fertiliaztion. Despite that low HsAp2 expression might occur independently form sperm concentration and that HspA2 was mainly a marker of sperm development and sperm cellular maturation [7] and adequate motile sperm concentrations alone are not proof of male fertility, we concluded that improved or high expression of HSPA2 was substantial nessessary for maintaining sperm concentration. Additionally, the high correlation coefficient among Johnsen score and HspA2 expression in seminiferous tubules means there is capable enough to detect small differences by image analyzer. So, it becomes possible to obtain an objective, repeatable and precise quantification for annotating spermatogenetic status in a short time without intensive labor.
HspA2 has been proved to be valuable predictor for IVF and the fertilization rate [7] and reduced HSPA2 has been positively correlated with defects in ZP adhesion [33] . Also, the relative levels of HSPA2 expression in human spermatozoa has been used as a highly accurate diagnostic marker of male infertility [34]. However, it is easy to measure HspA2 for sperm populations in IVF and very difficult in ICSI programme for a single sperm. So, we explored the possibility to judge spermatogenetic status and indirectly evaluate sperm cellular maturity via the HspA2 expression measurements in tissue samples. It has been demostrated that even normozoospermic specimens with low HspA2 level might cause no IVF pregnancies and had lower rates of fertilization [7]. In this investigation, fertilization rate with ICSI was found to be proportional to the testicular tissue HspA2 expression; but no statistical differences in the rates of fertilization, embryo cleavage and good quality embryo were found between normal spermatogenesis and hypospermatogenesis group. So, the speculation we could make here was that HspA2 expression on testicular tissue could mirror the sperm HspA2 content and cellular maturity and was subsequently correlated with oocyte fertilization. Given that the hypothesis was right, our present data would to some extent be consistent with the previous investgations [7, 34], but different mechanism must be noticed. As mentioned above, HspA2 regulates the expression of sperm surface receptors involved in human sperm-oocyte recognition [27]. So, the fertilization failure in IVF project might mainly due to immature sperm which was deficient in the oocyte binding site, however, in the ICSI programme, the prosess of sperm-oocyte recognition and binding was bypassed. On the basis of significant relation between HspA2 expression and fertilization rate and the hypothesis that testicular tissue could mirror the sperm HspA2 content and cellular maturity, we inferred that low levels of HspA2 might fail to repair spermatogenic and germ-cell damage and led to the interrupted delivery of DNA repair enzymes and other functional proteins chaperoned by the HspA2; then DNA damage could be linked to abnormal chromatin packaging and absence of histone–protamine replacement in spermatozoa nucleus; after that sperm nuclear DNA damage and altered chromatin structure substantially affect fertilization, embryo development even sperm was directly injected into oocyte. In our study, significant relation between HspA2 expression and fertilization rate was demonstrated, but no statistical differences in the rates of fertilization, embryo cleavage and high quality embryo was emphatically indicated between normal spermatogenesis and hypospermatogenesis group. Considering the restricted participants in our study, a larger sample trial and additional research was needed to make a substantial conclusion. Generally, very weak staining in maturational arrest testis is likely to be very lower fertilization indications. However, fertilization was observed with such a case in our work. The outcome may depend on heterogeneity of sperm production and sperm-to-sperm differences in the degree of cell immaturity.
It is worth while to note that a correlation is not indicative of cause-effects; however, it reflects the relation between subjects investigated. So, HspA2 IHC might be the easiest and possible reliable indirect approach for prediction of fertilization rate with ICSI. Moreover, the most important is that it becomes possible to combine evaluation of spermatogenetic status with prediction of fertilization in ICSI programme via HspA2 IHC of testicular tissue. In conclusion, the alteration of HspA2 expression had been involved in spermatogenic impairment and low HspA2 levels in testicular tissues might be related with diminished sperm cellular maturity and had possible predictive value of oocyte fertilization in ICSI programme.
Electronic supplementary material
Western blotting analysis of hsp70-2 protein in normal spermatogenesis, hypospermatogenesis and Sertoli cell only sydrome human testicular tissues. NS:normal spermatogenesis; HYPO: hypospermatogenesis; SCOS: Sertoli cell only sydrome. (GIF 38 kb)
There was no significant correlation between HSPA2 immunostaining intensity and oocyte cleavage rate. Solid line indicates the regression line for immunostaining intensity and fertilization rate, while the two dotted curves depict the 95% confidence limits for the population regression line. (GIF 57 kb)
There was no significant correlation between HSPA2 immunostaining intensity and high quality embryo rate. Solid line indicates the regression line for immunostaining intensity and fertilization rate, while the two dotted curves depict the 95% confidence limits for the population regression line. (GIF 56 kb)
Acknowledgments
Grant sponsor: National Natural Science Foundation, grant number: 30901493; National Key Technology Support Program, grant number: 2012BAI32B04 and the Ph.D. Programs Foundation of Ministry of Education of China, grant number: 200803351041.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Footnotes
Capsule Testicular HspA2 is related with spermatogenesis and fertilization.
References
- 1.Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–34. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 2.Huszar G, Stone K, Dix D, Vigue L. Putative creatine kinase M-isoform in human sperm is identifiedas the 70-kilodalton heat shock protein HspA2. Biol Reprod. 2000;63(3):925–32. doi: 10.1095/biolreprod63.3.925. [DOI] [PubMed] [Google Scholar]
- 3.Feng HL, Sandlow JI, Sparks AE. Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertil Steril. 2001;76(6):1136–9. doi: 10.1016/S0015-0282(01)02892-8. [DOI] [PubMed] [Google Scholar]
- 4.Son WY, Han CT, Hwang SH, Lee JH, Kim S, Kim YC. Repression of hspA2 messenger RNA in human testes with abnormal spermatogenesis. Fertil Steril. 2000;73(6):1138–44. doi: 10.1016/S0015-0282(00)00496-9. [DOI] [PubMed] [Google Scholar]
- 5.Huszar G, Vigue L, Morshedi M. Sperm creatine phosphokinase M-isoform ratios and fertilizing potential of men: a blinded study of 84 couples treated with in vitro fertilization. Fertil Steril. 1992;57(4):882–8. [PubMed] [Google Scholar]
- 6.Huszar G, Vigue L, Corrales M. Sperm creatine phosphokinase activity as a measure of sperm quality in normospermic, variablespermic, and oligospermic men. Biol Reprod. 1988;38(5):1061–6. doi: 10.1095/biolreprod38.5.1061. [DOI] [PubMed] [Google Scholar]
- 7.Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, Huszar G. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77(5):910–8. doi: 10.1016/S0015-0282(02)03073-X. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen SG. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1(1):2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 9.Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71(6):2012–21. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- 10.Veek LL. The morphological assessment of human oocytes and early conception. In: Keel BA, Webster BW, editors. Hand-book of the laboratory diagnosis and treatment of infertility. Voca Raton: CRC Press; 1990. pp. 353–69. [Google Scholar]
- 11.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, Blizard DR, et al. HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development. 1997;124(22):4595–603. doi: 10.1242/dev.124.22.4595. [DOI] [PubMed] [Google Scholar]
- 13.Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, et al. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A. 1996;93(8):3264–8. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, Weber JL, et al. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23(1):85–93. doi: 10.1006/geno.1994.1462. [DOI] [PubMed] [Google Scholar]
- 15.Cedenho AP, Lima SB, Cenedeze MA, Spaine DM, Ortiz V, Oehninger S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21(7):1791–4. doi: 10.1093/humrep/del055. [DOI] [PubMed] [Google Scholar]
- 16.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, Cedenho AP. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86(6):1659–63. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Motiei M, Tavalaee M, Rabiei F, Hajihosseini R, Nasr-Esfahani MH. Evaluation of HSPA2 in fertile and infertile individuals. Andrologia. 2013;45(1):66–72. doi: 10.1111/j.1439-0272.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 18.Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet. 2014;31(3):341–54. doi: 10.1007/s10815-013-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cayli S, Sakkas D, Vigue L, Demir R, Huszar G. Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Mol Hum Reprod. 2004;10(5):365–72. doi: 10.1093/molehr/gah050. [DOI] [PubMed] [Google Scholar]
- 20.Dix DJ, Rosario-Herrle M, Gotoh H, Mori C, Goulding EH, Barrett CV, et al. Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev Biol. 1996;174(2):310–21. doi: 10.1006/dbio.1996.0076. [DOI] [PubMed] [Google Scholar]
- 21.Afiyani AA, Deemeh MR, Tavalaee M, Razi M, Bahadorani M, Shokrollahi B, et al. Evaluation of heat-shock protein A2 (HSPA2) in male rats before and after varicocele induction. Mol Reprod Dev. 2014;81(8):766–76. doi: 10.1002/mrd.22345. [DOI] [PubMed] [Google Scholar]
- 22.Kovanci E, Kovacs T, Moretti E, Vigue L, Bray-Ward P, Ward DC, et al. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence or presence of cytoplasmic retention. Hum Reprod. 2001;16(6):1209–17. doi: 10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- 23.Nasr-Esfahani MHAH, Mirhosseini Z, Ghasemi N, Razavi SH, Tavalaei M, Tanhaei S, et al. Can altered expression of can altered expression of HSPA2 in varicocele patients lead to abnormal spermatogenesis? Int J Fertil Steril. 2010;4(3):104–13. [Google Scholar]
- 24.Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, Liu DY, et al. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS One. 2012;7(11):e50851. doi: 10.1371/journal.pone.0050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huszar G, Ozenci CC, Cayli S, Zavaczki Z, Hansch E, Vigue L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil Steril. 2003;79(Suppl 3):1616–24. doi: 10.1016/S0015-0282(03)00402-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li Y, Adell G, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17(6):530–5. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]
- 27.Redgrove KA, Anderson AL, McLaughlin EA, O’Bryan MK, Aitken RJ, Nixon B. Investigation of the mechanisms by which the molecular chaperone HSPA2 regulates the expression of sperm surface receptors involved in human sperm-oocyte recognition. Mol Hum Reprod. 2013;19(3):120–35. doi: 10.1093/molehr/gas064. [DOI] [PubMed] [Google Scholar]
- 28.Francisco JS, Moraes HP, Dias EP. Evaluation of the Image-Pro Plus 4.5 software for automatic counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral Res. 2004;18(2):100–4. doi: 10.1590/S1806-83242004000200002. [DOI] [PubMed] [Google Scholar]
- 29.Prasad K, Prabhu GK. Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research — a review. J Med Syst. 2012;36(4):2621–31. doi: 10.1007/s10916-011-9737-7. [DOI] [PubMed] [Google Scholar]
- 30.Eddy EM. HSP70-2 heat-shock protein of mouse spermatogenic cells. J Exp Zool. 1998;282(1–2):261–71. doi: 10.1002/(SICI)1097-010X(199809/10)282:1/2<261::AID-JEZ28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz D, Goldfinger N, Rotter V. Expression of p53 protein in spermatogenesis is confined to the tetraploid pachytene primary spermatocytes. Oncogene. 1993;8(6):1487–94. [PubMed] [Google Scholar]
- 32.Kwon YK, Hecht NB. Cytoplasmic protein binding to highly conserved sequences in the 3′ untranslated region of mouse protamine 2 mRNA, a translationally regulated transcript of male germ cells. Proc Natl Acad Sci U S A. 1991;88(9):3584–8. doi: 10.1073/pnas.88.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huszar G, Jakab A, Sakkas D, Ozenci CC, Cayli S, Delpiano E, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod BioMed Online. 2007;14(5):650–63. doi: 10.1016/S1472-6483(10)61060-7. [DOI] [PubMed] [Google Scholar]
- 34.Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18(3):260–7. doi: 10.1097/01.gco.0000193018.98061.2f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blotting analysis of hsp70-2 protein in normal spermatogenesis, hypospermatogenesis and Sertoli cell only sydrome human testicular tissues. NS:normal spermatogenesis; HYPO: hypospermatogenesis; SCOS: Sertoli cell only sydrome. (GIF 38 kb)
There was no significant correlation between HSPA2 immunostaining intensity and oocyte cleavage rate. Solid line indicates the regression line for immunostaining intensity and fertilization rate, while the two dotted curves depict the 95% confidence limits for the population regression line. (GIF 57 kb)
There was no significant correlation between HSPA2 immunostaining intensity and high quality embryo rate. Solid line indicates the regression line for immunostaining intensity and fertilization rate, while the two dotted curves depict the 95% confidence limits for the population regression line. (GIF 56 kb)