Abstract
Purpose
To evaluate whether sperm preparation (swim-up technique) before freezing improves the percentages of sperm motility, sperm viability, and non-apoptotic spermatozoa after freezing-thawing process compared with preparation after cryopreservation.
Methods
Semen samples from 65 infertile males were equally divided into two aliquots one of which was processed for swim-up prior to cryopreservation and one of which was processed following cryopreservation. Sperm count, motility, and apoptosis index were measured in each group.
Result (s)
The total sperm count and the total motile sperm count decreased after thawing in both the pre-preparation and non-preparation groups compared with neat semen group (P < 0.001). Moreover, the percentage of apoptotic sperm in the pre-preparation group after cryopreservation was lower than that in the non-preparation group (P < 0.05), whereas the percentage of vital sperm with progressive motility was higher than that in the pre-preparation group (P < 0.001).
Conclusion (s)
Semen preparation by swim-up before freezing resulted in better sperm quality and fewer apoptotic sperm than sperm preparation after thawing. Therefore, sperm preparation before cryopreservation should be considered in routine sperm cryopreservation.
Keywords: Apoptosis, Cryopreservation, Swim-up, Motility, Vitality
Introduction
The technology of assisted reproduction has been rapidly developed, especially in the last decade. Couples who would not have had the opportunity to conceive in the past can have a child using this technique. The cryopreservation technique offers the opportunity for an infertile couple to preserve their gametes for a period [1]. Male patients suffering from cancer and requiring chemotherapy for treatment, for example, can store their sperm for future fertility application by using sperm cryopreservation prior to chemotherapy [1]. Moreover, donor semen, which is frequently used by many infertile couples in situations with male factor problems such as severe oligospermia and azoospermia, need to be evaluated for sexually transmitted disease before utilization, so they would require cryopreservation for storage during that period [1, 2].
During cryopreservation, the dramatic physical and environmental changes of sperm detrimentally affect the sperm membrane, resulting in a large increase in the percentage of poorly motile sperm or sperm with abnormal morphology [2–4]. The negative effects relating to rapid decreases in temperature, such as osmotic injury, cellular dehydration, intra-cellular ice crystal formation, and oxidative stress, also lead to damage to sperm DNA, chromatin instability and DNA denaturation [4–8]. Therefore, the reproductive outcome following fertilization with the sperm containing the damaged genetic material may be poor [8–10]. In cases where pregnancy is achieved using this sperm, the risk of abortion is increased because of the damaged genetic material in the sperm [11].
Sperm preparation methods, e.g., the swim-up or gradient techniques, have been commonly used as part of infertility treatment, especially male factor-caused infertility [12]. The preparation of sperm aims to select normal spermatozoa to improve sperm quality prior to utilization. In abnormal seminal semen, previous studies suggested that sperm preparation can increase the percentage of normal morphological and motile sperm, leading to an increased success rate of assisted reproductive treatment in either IUI or ART [13]. Although semen analysis indicated normal results, a number of abnormal spermatozoa or leukocytes could be identified in the sample. Those cells generated reactive oxygen species (ROS), which can penetrate through the sperm plasma membrane and initiate intrinsic ROS formation inside the spermatozoa, causing sperm damage, e.g., DNA fragmentation [12,14–19]. After cryopreservation of spermatozoa, the percentage of abnormal spermatozoa in regards to motility, morphology, and DNA integrity significantly increases after freezing-thawing process [2, 19, 20]. Therefore, the preparation of post-thawed spermatozoa is most commonly performed for selecting the best quality of spermatozoa and discarding the cryoprotectant media before use for IUI or fertilization in IVF. Previous studies demonstrated that sperm preparation after cryopreservation can decrease the percentage of abnormal spermatozoa and increase the good motile spermatozoa [4, 5, 20].
The goal of sperm cryopreservation is to preserve a high number of post-thawing surviving normal sperms [21]. Maintenance of original pre-freezing structural integrity, viability and fertilization potential are also fundamentally required in post-thawing sperm [1, 22]. For sperm cryopreservation, several factors, such as the pre-frozen sperm quality baseline, freezing and thawing method, and cryopreservative medium, and volume of cryopreserved specimen, have been previously demonstrated to be the crucial factors relating to post-thawing sperm outcome [2, 7, 21, 23, 24]. Several attempts have been made to improve cryopreservation techniques and media, leading to the improvement of cryopreservation [6, 21, 22, 25]. Notably, the quality of ejaculated semen is also related to the outcome of cryopreservation. For example, dead spermatozoa or leukocytes in pre-freezing semen detrimentally affect the sperm survival rate and the fertility potential after thawing through the ROS generation process [6]. A number of studies have demonstrated that supplemental addition of antioxidative to cryoprotective medium, e.g., ascorbate or catalase, minimize DNA damage and preserve sperm integrity; however, the benefit of these supplementations is still debated [25, 26]. In this study, we hypothesized that sperm preparation before freezing, compared with sperm preparation after freezing-thawing, would improve post-thawed sperm, i.e., sperm motility, sperm vitality and non-apoptotic sperm.
Materials and methods
Semen samples were randomly collected from 65 husbands who attended an infertility clinic. Written informed consent was individually obtained before recruitment into the study. Consent forms and protocols were approved by the Siriraj Ethics Committee. Exclusion criteria included abnormal semen analysis according to the World Health Organization (WHO) guidelines [27], currently active sexual transmitted disease (HIV, syphilis, and hepatitis B) or genital tract infection (active prostatitis or urethritis), and semen collected from surgical sperm recovery techniques.
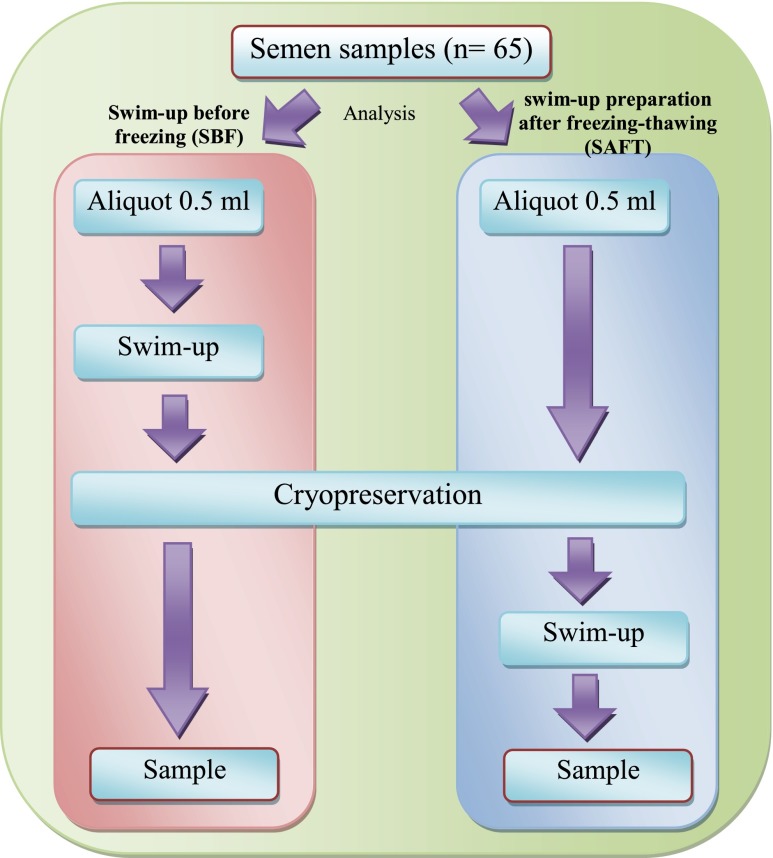
The semen samples were collected by masturbation, after an abstinence period of 3–5 days, and they were then kept in sterile containers. After semen liquefaction at room temperature, semen analysis was measured manually and automatically. Specifically, sperm count and motility were analyzed by computer-assisted semen analysis (CASA), whereas sperm morphology was evaluated using a manual method. At the same time, small aliquots of semen were analyzed for sperm apoptosis by flow cytometry. Subsequently, the semen was divided into two aliquots according to 2 different protocols of sperm preparation and cryopreservation (Fig. 1). The first protocol involved sperm preparation using the swim-up technique before freezing, whereas the later protocol described the sperm preparation after freezing-thawing. The final processing of semen in both groups was then analyzed for sperm apoptosis, sperm concentration and sperm motility using the same techniques.
Fig 1.
Schematic diagram to illustrate the division of sperm samples into two groups: sperm samples prepared (swim-up) before freezing (SBF) and sperm samples prepared (swim-up) after freezing-thawing (SAFT)
Computer-assisted semen analysis (CASA)
Sperm quality analysis was performed using the Sperm Analysis System version 12 IVOS (Hamilton Thorne Biosciences, MA) for all samples in this study. In brief, a 10 μl semen sample was dropped on both sides of the sperm analysis chamber for automatic analysis. With CASA, at least 200 sperm were evaluated regarding sperm concentration, sperm motility and different variable sperm motions, including sperm movement (rapid, medium, slow, static), average path velocity (VAP), straight line velocity (VSL), curvilinear velocity (VCL), amplitude of lateral head displacement (ALH), straightness (STR = VSL / VAP) and linearity (LIN = VSL/VCL). The CASA settings followed the manufacturer’s instruction.
First protocol: sperm preparation before freezing (SBF)
In this protocol, the best sperm were selected using the swim-up technique before cryopreservation with the liquid nitrogen vapor technique. In brief, 0.5 ml of fresh semen was diluted in 1 ml of sperm preparation medium (MediCult, DK) before being centrifuged at 300 g for 10 min. After discarding the supernatant, 1 ml of sperm preparation medium was gently added on top of the pellet and then incubated at 37 °C in 5 % CO2 in air for 30 min. Then, the medium on the top was collected and gently diluted with a small amount of sperm freezing medium (MediCult, DK) until reaching a ratio of 1:1 (V/V). Subsequently, the sperm suspension was loaded into two 500 μl straws. According to the liquid nitrogen vapor protocol [2], both straws were kept at 15 cm above the liquid nitrogen in a parallel position to the surface of liquid nitrogen for 30 min until cooling down to −80 °C and then were plunged into liquid nitrogen for cryostorage at −196 °C for a week. In the thawing procedures, both straws were taken out of liquid nitrogen and thawed by running through tap water for 10 min followed by maintenance in a 37 °C 5 % CO2 incubator for 10 min. Next, the media inside both straws were transferred to a 5-ml tube for further sperm washing. Finally, the thawing sperm samples were analyzed by flow cytometry and CASA.
Second protocol: Swim-up preparation afterfreezing- thawing (SAFT)
In the second protocol, the semen aliquot (0.5 ml) was frozen in liquid nitrogen vapor and then was prepared by the swim-up method after thawing. In brief, the second aliquot of semen (0.5 ml) was gently diluted with a small amount of sperm freezing medium until reaching a ratio of 1:1 (V/V) and a total volume of 1 ml. Then, the sperm suspension was loaded into two 500 μl straws. Both straws were frozen by using a liquid nitrogen vapor technique and were thawed after being kept under liquid nitrogen for a week. Both freezing and thawing methods were performed as the first protocol. After thawing, the thawed semen from both straws was transferred to a 5-ml tube and was diluted with 2 ml of sperm preparation medium. After centrifugation at 300 g for 10 min, the supernatant was removed, and sperm preparation medium was gently added on top of the pellet. Then, the tube was transferred to an incubator for storage at 37 °C in 5 % CO2 for 30 min. Finally, the suspension medium on the top level was collected and analyzed by CASA and flow cytometry in the same manner as the other protocol.
Flow cytometric analysis
To assess viable sperm, apoptotic sperm and necrotic sperm among the samples, an Annexin V/PI binding Assay (Annexin V-FITC Apoptosis Detection Kit I; BD Pharmingen, USA) was used for supravital staining in this study. Briefly, the semen sample was washed twice with phosphate buffer saline (PBS) and was resuspended in 1X Annexin V binding buffer at a concentration of 1 x 106/ml. The cell suspension was aliquoted into 100 μl in a 5 ml polystyrene tube. To stain the sperm, 5 μl of Annexin V-FITC (5 μg/ml) and 5 μl of PI (50 μg/ml) were added in the sample and gently mixed thoroughly until becoming homogeneous. After incubation at 25 ° C for 15 min in the dark, 400 μl of 1X Annexin V binding buffer was added into each tube and slightly mixed. All samples were analyzed by flow cytometry within 1 h. The results were categorized into four types of spermatozoa. First, non-apoptotic sperm or a viable sperm were considered live cells with no translocation of membrane PS and no PI binding and, as such, h demonstrated no fluorescence emission (AN- PI-). Second, early apoptotic sperm, which included live cells with translocation of membrane PS and no PI binding, presented green fluorescence (AN + PI-). Third, late apoptotic sperm, included dead cells with translocation of membrane PS and PI binding, displayed a combination of green and red fluorescences (AN + PI+). Lastly, necrotic sperm, which included dead cells with no binding with annexin V but contact PI, displayed only red fluorescence (AN- PI+). To evaluate the fluorescence signals of the labeled spermatozoa, a FAC Scan flow cytometer (BDB, CA) equipped with a 15 mW argon ion laser that operates at 488 nm was used in this study. In this assay, two-color flow cytometric analyses of green and orange fluorescence were used to detect Annexin V- and PI-labeled spermatozoa. Logarithmic green fluorescence (480–530 nm) was measured in the FL-1 channel, whereas orange fluorescence (580–630 nm) was evaluated in the FL-2 channel. A minimum of 10,000 spermatozoa were examined for each assay at a flow rate of less than 100 cells/s. The sperm population was gated using 90° and forward-angle light scatter to exclude debris and aggregates. The percentage of the mean fluorescence and the positive cells mentioned above were evaluated on a two-color dot plot using Cell Quest software (Becton Dickinson Biosciences, USA).
Statistical analysis
Statistical analysis was performed with SPSS for Windows 13.0 (SPSS, Inc., IL). All data are presented as the Mean ± SD. The normality of each reference was tested by the Kolmogorov-Smirnov normal distribution test. A repeated measure ANOVA was used for data analysis and post-hoc analysis was performed using Bonferroni multiple-comparisons test to assess statistical significance. A value of P < 0.05 was considered significant.
Results
Sixty-five semen samples were included in this study. The results indicated that the mean age of the male partner in the infertile couples was 33.2 ± 4.3 years old. The baseline characteristics of the semen analysis according to the WHO criteria and all parameters are shown in Table 1.
Table 1.
Baseline characteristics of semen analysis
| Parameters | Value | Range | |
|---|---|---|---|
| Mean | SD | ||
| Volume (ml) | 3.52 | 0.79 | 2.4 – 4.3 |
| pH | 7.4 | 0.2 | 7.2 – 7.6 |
| Total sperm count (x 106) | 184.44 | 76.63 | 70.0 – 349.0 |
| Sperm Concentration (x 106/ml) | 42.77 | 17.02 | 20.00 – 93.00 |
| Normal morphology (%) | 17.02 | 3.91 | 13.0 – 21.0 |
| Motility (%) | 53.0 | 11.0 | 41.9. – 64.2 |
| Vitality (%) | 74.2 | 4.9 | 69.6 – 88.5 |
| White blood cell (x 106/ml) | 0.4 | 0.2 | 0.0 – 0.6 |
The results of sperm analysis in both the post-treated and pre-treated spermatozoa groups are illustrated in Table 2. The cryopreservation resulted in a significant reduction of the mean values for total sperm count and total motile sperm when comparing the neat semen group and either the SBF or SAFT group. For the sperm motility, SBF (99.5 ± 2.3 %) or SAFT (93.9 ± 7.9 %) improved the percentage of highly motile spermatozoa compared to those in the untreated fresh sperm (53.0 ± 11.0 %, P < 0.001). The mean percentage of progressive sperm motility in SBF, however, was higher than that in the SAFT group (P < 0.001). Despite the fact the values of VAP, VSL, VCL, STR and LIN of spermatozoa in the neat semen group was significantly lower than those values in the SBF or SAFT groups, the ALH group differences were not significant.
Table 2.
Effect of sperm preparation on neat semen sample (NS) before cryopreservation on sperm motility evaluated by CASA, compared between sperm samples prepared before freezing group (SBF), and sperm samples prepared after freezing-thawing group (SAFT) in 65 semen samples
| Neat semen sample NS | Semen samples prepared before freezing group (SBF) | Semen samples prepared after freezing-thawing group SAFT | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Total Sperm Count (×106) a, b | 42.7 | 17.2 | 8.5 | 6.7 | 10.4 | 15.5 | < 0.001 |
| Total Motile Sperm Count (×106) a,b | 22.4 | 9.6 | 8.4 | 6.4 | 9.6 | 14.5 | < 0.001 |
| Progressive Motility (%) a,b,c | 53.0 | 11.0 | 99.5 | 2.3 | 93.9 | 7.9 | < 0.001 |
| Velocity distribution: Rapid (%) a,b,c | 36.7 | 2.4 | 94.6 | 10.8 | 65.9 | 32.5 | < 0.001 |
| Velocity distribution: Medium (%) b,c | 16.5 | 2.4 | 4.9 | 10.6 | 27.6 | 31.8 | < 0.001 |
| Velocity distribution: Slow (%) a,b | 27.6 | 1.9 | 0.2 | 1.4 | 2.4 | 5.0 | < 0.001 |
| Velocity distribution: Static (%) a,b,c | 19.3 | 3.2 | 0.3 | 1.5 | 4.1 | 7.3 | < 0.001 |
| Average path velocity (VAP) (μm/s) a,b | 46.4 | 2.3 | 89.1 | 55.6 | 70.9 | 56.0 | < 0.001 |
| Straight-line velocity (VSL) (μm/s) a,b | 36.0 | 1.8 | 81.1 | 57.2 | 67.0 | 56.4 | < 0.001 |
| Curvilinear velocity (VCL) (μm/s) a,b,c | 88.2 | 4.6 | 148.6 | 57.8 | 105.6 | 59.4 | < 0.001 |
| Amplitude of lateral head displacement (ALH) (μm) | 4.7 | 0.3 | 4.9 | 2.6 | 4.1 | 2.7 | < 0.347 |
| Straightness (STR) (%) a,b | 76.6 | 1.2 | 87.0 | 9.2 | 90.6 | 9.2 | < 0.001 |
| Linearity (LIN) (%) a,b | 42.1 | 1.5 | 52.7 | 16.2 | 61.0 | 22.1 | < 0.001 |
Hoc analysis using Bonferroni multiple-comparisons test (P < 0.05)
a Significance of difference between NS and SAFT
b Significance of difference between NS and SBF
c Significance of difference between SAFT and SBF
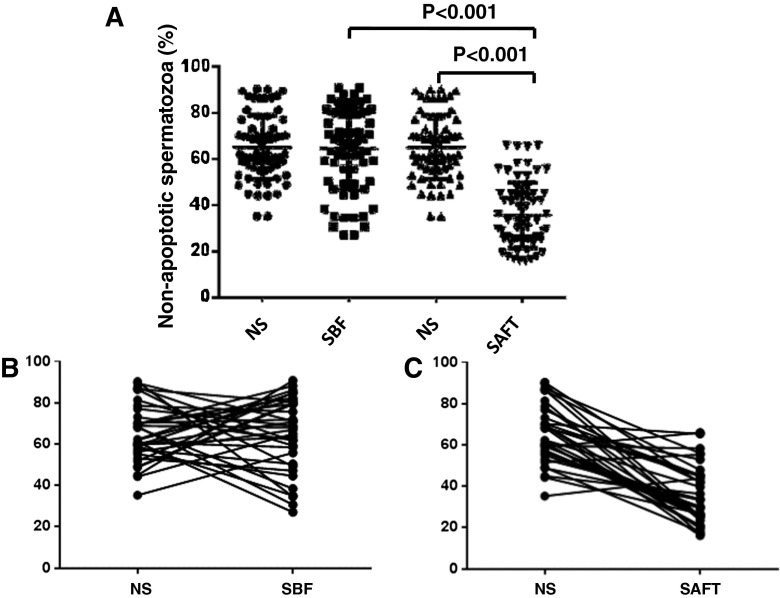
The comparison of apoptotic sperm among the neat semen, SBF, and SAFT groups is presented in Table 3. The percentage-rate of non-apoptotic spermatozoa in the neat semen group is similar to that of the SBF group (65.2 ± 4.3 % and 64.5 ± 18.0 %, respectively), whereas the percentage-rate of the non-apoptotic spermatozoa in the SAFT group (38.4 ± 18.6 %), which was prepared after cryopreservation, was decreased compared with the neat semen group as well as the SBF group (P < 0.001 and P < 0.001, respectively; Fig. 2A). Individual interpretations and plots are presented on the graphs in Fig. 2B. According to the percentage of non-apoptotic spermatozoa, the majority of non-apoptotic spermatozoa were improved in the SBF group but not in the SAFT group compared with the neat semen group. Individual variability, however, were observed in all groups.
Table 3.
Distribution of non-apoptotic sperm, apoptotic sperm, and necrotic sperm in neat semen samples and semen samples prepared by swim-up before and after cryopreservation in 65 semen samples, evaluated by flow cytometric analysis
| Neat semen sample (NS,%Rate) | Semen samples prepared before freezing group (SBF,% Rate) | Semen samples prepared after freezing- thawing group (SAFT,%Rate) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Non-apoptotic sperm a,c | 65.2 | 14.0 | 64.5 | 18.0 | 38.4 | 18.6 | < 0.001 |
| Early apoptotic spermb,c | 3.8 | 2.9 | 1.5 | 0.8 | 3.1 | 2.0 | < 0.001 |
| Late apoptotic sperma,b,c | 20.6 | 9.4 | 30.8 | 16.6 | 48.5 | 16.3 | < 0.001 |
| Total apoptotic sperma,c | 24.4 | 11.1 | 32.3 | 16.9 | 51.6 | 16.6 | < 0.001 |
| Necrotic spermb,c | 10.4 | 6.2 | 3.2 | 3.0 | 10.0 | 6.5 | < 0.001 |
Hoc analysis using Bonferroni multiple-comparisons test (P < 0.05)
a Significance of difference between NS and SAFT
b Significance of difference between NS and SBF
c Significance of difference between SAFT and SBF
Fig 2.
Percentage of non-apoptotic spermatozoa in neat semen sample, sperm sample prepared before freezing (SBF), and sperm sample prepared after freezing-thawing (SAFT) in 65 semen samples evaluated by Flow cytometric analysis. a. The percentage of non-apoptotic spermatozoa was not significantly difference between NS and SBF, but the percentage of non-apoptotic spermatozoa showed significantly decreased (P <0.001) in SAFT compared with NS. b. The percentage of non-apoptotic sperm before and after cryopreservation in each sample in SBF group. c. The percentage of non-apoptotic sperm before and after cryopreservation in each sample in SAFT group
Although the percentage-rate of total apoptotic spermatozoa in the SBF and SAFT groups was higher than that in the neat semen group (32.3 ± 16.9 %, 51.6 ± 16.6 %, and 24.4 ± 11.1 %, respectively), the percentage-rate of total apoptotic spermatozoa in the SBF group was decreased compared with the percentage in the SAFT group (P < 0.001). Regarding necrotic spermatozoa, the results in the SBF group indicated a significantly lower percentage-rate of necrotic spermatozoa than in the SAFT group (P < 0.001), whereas the percentage-rate in pre-freezing necrotic spermatozoa in SBF was nearly similar to the pre-freezing group value (Table 3).
Discussion
The present study aimed to evaluate if sperm preparation before cryopreservation enhances post-thawing sperm survival. Although pre-frozen sperm quality, especially sperm preparation prior to freezing, has been studies [18,22,28], the effects of sperm preparation before freezing on the post-thawing sperm quality, as part of optimizing sperm recovery, especially with regards to apoptosis, have not been established yet. Previous studies were only focused on the effect of cryopreservation in sperm when comparing fresh sperm and post-cryopreserved sperm [18,22]. The advantages of pre-frozen sperm preparation have been demonstrated in the previous studies, but the relevant experiments were designed to compare sperm preparation and a “no treatment” control group [28,29]. In general, post-thawing sperm, after cryopreservation, is routinely prepared during either sperm washing or sperm selection to discard dead sperm or cryoprotectant media. To identify the benefits of sperm preparation prior to sperm cryopreservation, sperm preparation after cryopreservation, as a control for the experiment, is required. In this study, the effect of sperm preparation on post-cryopreserved sperm was evaluated when comparing sperm preparation before and after cryopreservation.
Regarding sperm motility, the results indicated improvement of progressive motile sperm and curvilinear sperm velocity after swim-up both in the SBF group and SAFT group. These results suggest that sperm selection by swim-up is capable of selecting a good motile sperm in either fresh samples or post cryopreserved sperm. The total sperm count and total motile sperm count, however, were significantly decreased compared with the initial pre-freezing sperm. These findings are in accordance with the studies by Esteves [22,28]. The lower sperm count could be explained by cryopreservation-induced sperm damage. Poorly motile sperm and dead sperm were discarded, and only good motile sperm were selected by the swim-up technique [30]. Moreover, detrimental effects of freezing on spermatozoa have been reported, such as sperm mitochondria damage and sperm tail abnormality [2,31]. These lead to abnormal movement of sperm after cryopreservation, and these poorly motile sperm were eliminated by the swim-up technique. Notably, progressive sperm motility in the SAFT group, however, was significantly lower than that in the SBF group, suggesting sperm preparation prior to cryopreservation enhances post-thawed sperm motility. The explanation for this finding may relate to sperm washing selection of the sperm with good motility (e.g., resistant to cryoinjury) and elimination of the other components in semen (i.e., ROS, leukocytes, bacteria, etc.) that affect the post-thawing sperm outcome [12,16–19,30]. The percentages of sperm motility in the static, slow velocity, and medium velocity subgroups of the SAFT group were higher than in the SBF group. These results may indicate that the cryopreservation of the semen components and unprepared sperm negatively affected the post-thawing outcome. Although sperm preparation was performed after thawing in the SAFT group, the final post-thawing sperm outcome in the SAFT group (the percentage of progressive sperm motility) was still not as good as that in the SBF group.
In this study, the effect of cryopreservation on the plasma membrane was evaluated by Annexin V/PI using flow cytometric analysis to evaluate the percentage of apoptotic sperm. The specific binding of phosphatidylserine (PS) can be distinguished by using fluorescent dyes and other stains by a flow cytometer, which could detect cell apoptosis. Therefore, the technique is definitely very helpful for evaluating sperm viability and function after cell injury [32]. In addition, one of the most sensitive and widely used techniques to detect early cell death is “apoptosis detection” [33]. The results indicated apoptotic sperm were obviously detected in both groups. This suggests that the freezing and thawing processes could induce sperm injury, eventually leading to apoptosis [6]. Although the percentage of apoptotic sperm dramatically increased in both groups, the number of apoptotic sperm in the SBF group was significantly lower than in the other group, suggesting an advantage of sperm preparation prior to freezing. Freshly ejaculated spermatozoa may be damaged by oxygen radicals originating from seminal leucocytes and damaged spermatozoa [12,17], such that the stress caused by the cryopreservation procedure may add to damage already inflicted by free oxygen radicals [6,30]. Therefore, the application of SBF, which eliminates seminal plasma, cellular debris, leukocytes, and amorphous material, as well as immotile and dead sperm, should improve the survival rate of sperm after cryopreservation. Indeed, the results of this study confirmed that SBF provides a lower post-thawing apoptotic sperm percentage than SAFT.
In this study, staining with annexin V, together with PI, was used to detect apoptosis. A modification of the lipid architecture as a translocation of PS from inner to outer leaflet of the plasma membrane results in positive staining by annexin V, which is a very early sign of apoptosis [34–36]. In the present study, annexin V-positive stained spermatozoa was categorized as apoptotic sperm, whereas staining with PI indicated early apoptotic sperm (annexin V negative/PI positive) or late apoptotic sperm (annexin V positive /PI positive). Non-apoptotic sperm were negatively stained for both annexin V and PI. Dead sperm or necrotic sperm were positively stained only for PI. However, Martin et al. (2005) found a correlation between PS exposure and acrosome reaction, but not caspase activation, in an experiment involving treatment of sperm with the calcium ionophore A23187 [37]. It is still unknown whether calcium ionophore induction of PS externalization interacts with calcium-depending binding of Annexin V leading to non-specific results. Indeed, several studies still use Annexin V as a method for detecting early apoptosis of sperm [32,34,38]. An Annexin V magnetic-activated cell sorting recently has been used as a new tool to select sperm in assisted reproduction, and this technique has been demonstrated to improve the pregnancy rate in ICSI [35,36].
SBF displayed an advantage in apoptosis prevention. The outcomes of pre-preparation in post-thawing sperm with both SBF and SAFT, however, were most likely limited by low sperm concentrations or a low number of total motile sperm. When there is poor semen quality, an extremely low post-thawing sperm count would be obtained after swim-up; therefore, it is most likely not appropriate to use the technique is such cases. Notably, for couples who undergo in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm freezing, this sperm preparation before cryopreservation technique might be useful for sperm storage for obtaining good post-thawing sperm. Before using post freezing-thawing spermatozoa, additional Sperm washing step should be carefully performed to discard the remained cryoprotectants in these sample. However, the current study did not evaluate other parameters (such as sperm capaCitation, DNA integrity, and acrosome reaction) or other sperm functions (e.g., fertilization ability of post-thawed sperm). This lack of further investigation represents a limitation of this study, these parameters and functions should be evaluated in a future study.
Conclusion
Sperm preparation by swim-up before freezing improves sperm quality, including progressive motility, viability, and less apoptosis, compared with preparation after thawing. In semen cryopreservation, sperm preparation before sperm cryopreservation should be considered.
Acknowledgments
The study was financially supported by the Siriraj Grant for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University. I would like to thank Orawan Makemaharn, Suphadtra Phornwilardsiri, Suvana Sangchoo, Sukanya Sriiam, and all of the staff members in the Infertility Unit for their help in conducting the study. Finally, I would like to thank all participants in this research.
Conflict of interests
The study involves no conflict of interests and was financially supported by a Siriraj Grant for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University
Footnotes
Capsule Pre-freezing sperm preparation by swim-up technique improves sperm motility and reduces apoptosis in post-freezing-thawing sperm compared with post-thawing sperm preparation.
Author contributions
S.P. contributed to the conception and design of the study, conducted the experiments, performed data analysis and interpretation, and prepared the manuscript. C.N. participated in the experiments and data acquisition, conducted statistical analysis, and was involved in the manuscript preparation. I.T. was involved in revising the manuscript. P.L. and R.S. provided suggestions. All authors read and approved the final manuscript.
References
- 1.Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril. 2013;100:1203–9. doi: 10.1016/j.fertnstert.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Petyim S, Choavaratana R. Cryodamage on sperm chromatin according to different freezing methods, assessed by AO test. J Med Assoc Thai. 2006;89:306–13. [PubMed] [Google Scholar]
- 3.Critser JK, Arneson BW, Aaker DV, Huse-Benda AR, Ball GD. Cryopreservation of human spermatozoa. II. Postthaw chronology of motility and of zona-free hamster ova penetration. Fertil Steril. 1987;47:980–4. doi: 10.1016/s0015-0282(16)59233-4. [DOI] [PubMed] [Google Scholar]
- 4.Hammadeh ME, Askari AS, Georg T, Rosenbaum P, Schmidt W. Effect of freeze-thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Androl. 1999;22:155–62. doi: 10.1046/j.1365-2605.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 5.Royere D, Hamamah S, Nicolle JC, Lansac J. Chromatin alterations induced by freeze-thawing influence the fertilizing ability of human sperm. Int J Androl. 1991;14:328–32. doi: 10.1111/j.1365-2605.1991.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–70. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 7.Abush A, Hauser R, Paz G, Kleiman SE, Lehavi O, Yavetz H, et al. Thawed human sperm quality is influenced by the volume of the cryopreserved specimen. Fertil Steril. 2014;101:640–6. doi: 10.1016/j.fertnstert.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Ribas-Maynou J, Fernandez-Encinas A, Garcia-Peiro A, Prada E, Abad C, Amengual MJ, et al. Human semen cryopreservation: a sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology. 2014;2:83–7. doi: 10.1111/j.2047-2927.2013.00158.x. [DOI] [PubMed] [Google Scholar]
- 9.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–22. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–9. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 11.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–36. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 12.Ricci G, Perticarari S, Boscolo R, Simeone R, Martinelli M, Fischer-Tamaro L, et al. Leukocytospermia and sperm preparation–a flow cytometric study. Reprod Biol Endocrinol. 2009;7:128. doi: 10.1186/1477-7827-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demir B, Arikan II, Bozdag G, Esinler I, Karakoc Sokmensuer L, Gunalp S. Effect of sperm morphology on clinical outcome parameters in ICSI cycles. Clin Exp Obstet Gynecol. 2012;39:144–6. [PubMed] [Google Scholar]
- 14.Whittington K, Ford WC. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int J Androl. 1999;22:229–35. doi: 10.1046/j.1365-2605.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 15.Aitken RJ, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil. 1992;94:451–62. doi: 10.1530/jrf.0.0940451. [DOI] [PubMed] [Google Scholar]
- 16.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, Thomas AJ., Jr Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil Steril. 2004;82:871–7. doi: 10.1016/j.fertnstert.2004.02.132. [DOI] [PubMed] [Google Scholar]
- 18.Brugnon F, Ouchchane L, Pons-Rejraji H, Artonne C, Farigoule M, Janny L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum Reprod. 2013;28:2045–57. doi: 10.1093/humrep/det253. [DOI] [PubMed] [Google Scholar]
- 19.Valcarce DG, Carton-Garcia F, Riesco MF, Herraez MP, Robles V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology. 2013;1:723–30. doi: 10.1111/j.2047-2927.2013.00116.x. [DOI] [PubMed] [Google Scholar]
- 20.Rougier N, Uriondo H, Papier S, Checa MA, Sueldo C, Alvarez SC. Changes in DNA fragmentation during sperm preparation for intracytoplasmic sperm injection over time. Fertil Steril. 2013;100:69–74. doi: 10.1016/j.fertnstert.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Justice T, Christensen G. Sperm cryopreservation methods. Methods Mol Biol. 2013;927:209–15. doi: 10.1007/978-1-62703-038-0_18. [DOI] [PubMed] [Google Scholar]
- 22.Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6:139–48. [PubMed] [Google Scholar]
- 23.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Effect of swim-up sperm washing and subsequent capacitation on acrosome status and functional membrane integrity of normal sperm. Int J Fertil Womens Med. 2000;45:335–41. [PubMed] [Google Scholar]
- 24.Thomson LK, Fleming SD, Barone K, Zieschang JA, Clark AM. The effect of repeated freezing and thawing on human sperm DNA fragmentation. Fertil Steril. 2010;93:1147–56. doi: 10.1016/j.fertnstert.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2009;60:235–7. doi: 10.1016/j.cryobiol.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Lin Q, Liu R, Xiao W, Liu W. Protective Effects of Ascorbate and Catalase on Human Spermatozoa During Cryopreservation. J Androl. 2009;31:437–44. doi: 10.2164/jandrol.109.007849. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Cambridge; Cambridge University Press; 2010. WHO laboratory manual for the examination and processing of human semen.
- 28.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Improvement in motion characteristics and acrosome status in cryopreserved human spermatozoa by swim-up processing before freezing. Hum Reprod. 2000;15:2173–9. doi: 10.1093/humrep/15.10.2173. [DOI] [PubMed] [Google Scholar]
- 29.Counsel M, Bellinge R, Burton P. Vitality of oligozoospermic semen samples is improved by both swim-up and density gradient centrifugation before cryopreservation. J Assist Reprod Genet. 2004;21:137–42. doi: 10.1023/B:JARG.0000031245.39921.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abeysundara PK, Dissanayake D, Wijesinghe PS, Perera R, Nishad A. Efficacy of two sperm preparation techniques in reducing non-specific bacterial species from human semen. J Hum Reprod Sci. 2013;6:152–7. doi: 10.4103/0974-1208.117169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanic P, Tandara M, Sonicki Z, Simunic V, Radakovic B, Suchanek E. Comparison of protective media and freezing techniques for cryopreservation of human semen. Eur J Obstet Gynecol Reprod Biol. 2000;91:65–70. doi: 10.1016/S0301-2115(99)00255-9. [DOI] [PubMed] [Google Scholar]
- 32.Sion B, Janny L, Boucher D, Grizard G. Annexin V binding to plasma membrane predicts the quality of human cryopreserved spermatozoa. Int J Androl. 2004;27:108–14. doi: 10.1046/j.1365-2605.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Martinez H, Larsson B, Pertoft H. Evaluation of sperm damage and techniques for sperm clean-up. Reprod Fertil Dev. 1997;9:297–308. doi: 10.1071/R96081. [DOI] [PubMed] [Google Scholar]
- 34.Muratori M, Porazzi I, Luconi M, Marchiani S, Forti G, Baldi E. AnnexinV binding and merocyanine staining fail to detect human sperm capacitation. J Androl. 2004;25:797–810. doi: 10.1002/j.1939-4640.2004.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 35.Tavalaee M, Deemeh MR, Arbabian M, Nasr-Esfahani MH. Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assist Reprod Genet. 2012;29:31–8. doi: 10.1007/s10815-011-9686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunewald S, Paasch U. Sperm selection for ICSI using annexin V. Methods Mol Biol. 2013;927:257–62. doi: 10.1007/978-1-62703-038-0_23. [DOI] [PubMed] [Google Scholar]
- 37.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–68. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 38.Ricci G, Boscolo R, Martinelli M, Perticarari S. Methods for defecting sperm apoptosis. Fertil Steril. 2009;92(2):e19. doi: 10.1016/j.fertnstert.2008.12.109. [DOI] [PubMed] [Google Scholar]