Abstract
Purpose
To determine the composition of commercially available protein supplements for embryo culture media and test if differences in protein supplement composition are biologically relevant in a murine model.
Methods
Amino acid, organic acid, ion and metal content were determined for 6 protein supplements: recombinant human albumin (AlbIX), human serum albumin (HSA and Buminate), and three complex protein supplements (SSS, SPS, LGPS). To determine if differences in the composition of these supplements are biologically relevant, mouse one-cell embryos were collected and cultured for 120 hours in each protein supplement in Global media at 5 and 20 % oxygen in an EmbryoScope time-lapse incubator. The compositions of six protein supplements were analyzed for concentrations of 39 individual amino acids, organic acids, ions and elements. Blastocyst development and cell cycle timings were calculated at 96-hours of culture and the experiments were repeated in triplicate. Blastocyst gene expression was analyzed.
Results
Recombinant albumin had the fewest undefined components , the lowest concentration of elements detected, and resulted in high blastocyst development in both 5 and 20 % oxygen. Buminate, LGPS and SPS had high levels of transition metals whereas SSS had high concentrations of amino acids. Pre-compaction mouse embryo development was delayed relative to embryos in AlbIX for all supplements and blastocyst formation was reduced in Buminate, SPS and SSS.
Conclusions
The composition of protein supplements are variable, consisting of previously undescribed components. High concentrations of pro-oxidant transition metals were most notable. Blastocyst development was protein dependent and showed an interaction with oxygen concentration and pro-oxidant supplements.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0349-2) contains supplementary material, which is available to authorized users.
Keywords: Protein, Albumin, Quality control, Mouse embryo assay, Embryo culture
Introduction
Protein is an important component of embryo culture with documented lot-to-lot variability [1–7] that can adversely affect embryo development [1, 2, 4, 8–10]. Protein supplements are poorly defined and thus represent a gap in our control of quality in the clinical embryology laboratory. Closing this gap requires detailed analysis of protein supplements, analysis that can identify potentially harmful components that can then be avoided to minimize detrimental effects as well as beneficial components that can be selected for, or even supplemented, to improve embryo development.
Clinical laboratory practices for protein supplementation vary considerably for both the type and concentration of protein used. Menezo et al. [11] first demonstrated that albumin from human serum (HSA) could be used as a replacement for serum and today is the most common protein used in clinical embryology laboratories, whether alone or as part of a complex protein supplement. Adler et al. [12] showed that a plasma protein fraction containing a mixture of albumin and α and β globulins could support embryo development, leading to the first complex protein supplement developed specifically for clinical ART [13]. Though most pre-supplemented media contains HSA at 5 mg/mL, supplementation with varying levels of complex proteins is common and occurs either with protein-free media or with media containing HSA [14]. A recombinant albumin product is also available for supplementation of human embryo culture medium [15], although it is more expensive than human-derived protein products and has not been shown to improve clinical outcomes.
Little is known about the source of lot-to-lot variation in protein supplements. The albumin molecule itself contributes to variability as a potent antioxidant with lot-to-lot variation in REDOX activity [16]. Albumin is also a carrier of hormones, growth factors, energy substrates (citrate and fatty acids), medications, enzymes, and metals [6]. In addition, stabilizers added to HSA preparations show batch variation at concentrations that affect mouse embryo development [4]. Complex protein supplements show even more variability than HSA. Meintjes [3] reported routine batch testing of a complex supplement, finding variations in hormones, cytokines, electrolytes and endotoxins.
Protein supplements, including HSA, have heterogeneity that should be defined in order to limit adverse effects on clinical outcomes. To address this gap in knowledge, we performed a systematic analysis of the composition of six different protein supplements, including standard HSA, recombinant HSA lacking stabilizers, three complex protein supplements, and a clinical HSA product not prescreened by an ART supplier. We then determined if observed differences in supplement composition were biologically relevant using mouse embryo development. Studies were performed at both reduced (5 %) and ambient (20 %) oxygen to determine if there were any protein by oxygen interactions.
Materials and methods
Protein supplement analysis
Protein supplement sources
Six protein supplements were analyzed. Three albumin products were obtained and tested at 5 mg/mL: recombinant human serum albumin (rHSA; AlbIX, 100 mg/mL, Novozymes), human serum albumin (HSA) from a general supplier (Buminate, 50 mg/mL, Baxter Scientific) and one from an ART distributor (HSA, 100 mg/mL, IVFOnline). AlbIX differs from Vitrolife’s G-MM rHSA in that it does not contain either of the stabilizers n-acetyltryptophan and octanoic acid added to all protein supplements. Complex protein supplements from three different companies were obtained and tested at 10 % v:v: LGPS (IVFOnline); SPS (Origio a/s) and SSS (Irvine Scientific). SPS and LGPS contain 50 mg/mL protein consisting of 88 % HSA and 12 % α and β globulins, resulting in a final albumin concentration of 4.4 mg/mL. In contrast, SSS contains 60 mg/mL protein with 84 % albumin and 16 % α and β globulins, resulting in a final albumin concentration of 5.0 mg/mL. We chose the recombinant albumin AlbIX, which is not currently supplied for use in ART, as a control that lacks stabilizers and the variability of human derived products. Buminate was selected for testing because it is an FDA-approved HSA product that is used by some IVF clinics but does not go through quality control testing for use in ART.
Protein compositional analysis
Amino acids, organic acids, transferrin and elements were quantified in protein supplements as previously described (Morbeck et al., 2014). Two lots of each of the complex protein supplements were tested to determine lot-to-lot variability of observed components.
Mouse embryo assay
The Mayo Clinic Institutional Animal Care and Use Committee (IACUC) approved all procedures involving animals. All mice where obtained from Charles River Laboratories. Six to nine week old FVB female mice were superovulated with 5 IU of intraperitoneal pregnant mare serum (PMS; NHPP) followed 48 h later with 5 IU of intraperitoneal human chorionic gonadotropin (hCG; APP Pharmaceuticals). Females were individually caged with male CF1 mice overnight and mating was confirmed by the presence of a vaginal plug. Cumulus surrounded zygotes were isolated from oviducts obtained 18 h after hCG.
After denuding zygotes with hyaluronidase (250 u/mL; H3506 Sigma Chemical), embryos were transferred individually into 25 μL of Global medium containing one of the supplements in an EmbryoSlide (Unisense Fertilitech, Aarhus, Denmark) and each slide was inserted into an EmbryoScope (Unisense Fertilitech). A 6x2 factorial study was performed with 6 protein supplements (AlbIX, Buminate, HSA, LGPS, SPS, SSS) cultured at 20 % or 5 % oxygen. All experiments were performed in triplicate at 37 ° C and 6.2 % CO2 (pH of 7.2–7.3) with 10–11 embryos for each protein/condition combination. Expanded blastocyst at 96 h of culture was the primary endpoint. Data for precise cell division timings using time-lapse imaging were also obtained.
Quantitative PCR
Hatching blastocysts were frozen at −80 °C in four groups of four blastocysts per treatment in lysis buffer. RNA was extracted using the PicoPure RNA Isolation Kit (Applied Biosystems; Carlsbad, CA) with on-column DNase treatment (Qiagen; Valencia, CA). Total RNA extracted from blastocysts was loaded into the cDNA reaction. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) following the manufacture’s protocol. Primer design and qPCR were performed as previously described [17, 18]. Primers for genes previously correlated to embryo viability (bone morphogenic protein 15, Bmp15; caudal-type homeobox protein 2, Cdx2; cyclooxgenase 2, Cox2; DNA methyltransferase 3a, Dnmt3a; glutaredoxin 2, Glrx2; glucose transporter 1, Glut1; glucose regulated protein 78, Grp78; octamer-binding protein 4, Oct4; and placenta specific 8, Plac8) were designed for qPCR using Primer3 [19]. Accession number, primer sequence, and product length of target and reference genes are presented in Supplemental Table 1. Primer specificity was determined by melt curve analysis and PCR products were cloned into pCR 2.1 TOPO vectors and transformed into One Shot TOP10 chemically competent E. coli (Invitrogen Life Technologies; Grand Island, NY). Plasmids were sequenced by the DNA sequencing facility at Colorado State University (Fort Collins, CO) to confirm the identity of the transcript and plasmids were quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen Life Technologies). Quantitative PCR (qPCR) was performed on three-fold diluted sample cDNA run in duplicate. Target genes were analyzed using iQ SYBR Green Supermix (Bio-Rad; Hercules, CA, USA) and an Applied Biosystems 7300 Real Time PCR system. A reference gene was analyzed in each sample for accurate relative quantification and a standard curve was generated from serial dilutions of EcoRI digested plasmids (107 to 101 molecules).
Data and statistical analysis
Developmental and time-lapse data were analyzed using a one-way analysis of variance (ANOVA) with protein and oxygen (5 vs 20 %) in the model. Differences in blastocyst formation and cell cycle timings were determined with Dunnett’s test for pair-wise comparisons with AlbIX as the control. Statistical analyses were performed using JMP statistical software (SAS Institute, Cary, NC). For relative quantification of transcript abundance in hatching blastocysts, data were analyzed using the relative expression software tool, REST 2005 version 1.9.12 [20], as previously described [17, 18, 21, 22]. 18 s ribosomal RNA (18 s rRNA) was used as the reference gene as transcript abundance was not significantly different between treatment groups. Expression ratios of the target and reference genes were generated from Eq. 1 using the efficiency of the qPCR reactions (E) and the ΔCT values of the control and sample (treatment) oocytes. The levels of significance were calculated by pair-wise fixed reallocation randomization tests with 50,000 iterations and significance was determined with a P-value less than 0.05.
| 1 |
Results
Protein supplement composition
Glucose and organic acids
Two of the complex proteins (LGPS and SPS) contained citrate (Table 1) that was present at similar concentrations between the two lots tested. All of the supplements other than AlbIX contained the stabilizer octanoic acid at levels appropriate for the albumin concentration (Leonard et al., 2013). Other organic acids and glucose were not detected.
Table 1.
Concentrations of organic acids in protein supplements (values represent concentration in stock solutions as provided by manufacturer)
| AlbIX | Buminate | HSA | LGPS | SPS | SSS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lot 1 | Lot 2 | Lot 1 | Lot 2 | Lot 1 | Lot 2 | ||||
| Citrate (mM) | 0 | 0.2 | 0 | 2.5 | 2.9 | 2.3 | 2.9 | 0.1 | 0.1 |
| Lactate (mM) | 0 | 0 | 0 | 0.2 | 0.2 | 0.3 | 0.3 | 0 | 0 |
| Pyruvate (mM) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Octanoate (mM) | 0 | 6.6 | 10.1 | 5.4 | 5.8 | 5.2 | 6.6 | 4.2 | 4.4 |
Amino acids
No amino acids were detected in two of the albumin products, AlbIX and HSA. Low concentrations were present in Buminate, LGPS and SPS (Table 2). In contrast, SSS had high concentrations of both essential and non-essential amino acids. These concentrations were similar between the two lots tested and were highest for lysine.
Table 2.
Amino acid concentrations in protein supplements (uM; values represent concentration in stock solutions as provided by manufacturer)
| AlbX | Buminate | HSA | LGPS | SPS | SSS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Essential | |||||||||
| Arg | 0 | 49 | 0 | 19 | 20 | 28 | 31 | 402 | 438 |
| Cys | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| His | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 77 | 93 |
| Ile | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 103 | 118 |
| Leu | 0 | 31 | 0 | 0 | 0 | 16 | 13 | 382 | 441 |
| Lys | 0 | 44 | 0 | 13 | 15 | 24 | 20 | 619 | 684 |
| Met | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 12 |
| Phe | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 126 | 149 |
| Thr | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 120 | 143 |
| Trp | 0 | 13 | 0 | 0 | 0 | 14 | 12 | 80 | 90 |
| Tyr | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 155 | 177 |
| Val | 0 | 25 | 0 | 12 | 13 | 18 | 15 | 222 | 261 |
| Non-essential | |||||||||
| Ala | 0 | 37 | 0 | 22 | 24 | 37 | 30 | 383 | 436 |
| Asn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 23 |
| Asp | 0 | 15 | 0 | 0 | 0 | 21 | 12 | 60 | 73 |
| Glu | 0 | 0 | 0 | 0 | 0 | 12 | 11 | 49 | 60 |
| Gln | 0 | 10 | 0 | 15 | 14 | 0 | 0 | 166 | 191 |
| Gly | 0 | 28 | 0 | 17 | 18 | 26 | 23 | 180 | 215 |
| Pro | 0 | 18 | 0 | 14 | 15 | 36 | 26 | 223 | 257 |
| Ser | 0 | 23 | 0 | 0 | 0 | 13 | 10 | 293 | 331 |
Elements
Concentrations of chloride and sodium, constituents of saline used as the carrier for albumin, were similar among the human products but were higher for AlbIX (Table 3). Electrolytes common to culture media (Morbeck et al., 2014), such as calcium, potassium and magnesium were absent or present at concentrations that would have a minimal effect on the final working concentration in culture medium. This is in contrast to phosphorus, which was high in SSS (1.55 mM) and AlbIX (6.45 mM) versus the other supplements.
Table 3.
Electrolytes, metals and transferrin in protein supplements (values represent concentration in stock solutions as provided by manufacturer)
| Reference | AlbIX | Buminate | HSA | LGPS | SPS | SSS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range, Serum | Lot 1 | Lot 2 | Lot 1 | Lot 2 | Lot 1 | Lot 2 | ||||
| Calcium (mM) | 2.1–2.6 | 0 | 0 | 0 | 0.7 | 0.7 | 0.8 | 0.7 | 0 | 0 |
| Phosphorus (mM) | 0.9–1.4 | 82.0 | 0 | 0 | 0.16 | 0.17 | 0.19 | 0.16 | 1.55 | 1.81 |
| Potassium (mM) | 3.7–5.2 | 0 | 0 | 0 | 0.19 | 0.18 | 0.21 | 0.19 | 0 | 0 |
| Chloride (mM) | 96–106 | 202 | 94 | 119 | 100 | 99 | 109 | 107 | 131 | 131 |
| Sodium (mM) | 135–145 | 238 | 149 | 144 | 152 | 144 | 156 | 165 | 159 | 154 |
| Magnesium (mM) | 0.65–1.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Copper (mg/L) | 0.75–1.45 | 0 | 0.09 | 0.11 | 0.22 | 0.22 | 0.23 | 0.21 | 0.62 | 0.63 |
| Iron (mcg/L) | 600–1,700 | 126 | 523 | 297 | 1,195 | 1,138 | 1,150 | 1,176 | 206 | 215 |
| Selenium (mcg/L) | 0.5–1.4 | 0 | 40 | 79 | 42 | 45 | 40 | 42 | 75 | 69 |
| Zinc (mg/L) | 0.7–1.5 | 0 | 0.22 | 0.39 | 0.19 | 0.19 | 0.21 | 0.20 | 0.41 | 0.42 |
| Aluminum (mcg/L) | 0–6 | 0 | 25 | 0 | 80 | 77 | 817 | 85 | 9 | 9 |
| Chromium (mcg/L) | <0.3 | 4.7 | 1.5 | 2.9 | 5.5 | 5.5 | 7.7 | 5.7 | 1.4 | 1.3 |
| Cobalt (mcg/L) | 0–0.9 | 0 | 0.3 | 0 | 0.3 | 0.3 | 0.4 | 0.3 | 0.6 | 0.6 |
| Manganese (mcg/L) | 0.6–2.3 | 2.0 | 3.3 | 0.9 | 2.6 | 2.7 | 17.6 | 2.5 | 2.8 | 2.9 |
| Vanadium (mcg/L) | 0–0.9 | 0 | 117 | 21 | 68 | 68 | 68 | 110 | 11 | 11 |
| Transferrin (mg/dL) | 200–360 | 0 | 0 | 0 | 268 | 253 | 254 | 255 | 24 | 37 |
Reference range: http://www.nlm.nih.gov/medlineplus/ency/
Significant levels of trace metals were present in all 6 protein supplements with iron the most notable. AlbIX had the lowest iron concentration (126 mcg/L) whereas LGPS and SPS had >1 mg/L iron, levels that would result in >100 mcg/mL in culture medium if used at the standard 10 % v:v dilution. Chromium and manganese were present at concentrations 10-fold higher than found in culture medium; these levels would effectively double the concentration present during embryo culture after supplementation. Other trace elements present in human-derived protein supplements included copper, selenium, aluminum, and vanadium. Lot variation was minimal for all metals/electrolytes except aluminum, manganese and vanadium, which were 10-fold, 5-fold and 2-fold higher, respectively, in one lot of SPS relative to the other lot of SPS.
Three of the supplements, Buminate, LGPS and SPS, were notably pro-oxidant, with substantially higher concentrations of the transition metals copper, iron and vanadium. In addition, LGPS and SPS had higher levels of aluminum relative to the other supplements. AlbIX had the lowest overall amount of transition metals. The iron binding protein, transferrin, was detected in the complex protein supplements, present at plasma levels in SPS and LGPS (250–270 mg/dL) and low levels in SSS (24–37 mg/dL).
Embryo development
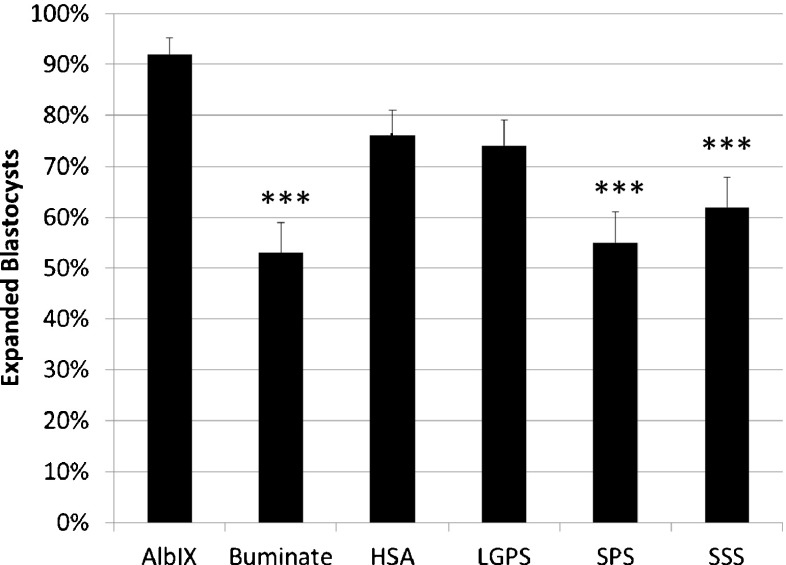
Embryo development was compared among the protein supplements at both 5 % and 20 % oxygen. While blastocyst development trended higher in 5 % oxygen (data not shown), the difference was not statistically significant in the model that included protein and oxygen as independent variables. Since there also was no protein by oxygen interaction, blastocyst development for the two oxygen concentrations was grouped for analysis. Significantly more embryos developed to the expanded blastocyst stage by 96 h of culture in the AlbIX group compared to embryos cultured with Buminate, SPS, or SSS (p < 0.001; Fig. 1). Compared to AlbIX, a similar number of embryos in the HSA (p = 0.10) and LGPS (p = 0.05) developed to the expanded blastocyst stage.
Fig. 1.
Development of individually cultured one-cell mouse embryos to the expanded blastocyst stage at 96 h cultured with six protein supplements. Each bar represents data from 6 replicates of 10–11 embryos per replicate. ***p < 0.001 vs AlbIX
Since oxygen concentration might interact with protein supplements that have high levels of pro-oxidant transition metals (iron, chromium and vanadium), we compared blastocyst development and cell cycle durations between control supplements (AlbIX, HSA and SSS) and supplements containing high levels of pro-oxidant transition metals (Buminate, LGPS, SPS). The duration of the first and second cell cycles were increased (p < 0.01 and p < 0.05, respectively) and the percentage of embryos that developed to the blastocyst stage decreased (p < 0.001) for embryos cultured in pro-oxidant supplements in 20 % oxygen (Table 4). This effect was not observed at 5 % oxygen.
Table 4.
Duration of the 2nd and 3rd cell cycles and blastocyst development (mean ± se) in 5 or 20 % oxygen for proteins with low (Control; AlbIX, HSA, SSS) and high (Pro-oxidant; Buminate, LGPS, SPS) concentrations of transition metals
| 5 % Oxygen | 20 % Oxygen | |||
|---|---|---|---|---|
| Control | Pro-oxidant | Control | Pro-oxidant | |
| cc2 (h) | 24.0 ± 0.3 | 24.7 ± 0.4 | 23.7 ± 0.5 | 26.2 ± 0.6** |
| cc3 (h) | 10.3 ± 0.2 | 10.6 ± 0.1 | 11.4 ± 0.3 | 12.4 ± 0.3* |
| Blastocyst rate (%) | 75.7 ± 4.2 | 67.6 ± 4.7 | 78.2 ± 4.1 | 53.5 ± 5.0*** |
*p < 0.05, **p < 0.01, ***p < 0.001 for Control vs Pro-oxidant within oxygen group
Time-lapse imaging provides an assessment of embryo development that is more sensitive than blastocyst development as well as providing evidence for either early or late effects on embryo growth. Though the time of the first cleavage to the 2-cell stage was similar among the protein groups (Supplemental Table 2), there was a trend for a shorter interval that favored AlbIX. Pre-compaction development was therefore assessed relative to time at the 2-cell stage (Supplemental Fig. 1). Relative to AlbIX, the second cell cycle (cc2; t3-t2) was delayed by Buminate (p < 0.001), SSS (p < 0.001) and SPS (p < 0.01). A cumulative effect resulted in a difference in time to the 8-cell stage of 2 to 6 h (Supplemental Table 2) that was significant for Buminate, SSS, LGPS and SPS (p < 0.001) but not for HSA (p = 0.06). A comparison of ART-only products (HSA, SSS, LGPS, SPS) illustrates that SSS caused a significant delay in pre-compaction cell divisions (Supplemental Fig. 1).
Gene expression
There was no significant difference in transcript abundance for Bmp15, Cox2, Dnmt3a, Glrx2, Oct4 or Grp78 when comparing gene expression of hatching blastocysts cultured in AlbIX, Buminate, HSA, LGPS, SPS or SSS at 5 % oxygen (p > 0.05). There was a significant increase in transcript abundance of Cdx2 in blastocysts cultured at 5 % oxygen in LGPS compared to blastocysts cultured in Buminate or SSS (p < 0.05; Supplemental Table 3). In 5 % oxygen, abundance of Glut1 was significantly increased in blastocysts cultured in SSS compared to LGPS (p < 0.05; Supplemental Table 4). Under optimal environmental conditions (5 % O2), expression of Plac8 was the most variable between blastocysts cultured with different protein supplements (Supplemental Table 5). There was a significant decrease in abundance of Plac8 in blastocysts cultured in Buminate, HSA or SSS compared to blastocysts cultured in AlbIX (p < 0.05).
Abundance of Plac8 was significantly increased in blastocysts cultured in SPS and LGPS (p < 0.05) compared to those cultured in Buminate (Supplemental Table 5). Furthermore, Plac8 abundance was significantly decreased in blastocysts cultured in SSS compared to blastocysts cultured in SPS or LGPS (p < 0.05; Supplemental Table 5).
At 20 % oxygen, there was no difference in transcript abundance in hatching blastocysts for Cdx2, Cox2, Dnmt3a, Glrx2, Grp78, Oct4 or Glut1 between protein supplements (p > 0.05). There was a significant increase in transcript abundance of Bmp15 in blastocysts cultured in SSS compared to blastocysts cultured in SPS (p < 0.05; Supplemental Table 6). There was a significant increase in Plac8 abundance in blastocysts cultured in HSA compared to blastocysts cultured in Buminate or SSS (p < 0.05; Supplemental Table 7).
When analyzing the qPCR data between O2 environments, there was no difference in transcript abundance for any of the genes analyzed within protein supplement for Buminate and HSA (p > 0.05; Supplemental Fig. 2). Blastocysts cultured in SPS where the most affected by O2 environment in terms of gene expression. Abundance of Glrx2 and Plac8 were significantly increased (p < 0.05) in blastocysts cultured at 5 % oxygen compared to blastocysts cultured at 20 % oxygen when SPS was utilized as the protein source (Supplemental Fig. 2).
Discussion
In this study we demonstrate that protein supplements contain elements and amino acids that vary among products used for embryo culture, components that also vary from lot-to-lot. In addition, human derived protein supplements slow pre-compaction mouse embryo development and result in reduced blastocyst formation relative to stabilizer-free recombinant HSA. Protein supplements in mouse culture medium also alter expression of genes known to be related to embryo quality in the resulting blastocysts. Even under optimal oxygen environment, Buminate and SSS reduced expression of several of these genes, while SPS, LGPS and AlbIX increased their expression, suggesting that protein supplement influences embryo quality. Numerous variables impact IVF outcomes and the importance of the quality of the embryo culture environment cannot be understated. Undefined products such as complex protein supplements represent a significant gap in our understanding of this environment. This study is a first step in providing a comprehensive definition of protein supplements in order to assure quality.
Protein is commonly added to culture media for human IVF and embryo culture, with HSA or a complex protein containing albumin and α- and β-globulins the two most common supplements. Two of the products, SPS and LGPS, are equivalent to USP Plasma Protein Fraction (PPF) whereas Irvine SSS is a mixture of USP HSA (84 %) and USP Human Normal Immunoglobulin (16 %). At 10 % v:v final dilution, SSS contains more albumin (5 mg/ml) than the two PPF products (4.4 mg/ml). The first reports of PPF for human IVF [12, 23] were followed closely by Irvine Scientific’s development of SSS [13]. Albumin from human serum was first used for embryo culture by Yves Menezo [11] and today is the most common protein supplement in use worldwide, though complex protein supplements are popular in the United States. Recombinant HSA (rHSA) is also commercially available [15], though its use is limited, perhaps due to additional cost without evidence of improved results.
All USP protein products contain stabilizers to protect the protein from oxidation during heat inactivation of viruses. One of these stabilizers, octanoic acid, varies among lots of HSA and adversely affects mouse embryo development [4]. In addition to batch variation, choice of protein supplement and concentration varies considerably among clinics, with one study adding SSS to media pre-supplemented with HSA [14]. Coupled with our findings on undefined contaminants, protein should be considered an important source of inter- and intra-clinic variation.
The high concentration of amino acids present in SSS was an unexpected finding. Free amino acids are present in plasma [24] but are not detected in HSA or PPF, indicating that the purification process for these proteins removes plasma amino acids. The source of amino acids in SSS is therefore unknown. Since the concentrations are high enough to alter the underlying composition of selected culture media, most of which contain a specific mix of both essential and non-essential amino acids [25], their source and potential impact on culture merit further investigation.
We did not obtain standard blastocyst development (80 % in our laboratory with inbred or hybrid mouse strains) for any protein other than the recombinant albumin, AlbIX. This could be because 1-cell mouse embryos are more sensitive to in vitro stress when cultured individually versus group culture (unpublished data). Given our previous report on improved sensitivity of mouse one-cell individual embryo culture to detect oil toxicity [26], this finding illustrates that individual culture should be considered as a more sensitive method of detecting toxicity in standard quality control testing.
Effects on embryo development, particularly at 20 % oxygen, may be due in part to contamination with pro-oxidant metals, resulting in an overtly pro-oxidant environment leading to an increase in DNA strand breaks [27]. Trace elements were detected in every protein supplement tested, with chromium, iron, manganese, selenium, and vanadium at concentrations higher than the normal range for human serum/plasma. Copper, present at levels considered normal for human serum, is another transition metal and was highest in the complex protein supplements (200–600 mcg/mL). The element aluminum, which has no known biological function, was also present at high concentrations and likely a contaminant introduced during the manufacturing process. The trace elements are considered essential for cell biology and are usually bound to proteins to form metallo- and selenoproteins [28]. Chromium, copper, iron, manganese and vanadium are transition metals which, along with the nonmetal selenium, contain unpaired electrons, rendering them highly reactive [29], particularly in cell culture [30]. Because of this oxidative reactivity, transition metals and selenium can participate in redox reactions that generate reactive oxygen species (ROS). Each of these elements has demonstrated toxicity in humans [31–33].
Little is known about trace element levels in proteins approved for use for embryo culture; however, metal ions such as aluminum, copper, iron and vanadium are known contaminants of commercial HSA [34]. The European Pharmacopeia (EP) sets limits for aluminum in HSA to < 200 ug/L. A test for aluminum is not included in the US Pharmacopeia’s (USP) monograph for HSA. In contrast, both the EP and USP limit the amount of heme in HSA. Heme is the prosthetic group of metalloproteins that contains iron (Fe2+) and porphyrin which, when released from the protein, is a highly reactive pro-oxidant [35]. In contrast, neither EP nor USP require testing for aluminum or heme in Immune Globulin (SSS), or USP’s Plasma Protein Fraction (LGPS, SPS). We found that one lot of SPS had significantly higher aluminum than the limit set by the EP for HSA. We also found iron was elevated in the products that were not tested for heme. The form of iron in protein supplements is not known, but is likely present as heme, free iron bound to albumin, transferrin and iron chelates, all of which are readily available for redox reactions and production of ROS [36]. Since iron and transferrin were both high in SPS and LGPS, the amount of free iron that may be reactive is reduced and harmful effects potentially mitigated.
Oxidative stress in cell culture can result from an imbalance of antioxidants and pro-oxidants [37] and is a concern for culture of cells in vitro [30, 38]. Embryo culture media contain several antioxidants, either as additives or essential components of the media’s composition [39] and are present at varying concentrations [25]. Antioxidants serve two functions: binding potential pro-oxidants and/or scavenging hydrogen peroxide (H2O2) and reactive oxygen species [40]. Given the variation in composition of culture media and protein supplements, the balance of pro- and antioxidant compounds and the resulting REDOX state of the culture environment is thus unknown.
Albumin is a potent antioxidant because it can bind pro-oxidants, such as metals, and scavenge ROS, but the latter is affected by the REDOX state of the albumin molecule. Albumin contains a primary oxidation site, Cys34, as well as 6 methionine residues that provide additional anti-oxidant potential [40]. However, this powerful antioxidant capacity is not consistent from lot-to-lot of protein [16], where some lots of HSA or rHSA contain an increased percentage of albumin in an oxidized state. It is apparent that the quality of the culture environment in terms of REDOX potential is dependent on both the amount of metals present in the supplement and the oxidation state of albumin. Furthermore, we demonstrate a potential interplay between oxygen concentration and transition metals since embryo development was compromised in supplements with high concentrations of pro-oxidant metals during culture at ambient oxygen.
One of the goals of this research is to define the undefined: identification of factors that contribute to variable quality. A limitation of this work is that we cannot determine cause and effect of any of the identified compounds. This limits the present work to be descriptive in nature. Along with research on the REDOX state of albumin [16] and our previous work on octanoic acid [4], there are now several candidates for further quality control testing. Several questions remain unanswered, however. For instance, why does SSS have high concentrations of amino acids and phosphorus, present at levels that will significantly alter the known amino acid composition of culture media [25] and may vary from lot-to-lot? Furthermore, microRNAs are present in SPS used for human embryo culture [41] and hormones and cytokines are also present in protein supplements [3, 42]. These remain as further undefined and unmonitored components that may affect embryo development. These findings are also of relevance to the field of stem cell derivation, where protein lot variation alters results observed between different laboratories [43–46].
In this work, we demonstrate that different protein supplements used for human clinical embryo culture affect blastocyst development and gene expression in the mouse. While the mechanism for these differences is unknown, we illustrate considerable variation among protein supplements and most notably, high concentrations of pro-oxidant transition metals. These compounds can vary from lot-to-lot and may have significant effects on clinical outcomes. The impact of high levels of transition metals may be particularly relevant for laboratories that culture in a pro-oxidant environment of 20 % oxygen.
Supported by a grant from Mayo Clinic Department of Obstetrics and Gynecology and equipment loan from Unisense Fertilitech.
Electronic supplementary material
(DOC 38 kb)
(DOC 39 kb)
(DOC 34 kb)
(DOC 34 kb)
(DOC 33 kb)
(DOC 35 kb)
(DOC 35 kb)
Specific cell division timings (hours; mean±se) from the 2-cell stage to 3-, 4-, 5- and 8 cells for embryos cultured with different protein supplements. Time to the 3-cell stage relative to AlbIX was longer for SPS (p<0.01) and Buminate and SSS (p<0.001). Time to 8-cell was delayed relative to AlbIX for Buminate, SSS, SPS and LGPS (p<0.01). (PPT 201 kb)
Gene expression analyses, relative to 18s rRNA, for hatching mouse blastocysts cultured in 5 mg/mL AlbIX,10% Buminate, 5 mg/mL HSA, 10% LGPS, 10% SPS, or 10% SSS at 5% O2 compared to 20% O2. Positive fold changes indicate an increase in transcript abundance in embryos cultured at 20% O2 as compared to 5% O2, whereas a negative fold change indicates a decrease in transcript abundance in embryos cultured at 20% O2 as compared to 5% O2. *, P < 0.05; ‡, P < 0.1. (DOC 190 kb)
Footnotes
Capsule
Protein supplements for IVF and embryo culture are highly heterogeneous and poorly defined. Some components of supplements adversely affect mouse embryo development and these effects are dependent on oxygen concentration used for culture.
References
- 1.Kane MT. Variability in different lots of commercial bovine serum albumin affects cell multiplication and hatching of rabbit blastocysts in culture. J Reprod Fertil. 1983;69:555–8. doi: 10.1530/jrf.0.0690555. [DOI] [PubMed] [Google Scholar]
- 2.McKiernan SH, Bavister BD. Different lots of bovine serum albumin inhibit or stimulate in vitro development of hamster embryos. In Vitro Cell Dev Biol. 1992;28A:154–6. doi: 10.1007/BF02631084. [DOI] [PubMed] [Google Scholar]
- 3.Meintjes M. Media composition: macromolecules and embryo growth. Methods Mol Biol. 2012;912:107–27. doi: 10.1007/978-1-61779-971-6_8. [DOI] [PubMed] [Google Scholar]
- 4.Leonard PH, Charlesworth MC, Benson L, Walker DL, Fredrickson JR, Morbeck DE. Variability in protein quality used for embryo culture: embryotoxicity of the stabilizer octanoic acid. Fertil Steril. 2013;100:544–9. doi: 10.1016/j.fertnstert.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Yu MW, Finlayson JS. Quantitative determination of the stabilizers octanoic acid and N-acetyl-DL-tryptophan in human albumin products. J Pharm Sci. 1984;73:82–6. doi: 10.1002/jps.2600730122. [DOI] [PubMed] [Google Scholar]
- 6.Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1:91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- 7.Blake D, Svalander P, Jin M, Silversand C, Hamberger L. Protein supplementation of human IVF culture media. J Assist Reprod Genet. 2002;19:137–43. doi: 10.1023/A:1014788821965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batt PA, Gardner DK, Cameron AW. Oxygen concentration and protein source affect the development of preimplantation goat embryos in vitro. Reprod Fertil Dev. 1991;3:601–7. doi: 10.1071/RD9910601. [DOI] [PubMed] [Google Scholar]
- 9.Caro CM, Trounson A. The effect of protein on preimplantation mouse embryo development in vitro. J In Vitro Fert Embryo Transf. 1984;1:183–7. doi: 10.1007/BF01139212. [DOI] [PubMed] [Google Scholar]
- 10.Leveille MC, Carnegie J, Tanphaichitr N. Effects of human sera and human serum albumin on mouse embryo culture. J Assist Reprod Genet. 1992;9:45–52. doi: 10.1007/BF01204114. [DOI] [PubMed] [Google Scholar]
- 11.Menezo Y, Testart J, Perrone D. Serum is not necessary in human in vitro fertilization, early embryo culture, and transfer. Fertil Steril. 1984;42:750–5. doi: 10.1016/s0015-0282(16)48202-6. [DOI] [PubMed] [Google Scholar]
- 12.Adler A, Reing AM, Bedford JM, Alikani M, Cohen J. Plasmanate as a medium supplement for in vitro fertilization. J Assist Reprod Genet. 1993;10:67–71. doi: 10.1007/BF01204443. [DOI] [PubMed] [Google Scholar]
- 13.Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J Assist Reprod Genet. 1995;12:354–60. doi: 10.1007/BF02215726. [DOI] [PubMed] [Google Scholar]
- 14.Meintjes M, Chantilis SJ, Ward DC, Douglas JD, Rodriguez AJ, Guerami AR, et al. A randomized controlled study of human serum albumin and serum substitute supplement as protein supplements for IVF culture and the effect on live birth rates. Hum Reprod. 2009;24:782–9. doi: 10.1093/humrep/den396. [DOI] [PubMed] [Google Scholar]
- 15.Bungum M, Humaidan P, Bungum L. Recombinant human albumin as protein source in culture media used for IVF: a prospective randomized study. Reprod Biomed Online. 2002;4:233–6. doi: 10.1016/S1472-6483(10)61811-1. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki J, Nagai Y, Matsuyama Y, Terada T, Era S. The redox state of recombinant human serum albumin and its optimal concentration for mouse embryo culture. Syst Biol Reprod Med. 2013;59:48–52. doi: 10.3109/19396368.2012.727946. [DOI] [PubMed] [Google Scholar]
- 17.Paczkowski M, Yuan Y, Fleming-Waddell J, Bidwell CA, Spurlock D, Krisher RL. Alterations in the transcriptome of porcine oocytes derived from prepubertal and cyclic females is associated with developmental potential. J Anim Sci. 2011;89:3561–71. doi: 10.2527/jas.2011-4193. [DOI] [PubMed] [Google Scholar]
- 18.Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod. 2013;88:111. doi: 10.1095/biolreprod.113.108548. [DOI] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Ida JM, Paczkowski M, Krisher RL. Identification of developmental competence-related genes in mature porcine oocytes. Mol Reprod Dev. 2011;78:565–75. doi: 10.1002/mrd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva E, Paczkowski M, Krisher RL. The effect of leptin on maturing porcine oocytes is dependent on glucose concentration. Mol Reprod Dev. 2012;79:296–307. doi: 10.1002/mrd.22029. [DOI] [PubMed] [Google Scholar]
- 23.Pool TB, Martin JE. High continuing pregnancy rates after in vitro fertilization-embryo transfer using medium supplemented with a plasma protein fraction containing alpha- and beta-globulins. Fertil Steril. 1994;61:714–9. doi: 10.1016/s0015-0282(16)56651-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaspar H, Dettmer K, Gronwald W, Oefner PJ. Automated GC-MS analysis of free amino acids in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:222–32. doi: 10.1016/j.jchromb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102:759–66 e9. doi: 10.1016/j.fertnstert.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Wolff HS, Fredrickson JR, Walker DL, Morbeck DE. Advances in quality control: mouse embryo morphokinetics are sensitive markers of in vitro stress. Hum Reprod. 2013;28:1776–82. doi: 10.1093/humrep/det102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meuter A, Rogmann LM, Winterhoff BJ, Tchkonia T, Kirkland JL, Morbeck DE. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J Assist Reprod Genet. 2014 epub. [DOI] [PMC free article] [PubMed]
- 28.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46:531–42. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37:99–105. doi: 10.4103/2319-4170.128725. [DOI] [PubMed] [Google Scholar]
- 31.Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. 2005;26:235–44. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Bal W, Kasprzak KS. Induction of oxidative DNA damage by carcinogenic metals. Toxicol Lett. 2002;127:55–62. doi: 10.1016/S0378-4274(01)00483-0. [DOI] [PubMed] [Google Scholar]
- 33.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan GJ, Coudray C, Hubbard A, Gutteridge JM. Vanadium and copper in clinical solutions of albumin and their potential to damage protein structure. J Pharm Sci. 1992;81:611–4. doi: 10.1002/jps.2600810703. [DOI] [PubMed] [Google Scholar]
- 35.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–37. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 36.Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–4. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/S0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 39.Combelles CM, Hennet ML. Media composition: antioxidants/chelators and cellular function. Methods Mol Biol. 2012;912:129–59. doi: 10.1007/978-1-61779-971-6_9. [DOI] [PubMed] [Google Scholar]
- 40.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–7. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. Human embryos secrete microRNAs into culture media-a potential biomarker for implantation. Fertil Steril. 2014;101:1493–500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 42.Menezo Y, Khatchadourian C. Peptides bound to albumin. Life Sci. 1986;39:1751–3. doi: 10.1016/0024-3205(86)90094-9. [DOI] [PubMed] [Google Scholar]
- 43.Akopian V, Andrews PW, Beil S, Benvenisty N, Brehm J, Christie M, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim. 2010;46:247–58. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Gonzalo FR, Izpisua Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skottman H, Hovatta O. Culture conditions for human embryonic stem cells. Reproduction. 2006;132:691–8. doi: 10.1530/rep.1.01079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 38 kb)
(DOC 39 kb)
(DOC 34 kb)
(DOC 34 kb)
(DOC 33 kb)
(DOC 35 kb)
(DOC 35 kb)
Specific cell division timings (hours; mean±se) from the 2-cell stage to 3-, 4-, 5- and 8 cells for embryos cultured with different protein supplements. Time to the 3-cell stage relative to AlbIX was longer for SPS (p<0.01) and Buminate and SSS (p<0.001). Time to 8-cell was delayed relative to AlbIX for Buminate, SSS, SPS and LGPS (p<0.01). (PPT 201 kb)
Gene expression analyses, relative to 18s rRNA, for hatching mouse blastocysts cultured in 5 mg/mL AlbIX,10% Buminate, 5 mg/mL HSA, 10% LGPS, 10% SPS, or 10% SSS at 5% O2 compared to 20% O2. Positive fold changes indicate an increase in transcript abundance in embryos cultured at 20% O2 as compared to 5% O2, whereas a negative fold change indicates a decrease in transcript abundance in embryos cultured at 20% O2 as compared to 5% O2. *, P < 0.05; ‡, P < 0.1. (DOC 190 kb)