Abstract
Purpose
Fertility preservation strategies warrant non-invasive viability assessment of preantral follicles (PAF) such as staining with Neutral Red (NR) that is incorporated by viable follicles. To optimize the procedure, we firstly determined the lowest concentration and shortest exposure time needed for optimal viability screening of isolated bovine PAF. Secondly, we combined this protocol to a vitrification procedure to assess cryotolerance of the stained follicles.
Methods
Isolated PAF (900, divided over 6 replicates) were cultured in DMEM/Ham’s F12 (Culture Medium - Cm) for 4 days (38.5 °C, 5 % CO2). On D0, D2 and D4, follicles were stained, by adding NR medium (NRm = Cm with different concentrations NR) after which viability was assessed by counting stained/non-stained PAF every 30 min for a period of 2 h.
Results
Following a binary logistic regression analysis with staining as a result (yes/no) versus log-concentration, a probability model could be fitted, indicating that the proportion of stained follicles remained stable after 30 min when 15 μg/ml NR was used, without compromising follicular health and viability. Consequently, using this protocol, no significant effect of staining prior to vitrification, was found on PAF viability immediately after warming or following 4 days of culture.
Conclusions
In conclusion, we propose NR staining as a non-invasive, non-detrimental viability assessment tool for PAF, when applied at 15 μg/ml for 30 min, being perfectly compatible with PAF vitrification.
Keywords: Viability assessment, Isolated (bovine) preantral follicles, Vitrification, Neutral red (NR)
Introduction
Preantral follicle physiology (PAF) is an intriguing field of study from a fertility preservation point of view [1, 2]. They represent an untapped source of reproductive potential, offering hope to women suffering from a decreased fertility [3]. However, limited knowledge about PAF dynamics, combined to a lack of standardized non-invasive retrieval and viability assessment protocols [3, 4] and reliable cryopreservation procedures are hampering progress of clinical applications. An efficacious integration of these different processes is a prerequisite for success, and therefore, their interactions need to be studied and characterized. Because human tissue is only scarcely available, the bovine ovary was used as a well-established in vitro model for pre-implantation reproductive research [5–7].
While the method of retrieval can have a huge impact on follicle quality [8, 9], this paper will focus on the nexus ‘follicle viability testing’ and its possible effect on ‘cryopreservation through vitrification’. Assessment of follicle viability is crucial to increase the efficiency of fertility preservation strategies [10]. Until now, mostly invasive methods using light or transmission electron microscopy are applied to tissue fragments to assess follicle viability by extrapolation [11, 12]. As a consequence, studied follicles are destroyed and rendered useless for subsequent culture or transplantation purposes. To our knowledge, ‘follicle saving’ protocols, allowing repeated non-invasive follicle viability assessment during culture, haven’t been reported yet.
Quite recently [10, 13], Neutral Red (NR), a water-soluble and non-toxic dye was proposed as a non-invasive tool to perform live/dead assays [14–16], based on the ability of living cells to incorporate it in their lysosomes [10, 15]. However, nothing is known about the effect of NR staining on the viability of subsequent cryopreserved PAF [17]. Cryopreservation strategies can involve the whole ovary [18–20], pieces of ovarian cortex [7, 21, 22] or isolated follicles. Based on earlier work [7], vitrification was chosen, although, to our knowledge, specific data on the use of isolated, bovine early PAF in this context are lacking.
Aiming to contribute in a clinical context, we hypothesized that NR staining, as a non-invasive tool for (repeated) viability testing, had no effect on the viability of mechanically isolated PAF after vitrification. Therefore, in the present study we aimed: 1) to determine the lowest NR concentration and shortest incubation time allowing a safe viability assessment of mechanically isolated, early preantral bovine follicles, 2) to confirm the relative non-toxicity of NR when repeatedly used during culture in appropriate dosage, for a limited time period and 3) to study the possible effects of the optimized NR protocol on follicle viability following vitrification.
Materials and methods
Collection of ovaries and isolation of early PAFs
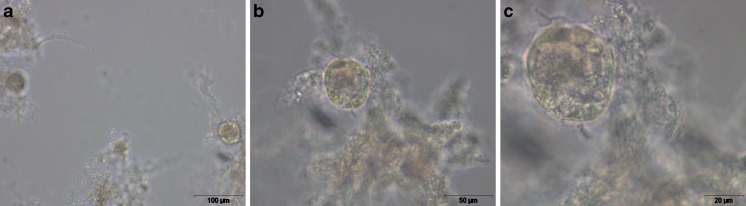
All chemicals were purchased from Life Technologies, unless stated otherwise. Batches (10–40) bovine ovaries were collected at local slaughterhouses and transported to the laboratory in physiologic saline (0.9 % NaCl, Braun) at 25 °C. Upon arrival, 3–4 ovaries were selected, free of abundant antral follicles or a corpus luteum. Following removal of the adnexa, the ovaries were washed in warm physiologic solution supplemented with Kanamycin (0.25 %). Follicle retrieval was described extensively elsewhere [23]. Briefly, ovarian cortex tissue was cut in pieces of approximately 1 mm3, which were dispersed (UltraTurrax, IKA, Germany) in isolation medium (Leibovitz, L15 with GlutaMAX, supplemented with 10 μg/ml penicillin-streptomycin and 0.3 mg/ml BSA, Sigma). The resulting follicle suspension was filtered through 100 and 25 μm mesh filters (BD Falcon). The follicles of interest (diameter between 25–100 μm) remained on the 25 μm filter and were transferred to a petri dish filled with isolation medium and visualized by means of an inverted phase-contrast microscope (Olympus CKX 41, magnification: x100 up to x400) (Fig. 1). In total, 900 PAFs, divided over six (2x3) replicates, were used.
Fig. 1.
Preantral follicles after isolation of bovine ovarian cortex tissue. a: enlargement x100; b: enlargement x200; c: enlargement x400
Determination of the lowest concentration and the shortest possible incubation time for Neutral Red (NR)
Half area 96-well plates were filled with 35 μl Culture (Cm) and 35 μl Neutral Red medium (NRm) and equilibrated for 2 h (38.5 °C, 5 % CO2). Based on Jorssen et al. [24], Cm consisted of DMEM and Ham’s F12 in equal amounts, Penicillin-Streptomycin 10 μg/ml, Fungizone 20 μg/ml, FBS (Greiner) 2.5 v/v%, NCS (Sigma) 2.5 v/v%, a mixture of Insulin, Transferrin and Selenium (ITS) (0.01 μg/ml, 0.55 μg/ml and 6.7 ng/ml respectively) and BSA (Sigma) 0.75 w/v%. NRm was composed of Cm supplemented with relevant NR test concentrations, obtained by diluting the original NR stock solution of 0.05 mg/ml (the concentration of NR as used by Chambers et al. [10]). Initially, three replicates were performed for each concentration of 0, 0.0025, 0.025, 0.25, 2.5 and 25 μg/ml NR. Each concentration was tested twice per replicate. Because follicles were only stained within the concentration range of 2.5 to 25 μg/ml NR, the same experiment was repeated in the same way as the former experiment within the range of 2.5-25 μg/ml (0, 2.5, 5, 10, 15, 20 and 25 μg/ml). All evaluations were performed by the same operator, using inverted phase-contrast microscopy (Olympus CKX 41) (Figs. 2 and 3). NR-stained follicles were considered viable when at least 75 % of the granulosa cells surrounding the oocyte and the oocyte itself were stained red. Briefly, PAF between 25–100 μm were selected (10/well) and viability was assessed every 30 min for 2 h following the onset of staining. At each time point, the number of NR + follicles was counted. Following the last count, follicles were cultured in 70 μl Cm for 48 h. On Day 2, follicles were again exposed to NR at the same dosage and viability was re-assessed (cfr. Day 0). Following the final count on D2, follicles were again transferred to Cm and re-incubated for another 48 h. On Day 4, the procedure was repeated for a third time as described above. Following the last count on D4, the highest NR concentration was added to all wells, as a positive control. For each replicate, an internal control for culture effects was included: PAFs were not exposed to NR at D0 but were exposed to maximal NR concentration on D2 or on D4 of culture.
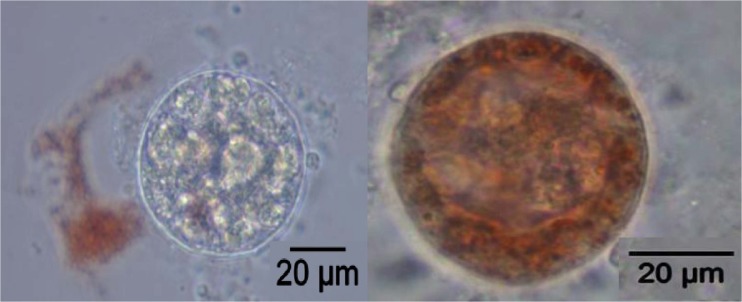
Fig. 2.
Metabolically inactive (or dead - left) and metabolically active (or live - right) isolated follicle stained with NR (Neutral Red)
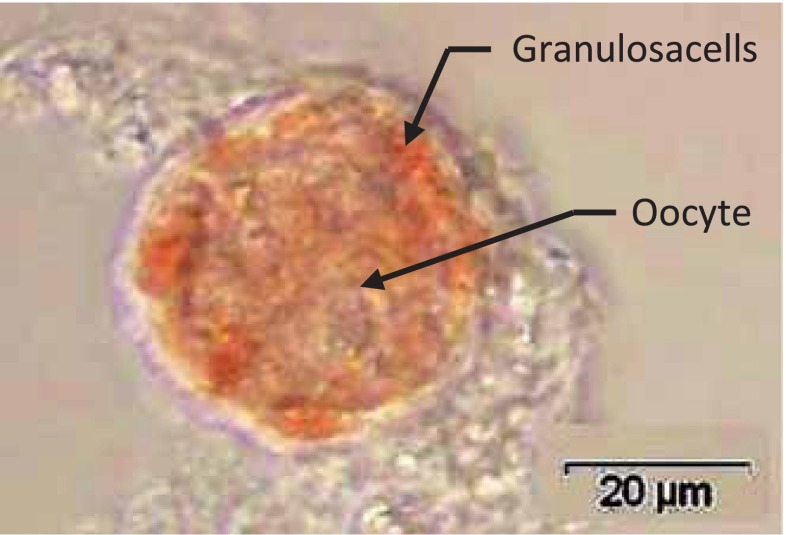
Fig. 3.
An enlarged picture of a preantral follicle positively stained for NR. Oocyte and granulosacells surrounding the ooccyte are pointed out
Two factorial crossover design to test the effect of vitrification and NR staining on the viability of isolated PAFs
In a crossover design (see Fig. 4), viability was assessed by staining the follicles using the optimized NR protocol (15 μg/ml, 30 min) on different time points: 1) prior to vitrification (time point 1, T1), treatments were defined as pre/postCRYO and preCRYO. Subsequently, both groups were vitrified. Following vitrification and warming (time point 2, T2), one group was re-stained with NR to re-assess viability and subsequently cultured for 4 days (treatment pre/postCRYO) while the other group (treatment preCRYO) was immediately cultured for 4 days; 2) only following vitrification and warming (T2), treatment defined as postCRYO; 3) nor prior to or after vitrification, treatment defined as nonSTAINED.
Fig. 4.

Experimental cross-over design to study the effect of NR on the viability of early preantral follicles after vitrification. (NR = Neutral Red; T1, T2, T3 = timepoint 1, 2 and 3)
Finally, all groups were stained with NR following 4 days of culture (time point 3, T3) to assess the remnant proportion of viable follicles. An additional group of follicles (CONTROL) was cultured for 4 days, without being vitrified or stained, except at the end of the trial at T3.
Vitrification of isolated PAFs
Preantral follicles were isolated as described earlier (2.1). Halfarea 96 well-plates were prepared as stated under 2.2. Preantral follicles were subjected to different treatments (10 PAFs/well) (pre/postCRYO, preCRYO, postCRYO and nonSTAINED, see 2.3 and Fig. 4). The vitrification protocol was based on Aerts et al. [7]. Manipulations were performed at room temperature, limiting the amount of transferring medium and grouping the PAFs to shorten handling time. After isolated PAFs were stained and placed in culture medium, viability was assessed 30 min later. Following viability assessment or culture, follicles were vitrified. Briefly, 10 follicles per well (8 wells in total per replicate, 3 replicates, 240 PAFs) were placed for 1 min in 50 μL of Hm (holding medium), consisting of L15 with GlutaMAX supplemented with penicillin-streptomycin (10 μg/ml) and 0.5 % polyvinylpyrrolidone (PVP, Sigma). Subsequently, follicles were placed in 250 μL Hm for 1 min and transferred to 50 μL equilibration medium (Em) consisting of Hm supplemented with ethylene glycol (EG, 1.8 M, Sigma) and dimethyl sulfoxide (DMSO, 1.4 M, Sigma). After 1 min, follicles were plunged into 20 μL of vitrification medium (Vm) consisting of Hm to which sucrose (0.2 M, Sigma), EG (3.6 M) and DMSO (2.8 M) had been added. After 20 s, follicles were picked up and placed in a fibreplug in a maximum of 3 μl Vm. Follicles were vitrified by making contact between the fibreplug and the CVM (cryologic vitrification method) block and subsequently stored in LN2 for at least 2 days.
Warming and NR staining of the vitrified PAF
Before initiating the warming procedure, half area 96-well plates were prepared as stated under 2.2. Fibreplugs were recovered from the LN2 and warmed for 1 min in a drop of 250 μL of thawing medium (Tm) consisting of Hm with sucrose (0.3 M). The follicles were then transferred to 50 μL of Tm for 5 min and transferred to 50 μL of dilution medium (Dm) consisting of Hm with sucrose (0.2 M) for 5 min. Finally, all follicles were transferred to 50 μL of Hm for 5 min and then transferred to the corresponding staining or culture wells (Figs. 2 and 3). Viability was assessed after 30 min and follicles were cultured in Cm during 4 days.
Culture and viability re-assessment of PAFs after 4d ‘in vitro’ culture
Finally, follicles of all treatments were cultured for 4 days in Cm [25]. Half of the culture medium was changed the second day. On the 4th day, follicle viability was assessed by replacing half of the volume of Cm with NRm (final concentration NR is 15 μg/ml). Dead or alive status was assessed after 30 min following the uptake of NR.
Statistical analysis
SPSS Statistics 20.0 (for Windows, Chicago, IL, USA) and R, version 2.13.1 (http://www.r-project.org), were used to perform statistical analysis. The effects of concentration, replicate and incubation time were tested using logistic regression analysis. A stepwise backward model building strategy was used, starting from a model including replicate, incubation time and concentration (categorical), as well as all pairwise interactions. Significance testing was performed using a likelihood ratio test. The optimum concentration 15 μg/ml, was obtained by calculating the mean fraction of stained follicles at each concentration. Significance level was set at P < 0.05.
To study the effect of vitrification on follicle viability, PAFs were stained and counted before and after the cryopreservation procedure and after 4 days of culture. The percentage of viable follicles was measured per well and per replicate on the total number of PAFs retrieved at the different time points. No interaction between replicate and treatment was noticed. Percentages were compared with a paired Student’s T-test to address the net effect of vitrification on viability at T2 (observations within treatment pre/postCRYO). In order to cover the comparisons between treatment nonSTAINED and the CONTROL (the net effect of vitrification on viability at T3), treatment pre/postCRYO and postCRYO (the effect of NR staining before cryopreservation on viability at T2) and nonSTAINED and preCRYO (the effect of NR staining before cryopreservation on viability at T3), a binary logistic regression analysis was performed. Significance level was set at P < 0.05.
Results
Optimizing the Neutral Red protocol (aim 1 and 2)
Determination of the lowest NR concentration for PAF viability testing
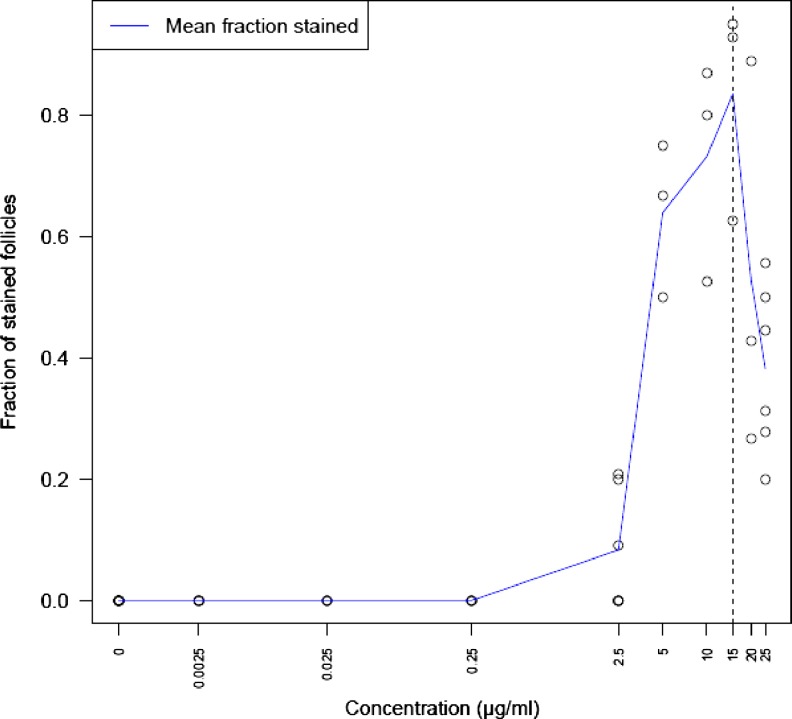
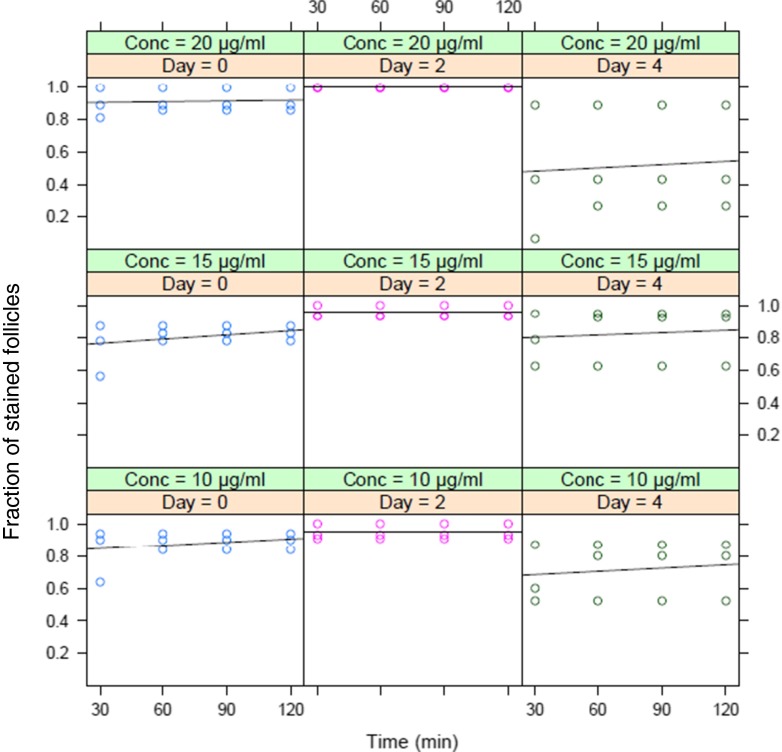
The first trial (2.2) showed that follicles stained positive at NR concentrations between 2.5 and 25 μg/ml. The experiment was therefore repeated within this narrower dose range. Plotting the observed fraction of stained follicles versus the concentration of NR clearly showed an optimal NR concentration at 15 μg/ml. When increasing the NR concentration beyond 15 μg/ml the number of stained follicles decreased (Fig. 5).
Fig. 5.
Optimum concentration (blue line). Concentrations of NR in the X-axis are plotted against the observed fraction of stained follicles in the Y-axis. The solid line shows the mean fraction of stained cells at each concentration. A clear optimum can be seen at 15 μg/ml
Determination of the shortest NR incubation time with the highest specificity
The effect of incubation time on the specificity of staining and further PAF development was not significant (P = 0.07). Therefore, staining was assessed at 30, 60, 90 and 120 min, respectively. To analyze the effect of concentration on incubation time, the interaction between both was tested, with one concentration above and one below the optimal concentration (10-15-20 μg/ml), during 4 different incubation times and at Day 0, 2 and 4 (Fig. 6). No significant interaction was found (P = 0.98), meaning that the slopes of the 9 lines depicted did not differ significantly between the 3 NR concentrations, and that within each concentration, the proportion of follicles stained after 30, 60, 90 or 120 min did not increase over time. Looking at Day 4 demonstrated (P = 0.06) a lower proportion of stained follicles, illustrating that more non-stained, thus metabolically inactive, follicles were present, especially at 20 μg/ml. In addition, the experiments revealed that, in total, about 80 % of the follicles were successfully processed (manipulation, changing medium, transfer from well to well), while again 80 % of the present PAFs survived 4 days of culture including repeated NR stainings.
Fig. 6.
Effect of NR concentration (green bar), incubation time (X-axis) and day (pink bar) on the fraction of stained follicles (Y-axis). The solid line shows the relation between incubation time and staining within each of the 3 concentrations on the three timepoints (D0, D2 and D4)
The effects of NR before vitrification on the viability of PAFs (aim 3)
Overall averages of the percentage ± SEM of viable follicles per treatment are presented in Fig. 7. Pictures of PAF during the cross-over design (2.3) at the different time points are depicted in Fig. 8.
Fig. 7.

Overall averages of viability percentages ± SEM at T1, T2, T3 for the different treatment groups
Fig. 8.
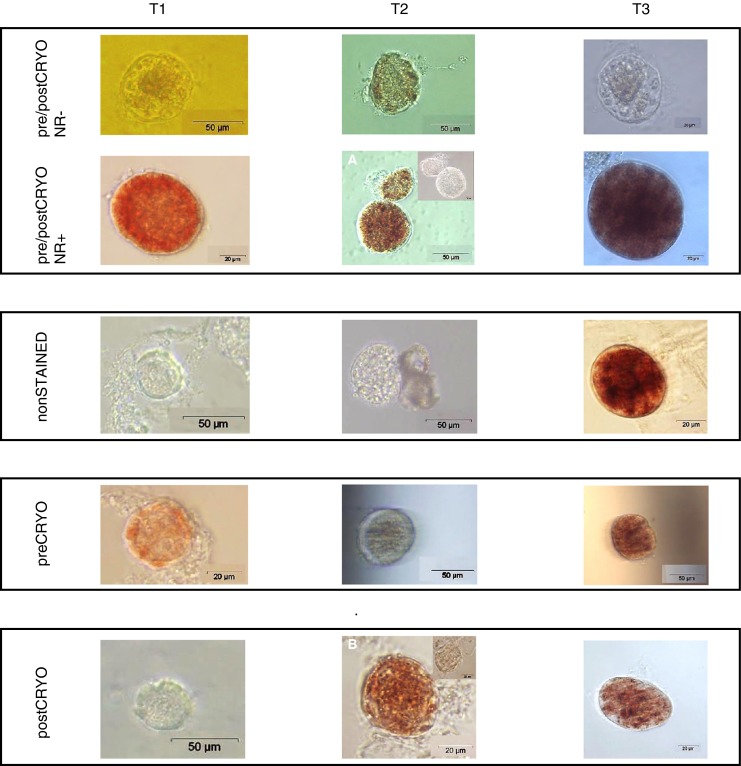
Pictures of NR positive and negative preantral follicles at time points T1, T2 and T3 (vertically). Horizontally, the different treatments are displayed. The inlets in pictures A and B display the elimination of NR 2 h after transferring the follicles to culture medium
Net effect of vitrification on PAF viability immediately after cryopreservation and after 4 days of culture
Comparing observations within treatment pre/postCRYO pointed out that significantly less follicles were assessed as ‘viable’ immediately after thawing (77 ± 6 % at T2 versus 90.5 ± 3 % at T1, P = 0.008). A negative effect of cryopreservation on PAF viability was found: 76 ± 9 % of the nonSTAINED treatment group was assessed as ‘viable’ at T3, while in the CONTROL group without vitrification 93.4 ± 2 % survived 4 days of culture (P = 0.003).
Effect of NR staining before cryopreservation on the viability immediately after warming and after 4 days of culture
Comparing treatments pre/postCRYO and postCRYO showed that there was no significant difference between the viability of follicles after warming, whether they were stained before vitrification (77.2 ± 6 %) or not (82.7 ± 5.3 %) (P = 0.551). In addition, equal proportions of the originally non-stained (70 ± 5 %) and stained (65 ± 5 %) follicles could be retrieved (P = 0.713) immediately after warming. Finally, when comparing treatment nonSTAINED to treatment preCRYO, NR staining prior to vitrification did not seem to have an effect on the viability of vitrified PAFs after 4 days of culture: 76 ± 9 % of the stained versus 92 ± 5 % of the non-stained follicles were determined as viable (P = 0.140).
Discussion
As described above, our results show that NR staining had no effect on the viability of isolated preantral follicles following vitrification. From a precautionary point of view, 15 μg/ml is proposed as the lowest NR concentration and 30 min as the shortest incubation time needed for reliable viability assessment of isolated bovine PAFs. To our knowledge, this is the first report studying the combination of non-invasive PAF NR assisted viability assessment followed by vitrification of isolated preantral follicles and short-term follicle culture.
Since the first description of its use by Bensley in 1911 [26, 27], Neutral Red has been used for viability testing in different cell types [28–31] before it was turned into a viability assessment method by measuring its uptake by metabolically active cells (NRU, Neutral Red Uptake-assay). While the residual NR in the medium [32, 33] was originally quantified by spectrophotometry, we determined cell viability directly by light microscopy based on NR uptake to facilitate clinical use. Currently, there are only a few papers available that study the effects of NR and its suitability for isolated follicle viability assessment [10, 13]. None of these used the bovine model, which is increasingly considered to be very relevant for human pre-implantation studies on follicle, oocyte and embryo developmental competence [6, 34, 35]. A major advantage of using NR is the reversibility of the process because follicles eliminate residual NR when subsequently cultured in NR free medium. We believe to be the first to report on the repeated use of NR as a possible sentinel for quality changes and a built-in viability assessment tool during short-term in vitro culture. The development of long-term culture for PAFs of larger mammals including human will be one of the next necessary steps [36–40]. Because nowadays, long-term in vitro culture methods for PAFs, ultimately leading to viable offspring, are only available in mice [41–43], we chose to assess NR toxicity by repeated staining during short-term in vitro culture of isolated bovine PAFs, with the fourth day of culture as an endpoint. From a precautionary point of view, NR concentration and the length of exposure time needed to be studied intensively. Reported NR toxicity was linked to the formation of reactive oxygen species in the presence of light [44]. Although we repeatedly stained PAFs and assessed viability through light-microscopy, only a slightly negative trend on viability could be seen after 4 days of culture (at the high end concentrations), while 80 % of the follicles were still metabolically active after repeated staining. Other studies [13] did not show a detrimental effect of NR on isolated PAFs with an incubation time of 4 h. This was validated with a carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) assay following the NR staining. In the current study, the proportion of stained follicles decreases at concentrations higher than 15 μg/ml. This might be due to the fact that a higher amount of follicles do not survive the highest NR concentrations, as substantiated by earlier reports [44] and due to the repeated viability assessment. When culturing a longer period and at higher NR concentrations, the number of metabolically active PAFs decreases. We do not see this decline for 10 nor 15 μg/ml NR, which is an extra argument in favor for the NR concentration chosen. An additional plus of NR is the easiness of use, with limited follicle loss and short handling times as compared to the use of rhodamine 123 [45, 46]. Our data indicate that the duration of NR exposure, whether it was 30 or 120 min, had no significant effect on the proportion of stained follicles. Therefore, we propose a NR exposure time of 30 min, being on the safe side and allowing to process high numbers of follicles while gaining a specific idea about their viability in a non-invasive way. However, because of manipulation restrictions (great amount of follicles, different concentrations NR) an even shorter incubation time could not be tested in the current set-up.
Cryopreservation is a crucial component of state of the art fertility preservation strategies [47]. While it is beyond the scope of this paper to review all available cryopreservation methods, vitrification showed to be a valuable option for the preservation of follicles and individual oocytes [48, 49]. Earlier own work (CVM, Cryologic Vitrification Method [7]), confirmed the suitability of vitrification to cryopreserve murine PAFs. Often, ovarian cortical tissue pieces are used [7, 50–53], while others turned to mechanically or enzymatically isolated PAFs [54, 55]. The choice to work on isolated follicles was inspired by the fact that ultimately, this will be the most rewarding strategy when dealing with fertility preservation in oncology patients, minimizing the risk of reintroducing cancerous cells following cryopreserved PAF transfer. In addition, our protocol demonstrated that individual PAFs are much easier to quantify and assess on viability as agreed on by Kristensen et al. [13]. Fortunately, high survival rates were seen in follicles stained prior to cryopreservation (preCRYO) as compared to all other groups, including the CONTROL and the repeated stained group. This aberrant positive result (92 %) substantiates the need for additional insights to increase survival rates for all cryopreserved groups. To our knowledge, we are the first to report on successfully vitrified isolated bovine follicles, in combination with repeated NR viability assessment prior and following cryopreservation and subsequently cultured for a short-term period. In line with earlier work on follicles embedded in cortical tissue strips [56, 57], we showed that cryopreservation, as compared with non-cryopreserved follicles, has an overall negative effect on the viability of isolated (bovine) PAFs, as noticed immediately after warming and at 4 days of culture. We have used NR as an indicator or build-in sentinel for easy and routine applicable assessment of PAF’s metabolic activity. Comparable amounts of follicles were determined ‘viable’ immediately after warming and at day 4 of culture. Although measuring cellular metabolism and the exact extend of NR uptake would generate extra information, invasive analyses would interrupt the high throughput approach. Nevertheless, our results suggest that even if metabolism in follicles decreased immediately after vitrification, our protocol used sufficient NR and a relevant incubation time to detect viable PAFs. While additional research is needed to minimize cryopreservation damage or test the impact of NR on long-term culture outcome, our major goal was to investigate if the use of NR has deleterious effects when used in combination with vitrification. The vitrification process can cause the formation of reactive oxygen species (ROS) in follicles [58] which can add up to ROS generated by using NR combined to light exposure [14] and therefore could aggravate negative effects on (long-term) follicle/oocyte viability. Human cortical tissue strips are often removed and cryopreserved before anti-cancer treatment [13, 59–61], without any knowledge on the presence of follicles and their viability. However, assessment of isolated follicle viability is a crucial component of a functional fertility preservation strategy. PAF viability assessment prior to vitrification is possible without hampering follicle quality, as indicated by our findings. A second NR assisted assessment, following warming and before transfer, can give an indication on follicle survival rate and the expected chance of the successful restoration of fertility.
In conclusion, our results illustrate in a bovine model that NR staining is a valuable tool to repeatedly assess isolated PAF viability, with limited toxic properties if used at 15 μg/ml and an exposure time of 30 min. Combined to cryopreservation, NR staining can be integrated in fertility preservation strategies without compromising PAF survival after vitrification. NR staining can be considered an added value to fertility preservation strategies to select viable follicles, provided that additional research on possible long-term toxicity is performed as soon as successful long-term in vitro culture protocols are available.
Acknowledgments
The authors thank Silke Andries and Els Merckx for their excellent technical assistance and the local slaughterhouses for their cooperation in sample collection.
Conflict of interest
All (co-)authors state that the funding of this research is provided by the independent Operational Costs of the University of Antwerp. E. P. A. Jorssen acknowledges support from a Research Grant from the Belgian Government (Federale Overheidsdienst Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu, Cel Contractueel Onderzoek) ‘Embryoscreen RF6222’. There is nothing to disclose and there are no conflicts of interest, financially nor personal, for none of the (co-)authors.
Footnotes
Capsule Applied at a concentration of 15 μg/ml for 30 minutes, Neutral Red is proposed as a non-invasive viability assessment tool for isolated bovine preantral follicles, without compromising follicle cryotolerance.
References
- 1.Fortune JE, Kito S, Wandji SA, Srsen V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology. 1998;49(2):441–9. doi: 10.1016/S0093-691X(97)00416-0. [DOI] [PubMed] [Google Scholar]
- 2.Campos JR, Rosa ESAC. Cryopreservation and fertility: current and prospective possibilities for female cancer patients. ISRN Obstet Gynecol. 2011;2011:350813. doi: 10.5402/2011/350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanacker J, Luyckx V, Amorim C, Dolmans MM, Van Langendonckt A, Donnez J, et al. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril. 2013;99(5):1363–8 e2. doi: 10.1016/j.fertnstert.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Amorim CA, Goncalves PB, Figueiredo JR. Cryopreservation of oocytes from pre-antral follicles. Hum Reprod Update. 2003;9(2):119–29. doi: 10.1093/humupd/dmg014. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, et al. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl. 2003;61:429–43. [PubMed] [Google Scholar]
- 6.Malhi PS, Adams GP, Singh J. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol Reprod. 2005;73(1):45–53. doi: 10.1095/biolreprod.104.038745. [DOI] [PubMed] [Google Scholar]
- 7.Aerts JM, De Clercq JB, Andries S, Leroy JL, Van Aelst S, Bols PE. Follicle survival and growth to antral stages in short-term murine ovarian cortical transplants after Cryologic solid surface vitrification or slow-rate freezing. Cryobiology. 2008;57(2):163–9. doi: 10.1016/j.cryobiol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Human reproduction (Oxford, England). 1999;14(10):2519–24. [DOI] [PubMed]
- 9.Park KS, Lee TH, Park YK, Song HB, Chun SS. Effects of isolating methods (mechanical or enzymatical) on structure of pre-antral follicles in mouse. J Assist Reprod Genet. 2005;22(9–10):355–9. doi: 10.1007/s10815-005-6796-z. [DOI] [PubMed] [Google Scholar]
- 10.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod (Oxford, England) 2010;25(10):2559–68. doi: 10.1093/humrep/deq192. [DOI] [PubMed] [Google Scholar]
- 11.Sanfilippo S, Canis M, Ouchchane L, Botchorishvili R, Artonne C, Janny L, et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1) J Assist Reprod Genet. 2011;28(12):1151–6. doi: 10.1007/s10815-011-9649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fransolet M, Labied S, Henry L, Masereel MC, Rozet E, Kirschvink N, et al. Strategies for using the sheep ovarian cortex as a model in reproductive medicine. PloS one. 2014;9(3):e91073. doi: 10.1371/journal.pone.0091073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod (Oxford, England) 2011;26(1):157–66. doi: 10.1093/humrep/deq318. [DOI] [PubMed] [Google Scholar]
- 14.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 15.Elliott WM, Auersperg N. Comparison of the neutral red and methylene blue assays to study cell growth in culture. Biotechnic Histochem : Off Publ Biol Stain Comm. 1993;68(1):29–35. doi: 10.3109/10520299309105573. [DOI] [PubMed] [Google Scholar]
- 16.Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24(2–3):119–24. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 17.Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, et al. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci. 2010;122(3–4):151–63. doi: 10.1016/j.anireprosci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Torre A, Momier M, Mazoyer C, Selva J, Salle B, Lornage J. Validation of a new metabolic marker to assess the vascular viability of vitrified whole sheep ovaries. Hum Reprod (Oxford, England) 2012;27(6):1811–21. doi: 10.1093/humrep/des100. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Wang X, Wu Y, Meng Y, Wu F, Zhou N, et al. Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries. Theriogenology. 2012;77(3):483–91. doi: 10.1016/j.theriogenology.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27(6):465–71. doi: 10.1055/s-0029-1241056. [DOI] [PubMed] [Google Scholar]
- 21.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod (Oxford, England) 2013;28(5):1267–79. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langbeen A, Jorssen EPA, Fransen E, Rodriguez APA, Chong Garcìa M, Leroy JLMR et al. Characterization of freshly retrieved preantral follicles using a low-invasive, mechanical isolation method extended to different ruminant species. Zygote. 2014. [DOI] [PubMed]
- 24.E.P.A. Jorssen AL, J.L.M.R. Leroy, S. Andries, E. Merckx, P.E.J. Bols, editor. The effect of FSH on the survival and growth of individually in vitro cultured early pre-antral bovine follicles. REPRODUCTION IN DOMESTIC ANIMALS 2013 september 2013; Bologna.
- 25.Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE. Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology. 2014 doi: 10.1016/j.theriogenology.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Studies RRB. on the pancreas of the Guinea Pig. American. J Anat. 1911;12:297. doi: 10.1002/aja.1000120304. [DOI] [Google Scholar]
- 27.Gray DW, Millard PR, McShane P, Morris PJ. The use of the dye neutral red as a specific, non-toxic, intra-vital stain of islets of Langerhans. Br J Exp Pathol. 1983;64(5):553–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WH, Jr, Hagerty RF, Braid HL. Measurements of cellular viability. A comparative study of neutral red and radioactive sulfate in the examination of the viability of cartilage. Plast Reconstr Surg Transplant Bull. 1960;26:280–5. doi: 10.1097/00006534-196009000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Maier K, Schmitt-Landgraf R, Siegemund B. Development of an in vitro test system with human skin cells for evaluation of phototoxicity. Toxicol In Vitro : Int J Published Assoc BIBRA. 1991;5(5–6):457–61. doi: 10.1016/0887-2333(91)90072-L. [DOI] [PubMed] [Google Scholar]
- 30.Fautz R, Husein B, Efstathiou E, Hechenberger-Freudl C. Assessment of the relation between the initial viability and the attachment of freshly isolated rat hepatocytes used for the in vivo/in vitro DNA repair assay (UDS) Mutat Res. 1993;291(1):21–7. doi: 10.1016/0165-1161(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SZ, Lipsky MM, Trump BF, Hsu IC. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes. Cell Biol Toxicol. 1990;6(2):219–34. doi: 10.1007/BF00249595. [DOI] [PubMed] [Google Scholar]
- 32.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160(2):171–7. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues RM, Bouhifd M, Bories G, Sacco MG, Gribaldo L, Fabbri M, et al. Assessment of an automated in vitro basal cytotoxicity test system based on metabolically-competent cells. Toxicol In Vitro : Int J Published Assoc BIBRA. 2013;27(2):760–7. doi: 10.1016/j.tiv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Adams GP, Pierson RA. Bovine model for study of ovarian follicular dynamics in humans. Theriogenology. 1995;43(1):113–20. doi: 10.1016/0093-691X(94)00015-M. [DOI] [Google Scholar]
- 35.Menezo YJ, Herubel F. Mouse and bovine models for human IVF. Reprod Biomed Online. 2002;4(2):170–5. doi: 10.1016/S1472-6483(10)61936-0. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62(5):1322–8. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reprod (Cambridge, England) 2010;139(6):971–8. doi: 10.1530/REP-10-0025. [DOI] [PubMed] [Google Scholar]
- 38.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59(4):783–90. [PubMed] [Google Scholar]
- 39.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod (Oxford, England) 2008;23(5):1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod (Oxford, England) 2011;26(5):1061–72. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albertini DF, Akkoyunlu G. Ovarian follicle culture systems for mammals. Methods Enzymol. 2010;476:107–21. doi: 10.1016/S0076-6879(10)76007-9. [DOI] [PubMed] [Google Scholar]
- 42.Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2(2):94–104. doi: 10.1530/ror.0.0020094. [DOI] [PubMed] [Google Scholar]
- 43.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod (Oxford, England) 1996;11(12):2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 44.Guerard M, Zeller A, Singer T, Gocke E. In vitro genotoxicity of neutral red after photo-activation and metabolic activation in the Ames test, the micronucleus test and the comet assay. Mutat Res. 2012;746(1):15–20. doi: 10.1016/j.mrgentox.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Soleimani R, De Vos W, Van Oostveldt P, Lierman S, Van den Broecke R, De Sutter P, et al. Two novel techniques to detect follicles in human ovarian cortical tissue. Hum Reprod (Oxford, England) 2006;21(7):1720–4. doi: 10.1093/humrep/del057. [DOI] [PubMed] [Google Scholar]
- 46.Norins AL, Gould WM. The photodynamic action of neutral red on rabbit basophils. J Investig Dermatol. 1964;42:257–9. doi: 10.1038/jid.1964.58. [DOI] [PubMed] [Google Scholar]
- 47.Varghese AC, du Plessis SS, Falcone T, Agarwal A. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol : RB&E. 2008;6:47. doi: 10.1186/1477-7827-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arav A. Cryopreservation of oocytes and embryos. Theriogenology. 2014;81(1):96–102. doi: 10.1016/j.theriogenology.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Celestino JJ, dos Santos RR, Lopes CA, Martins FS, Matos MH, Melo MA, et al. Preservation of bovine preantral follicle viability and ultra-structure after cooling and freezing of ovarian tissue. Anim Reprod Sci. 2008;108(3–4):309–18. doi: 10.1016/j.anireprosci.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Liebenthron J, Koster M, Drengner C, Reinsberg J, van der Ven H, Montag M. The impact of culture conditions on early follicle recruitment and growth from human ovarian cortex biopsies in vitro. Fertility and sterility. 2013:483–91. doi:10.1016/j.fertnstert.2013.03.046. [DOI] [PubMed]
- 51.Oskam IC, Lund T, Santos RR. Irreversible damage in ovine ovarian tissue after cryopreservation in propanediol: analyses after in vitro culture and xenotransplantation. Reprod Domest Anim. 2011;46(5):793–9. doi: 10.1111/j.1439-0531.2010.01743.x. [DOI] [PubMed] [Google Scholar]
- 52.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Preservation of human ovarian follicles within tissue frozen by vitrification in a xeno-free closed system using only ethylene glycol as a permeating cryoprotectant. Fertility and sterility. 2013:170–7. doi:10.1016/j.fertnstert.2013.03.018. [DOI] [PubMed]
- 53.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103(2):378–86. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amorim CA, Rondina D, Rodrigues AP, Costa SH, Goncalves PB, de Figueiredo JR, et al. Isolated ovine primordial follicles cryopreserved in different concentrations of ethylene glycol. Theriogenology. 2003;60(4):735–42. doi: 10.1016/S0093-691X(03)00089-X. [DOI] [PubMed] [Google Scholar]
- 55.Amorim CA, Rodrigues AP, Rondina D, Goncalves PB, de Figueiredo JR, Giorgetti A. Cryopreservation of ovine primordial follicles using dimethyl sulfoxide. Fertil Steril. 2003;79(Suppl 1):682–6. doi: 10.1016/S0015-0282(02)04820-3. [DOI] [PubMed] [Google Scholar]
- 56.Lamaita RM, Bambirra EA, Camargos M, Silva-Filho AL, Reis FM, Camargos AF. Histological evaluation of the effects of cryopreservation in bovine ovarian tissue. J Assist Reprod Genet. 2005;22(2):105–6. doi: 10.1007/s10815-005-1501-9. [DOI] [PubMed] [Google Scholar]
- 57.Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology. 1999;38(4):301–9. doi: 10.1006/cryo.1999.2170. [DOI] [PubMed] [Google Scholar]
- 58.Abedelahi A, Salehnia M, Allameh AA, Davoodi D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod (Oxford, England) 2010;25(4):977–85. doi: 10.1093/humrep/deq002. [DOI] [PubMed] [Google Scholar]
- 59.Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21(8):887–98. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- 60.Donnez J, Dolmans MM. Cryopreservation of ovarian tissue: an overview. Minerva Med. 2009;100(5):401–13. [PubMed] [Google Scholar]
- 61.Oktem O, Oktay K. Fertility preservation for breast cancer patients. Semin Reprod Med. 2009;27(6):486–92. doi: 10.1055/s-0029-1241059. [DOI] [PubMed] [Google Scholar]