Abstract
Purpose
Nowadays sperm analysis is used routinely to assess infertile men, but it’s predictive value is still restricted. For this reason, new markers are needed for diagnosis and guide treatment. A DNA damage evaluation method, which is easily applicable for routine analysis is described in this paper to provide an important support in clinical evaluation.
Methods
In this study, DNA damage evaluation was planned in infertile males with the COMET assay, a method which is simple, cheap, sensitive and reliable. For this purpose, the sperm DNA damage of normospermic, oligospermic, asthenospermic and teratospermic cases were investigated and the relationship between sperm parameters (sperm count, motility, morphology) and DNA damage was assessed.
Results and conclusion
According to our results we suggest that the factors to blame for sperm DNA damage and semen quality are the same. Also it was found that the infertile males possess substantially more sperm DNA damage than fertile men do. Improving and using the DNA damage evaluation tests seem to improve the results in ART clinics.
Keywords: Assisted reproductive technologies (ART), COMET assay, Male infertility, Sperm, DNA damage
Introductıon
The World Health Organization (WHO) and the American Society for Reproductive Medicine (ASRM) described infertility as when pregnancy is not achieved within a 1-year period in couples who do not use any contraceptive method. Male infertility is the only reason in 20 % of the infertile couples, and in 30–40 % of the couples goes together with a problem in the female so it is found in about 50 % of cases [1].
Sperm chromatin structure and sperm DNA integrity are essential for successful fertilization and heritage of the genome to the next generation [2]. In most studies it was shown that the paternal genome is responsible for the recurrent unsuccessful results in Assisted Reproductive Technologies (ART) [3–7]. Nowadays there are plenty of researches which support the idea that infertile men possess substantially more sperm DNA damage than fertile men do [8–10].
Although many techniques have been described to evaluate the status of the sperm DNA damage (toluidine blue staining, chromomycin A3 (CMA3), sperm chromatin structure assay (SCSA), the deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) test, sperm chromatin dispersion (SCD) test, DNA breakage detection-fluorescent in situ hybridization (DBD-FISH) assay, in situ nick translation (NT), COMET assay and the measurement of 8-hydroxy-2-deoxyguanosine (8-OHdG) by high performance liquid chromatography (HPLC)) [11], only a few researches compared these methods. According to these researches results “alkaline comet assay” is the best discriminative test for male infertility [12–16].
COMET assay, is a fluorescent technique for measurement of DNA strand breaks in individual cells. Since its first introduction in the late 1970s, the assay has been widely used as a simple and sensitive method for assessing DNA damage in somatic cells. This technique recently has been adapted for measuring DNA strand breaks in human sperm [17–19].
In this study, the sperm DNA damage values of normospermic, oligospermic, asthenospermic and teratospermic cases were compared. Also the relation between sperm parameters (sperm count, motility, morphology) and the relation of these parameters to DNA damage was investigated.
Materials and methods
Patient selection and sample collection
Male cases who came to the Çukurova University, Faculty of Medicine, Urology Dept. and Ege University Family Planning, Infertility Research and Treatment Center between 2008 and 2010 with complaints of infertility were involved in this study within the framework of decision #5 of the Ethics Committee of Çukurova University Faculty of Medicine dated June 12, 2007. 60 infertile males aged 26–47 years, undergoing IVF treatment, were included in the study with the following criteria: 1) a minimum of 1 year unprotected intercourse without pregnancy, 2) no physiological disorders leading to male factor infertility, 3) subnormal semen analysis according to WHO (1999) criteria (samples with leukocytes were excluded) 4) female partners are healthy with no known cause of infertility. The control group consisted of 20 normospermic fertile cases who were married with children.
Semen analysis
Semen sample was collected by masturbation after 4 days of sexual abstinence. The ejaculate was fully liquefied and the semen analysis was performed in our laboratory according to standard WHO (1999) criteria.
Comet assay
The alkaline COMET assay was performed as previously described methods [20, 21] with some modifications. Briefly, the fully frosted microscope slides were covered with 100 μl of 0.75 % normal melting-point agarose (NMPA; Sigma A9539, USA) in PBS and then dried at room temperature. 2 X106 cells/ml (8 μl) were mixed with 0.75 % (w/v) low melting agarose (LMPA; Sigma A9414, USA) (72 μl) to form a cell suspension, which was pipetted onto precoated slides. The slides were immediately covered with a cover slip and kept at 4 °C for 8 min to allow the agarose to solidify. Then the third layer of 0.75 % LMPA was added and the slides were kept at 4 °C for another 8 min. After the cover slips were removed, the slides were immersed in ice cold, freshly prepared lysing solution (2,5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 10 % DMSO (Sigma D5879, China), 1 % Triton X-100 (Fluka 93420, France) and 200 μg/ml proteinase K (Sigma-Aldrich P6556,USA) at a pH of 10) at 37 °C overnight.
After draining the proteinase K solution from the slides, they were placed in a horizontal electrophoresis unit filled with fresh alkaline electrophoresis buffer containing 300 mM NaOH and 1 mM EDTA for 20 min to allow the DNA from the cells to unwind. Electrophoresis was performed at 50 V and 300 mA for 10 min.
The results were analyzed with TriTek CometScore Freeware v1.5. One hundred cells per slide were scored and the COMET measurements “% Tail DNA and Tail Moment” were used to evaluate sperm DNA damage.
Statistical analysis
SPSS v.15 software was used to analyze and to graph the data collected in the study. Descriptive statistics was used to describe the basic features of semen volume, sperm count, motility, morphology, % Tail DNA and Tail Moment. The relation between semen parameters and the relation of these parameters to the percentage of tail DNA and Tail Moment was analyzed with the Pearson Correlation test. Comparisons of “% Tail DNA” and “Tail Moment” between groups were analyzed using the Post Hoc Dunnett’s T3 test. Reciever Operating Characteristic (ROC) analysis was performed to obtain the specificity, sensitivity and the cut-off values of %Tail DNA and Tail Moment. All statistical tests were performed taking into account the 95 % confidence interval and p < 0.05 was considered to be statistically significant.
Results
When the association of spermiogram parameters was investigated, it was determined that motility showed a positive correlation to sperm count (r = 0,28; p < 0,05) and morphology proved to have a negative correlation to age (r = −0,25; p < 0,05), and a positive and statistically significant correlation to sperm count (r = 0,53; p < 0,01) and motility (r = 0,34; p < 0,01).
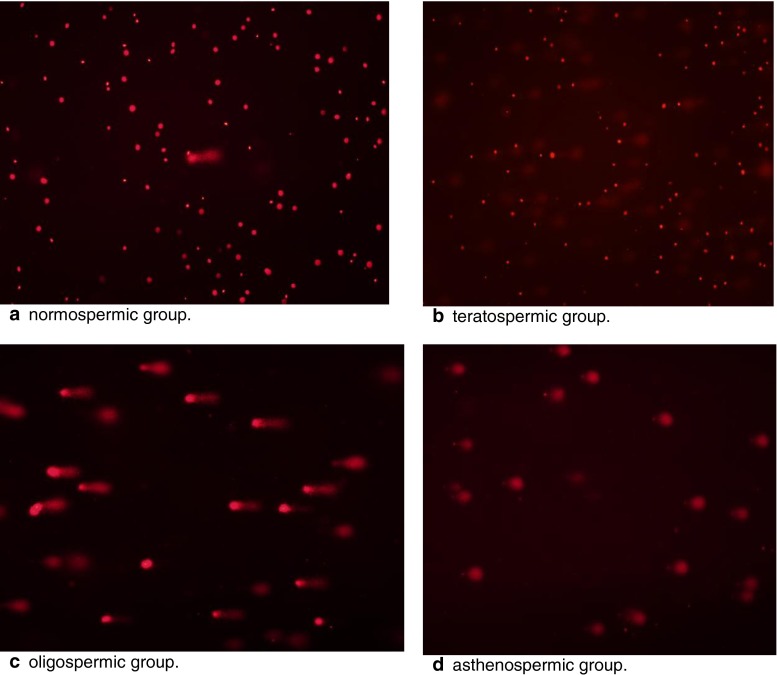
DNA damage in 80 cases, was studied with COMET assay. A gel image of each group is shown in Fig. 1.
Fig. 1.
Comet Assay gel representative images per study group
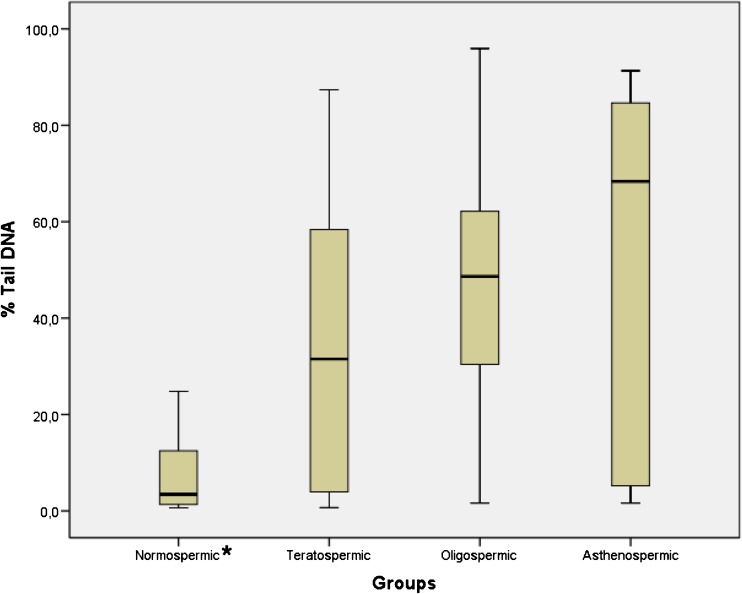
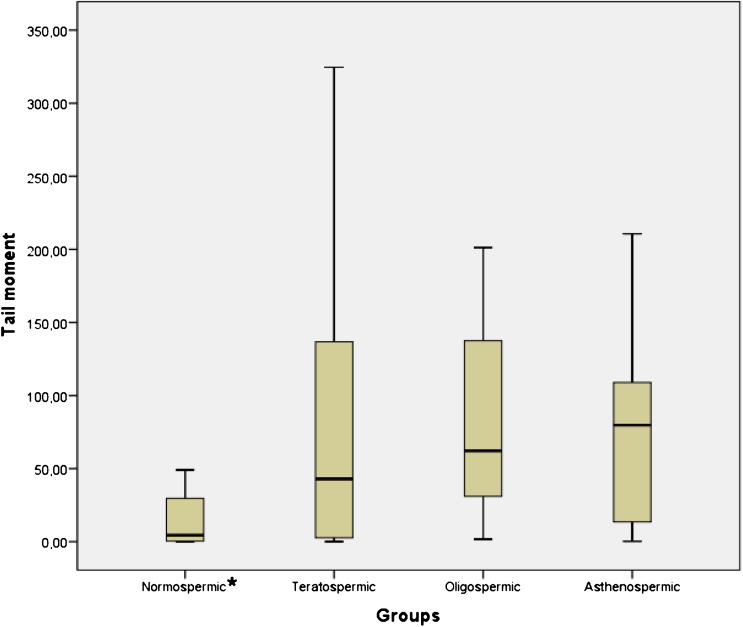
There was a statistically significant difference between groups in terms of the % Tail DNA (Fig. 2; p < 0,05) and Tail Moment (Fig. 3; p < 0,05). % Tail DNA in the normospermic group was significantly lower than in the other groups (p < 0,05). However, Tail Moment in the normospermic group was significantly lower than oligospermic and asthenospermic groups (p < 0,05) but there is no statistically significant difference between normospermic and teratospermic groups.
Fig. 2.
Boxplot representation of % Tail DNA values in groups. Mean ± SD for normospermic, teratospermic, oligospermic and asthenospermic goups are 7,21 ± 7,52; 35,69 ± 32,08; 47,69 ± 26,59; 52,88 ± 35,45 respectively
Fig. 3.
Boxplot representation of Tail Moment values in groups. Mean ± SD for normospermic, teratospermic, oligospermic and asthenospermic goups are 17,74 ± 25,29; 78,05 ± 95,96; 88,12 ± 77,07; 69,78 ± 56,85 respectively
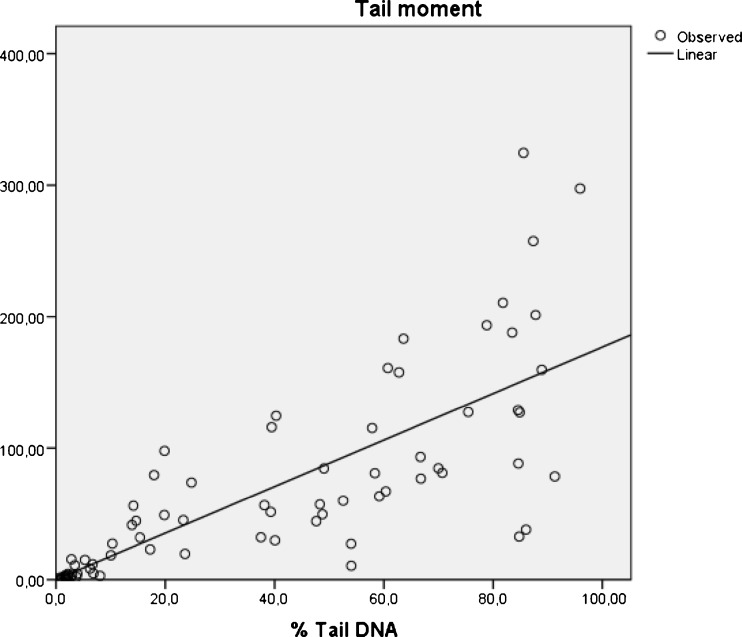
When the relationship between age, spermiogram parameters, % Tail DNA and Tail Moment was investigated, it was found that sperm count had a negative correlation with the % Tail DNA (r = −0,41; p < 0,01) and a negative correlation with Tail Moment (r = −0,23; p < 0,05). Motility had a negative correlation with the % Tail DNA (r = −0,31; p < 0.01). Morphology had a negative correlation with the % Tail DNA (r = −0,49; p < 0,01) and a negative correlation with Tail Moment (r = −0,35; p < 0,01). Moreover, a highly positive correlation (r = 0,79; p < 0,01) was detected in the relationship between the % Tail DNA and Tail Moment, as expected (Fig. 4).
Fig. 4.
The relationship between % Tail DNA and Tail Moment
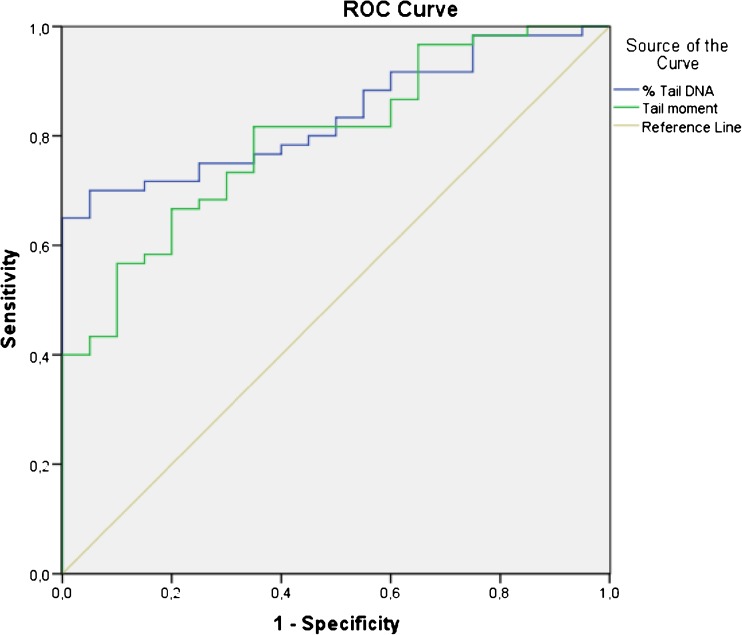
The sensitivity, specificity, the cut-off values and the area under the curve for % Tail DNA and Tail Moment obtained by the ROC analysis are shown in Table 1. Graphical representation of ROC curve for % Tail DNA and Tail Moment is shown in Fig. 5.
Table 1.
Cut-off values with sensitivity and specificity observerd for % Tail DNA and Tail moment
| Comet measurement | n | Areab | Cut-off valuea (%) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| % Tail DNA | 60 | 0,837 | 15,00 | 0,717 | 0,800 |
| Tail moment | 60 | 0,796 | 15,21 | 0,750 | 0,650 |
aCut-off values for male infertility were obtained from 80 cases; 20 normospermic fertil individuals vs. 60 infertile patient
bArea under the ROC curve
Fig. 5.
ROC curve comparing the comet measurements “% Tail DNA and Tail Moment”
Discussion
Sperm DNA damage has an important effect on in vivo fertilization [2]. Many studies have shown that the levels of sperm DNA damage in fertile and infertile males were considerably different [22]. In fact, the sperm of infertile individuals has been found to be more sensitive to agents like H2O2 and radiation which cause damage to DNA [21]. If the sperm DNA damage exceeds 30 % as detected by the SCSA [23] or 20 % by TUNEL [24], the in vivo fertilization rate seems to be close to zero. For all these reasons, diagnosis of sperm DNA damage can be taken as a new indicator for the assessment of male infertility.
WHO guideline categorized infertile individuals into four groups as; normospermics, teratospermics, oligospermics and asthenospermics. In this study we compare sperm DNA damage (% Tail DNA) values of these groups to investigate whether there is any difference among them. As a result we found that the DNA damage in normospermic group was significantly lower than the others (p < 0,05) but there is no statistically significant difference between teratospermic, oligospermic and asthenospermic groups. These results suggested that the sperm DNA damage has the same deleterious effects on sperm count, motility and morphology.
There have been many studies which investigated the correlation between human sperm DNA damage and semen parameters using different methods. For example; Kodama et al. reported that 8-OHdG quantitation had a negative correlation to sperm count [25], while Shen et al. stated that it had a negative correlation to sperm count, motility and morphology [26]. Oosterhuis et al. indicated that phosphatidylserine expression had a reverse correlation to sperm count and motility [27]. Benchaib et al. used the TUNEL method in their study in 104 couples who applied for IVF (n = 50) and ICSI (n = 54) treatment, and stated a negative correlation between DNA damage and sperm count and motility [28]. Sun et al. carried out a study in which the TUNEL method was applied by flow cytometry. They found that DNA damage had a negative correlation to sperm motility and morphology, but no significant correlation to age [29].
On the other hand Morris et al. carried out a study using the alkaline COMET assay method on 60 males who had applied for IVF treatment, and found out that DNA damage increased with age. They also revealed that DNA damage had a positive correlation to abnormal sperm morphology but a negative correlation to sperm count. Unexpectedly, a positive correlation between DNA damage and motility was shown in the study [12].
In a study performed using the alkaline COMET assay, NT-condensed and NT-decondensed methods, Irvine et al. found a significant negative correlation between sperm DNA damage and semen quality, especially in sperm count. When they compared the three techniques of measuring DNA damage, COMET ASSAY was discovered to be better at differentiating the fertile and infertile groups [16].
As in our study, Trisini et al. revealed a negative correlation between DNA damage and sperm count, motility and morphology in a study carried out using neutral COMET assay [30]. We also found a positive but not significant correlation (p = 0,051192) between age and DNA damage. Although these findings suggested that the factors to blame sperm DNA damage and semen quality are the same, there is still idiopathic male infertility existing with normal sperm parameters. In a recent study carried out with SCSA, DNA damage in infertile males with normal sperm parameters was shown to be significantly higher than that of fertile males. Saleh et al. examined 92 infertile males receiving infertility treatment in a SCSA study. While 21 of them had normal sperm parameters, the spermiogram results of 71 were not within normal limits. The control group consisted of 16 fertile volunteers. DNA damage was measured as DNA fragmentation index (DFI) and found that DFI (%) was significantly higher in infertile males with normal sperm parameters than it was in fertile males. They reported that they did not determine a significant difference between normospermic infertile males and other infertile males with abnormal semen parameters [22]. So we can conclude that infertile men possess substantially more sperm DNA damage than fertile men do.
In the light of these findings, sperm DNA damage analysis can be a discriminating test to identify hidden sperm anomalies in patients with normal semen parameters who were diagnosed with idiopathic infertility.
At present, the clinical value of comet measurements on predicting male infertility have not been reported yet. For this purpose we test comet measurements, % Tail DNA and Tail Moment, with ROC analysis. % Tail DNA represented a threshold value of 15 % for predicting male infertility with an area under the curve 0,837 and Tail Moment represented a threshold value of 15,21 % with an area under the curve 0,796. However, these results are not comparable with previous studies because it is not mentioned which comet measurement were used in these studies [15, 18, 31]. Higher area under the ROC curve has been shown by % Tail DNA with a high sensitivity and specificity of 0,717 and 0,800 respectively (Table 1, Fig. 5). Moreover, when taking Tail Moment into account we found that there is no statistically significant difference between normospermic and teratospermic groups and also we found there is no correlation between motility and Tail Moment in our patient population. According to these results % Tail DNA seems the best predictor comet measurement for alkaline comet assay but further studies are needed to reach precise results.
Before using the tests of sperm DNA damage in routine analysis, the correlation between semen parameters and sperm DNA integrity must be investigated. If sperm DNA integrity can determine a diagnosis, it should be used in routine practice. When the method for measuring DNA damage is improved and the results are used in practice, this development may answer both patients’ and doctors’ questions and help their treatment modality.
Footnotes
Capsule An accurate sperm DNA damage analysis provides an important support in clinical evaluation so it is highly recommended before assisted reproductive technologies.
References
- 1.Hamada A, Esteves SC, Agarwal A. Unexplained male infertility: potential causes and management. Hum Androl. 2011;1:2–16. doi: 10.1097/01.XHA.0000397686.82729.09. [DOI] [Google Scholar]
- 2.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 3.Avendano C, Oehninger S. DNA fragmentation in morphologically normal spermatozoa: how much should we be concerned in the ICSI era? J Androl. 2011;32:356–63. doi: 10.2164/jandrol.110.012005. [DOI] [PubMed] [Google Scholar]
- 4.Avendano C, Franchi C, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94:549–57. doi: 10.1016/j.fertnstert.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011;13:69–75. doi: 10.1038/aja.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meseguer M, Martinez-Conejero JA, O’Connor EJ, Pellicer A, Remohi J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89:1191–9. doi: 10.1016/j.fertnstert.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/S0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 9.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, infertile and infertile men. Fertil Steril. 2001;75:674–7. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 10.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. The Danish first pregnancy planner study team. Sperm chromatin damage impairs human fertility. Fertil Steril. 2000;73:43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Erenpreiss J, Sharma R. Sperm chromatin assessment. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of assisted reproductive technologies. UK: Informa Healthcare; 2009. pp. 67–84. [Google Scholar]
- 12.Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–8. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 13.Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27:53–9. doi: 10.2164/jandrol.05068. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Peiro A, Oliver-Bonet M, Navarro J, Abad C, Guitart SM, Amengual MJ, et al. Dynamics of sperm DNA fragmentation in patients carrying structurally rearranged chromosomes. Int J Androl. 2011;34:e546–53. doi: 10.1111/j.1365-2605.2011.01153.x. [DOI] [PubMed] [Google Scholar]
- 15.Ribas-Maynou J, Garcia-Peiro A, Fernandez-Encinas A, Abad C, Amengual MJ, Prada E et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: tunel assay, SCSA, SCD test and alkaline and neutral comet assay. Andrology 2013;1–8. [DOI] [PubMed]
- 16.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 17.Liao W, McNutt MA, Zhu WG. The comet assay: a sensitive method for detecting DNA damage in individual cells. Methods. 2009;48:46–53. doi: 10.1016/j.ymeth.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Simon L, Lutton D, McManus J, Lewis SEM. Sperm DNA damage measured by the alkaline comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril. 2011;95:652–7. doi: 10.1016/j.fertnstert.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 20.VJ M-M, Green MH, Schmezer P, Pool-Zobel BL, DeMeo MP, Collins A. The single cell gel electrophoresis assay (comet assay): a European review. Mutat Res. 1993;288:47–63. doi: 10.1016/0027-5107(93)90207-V. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CM, Lewis SEM, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Reprod Dev. 1996;2:613–9. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 22.Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–8. doi: 10.1016/S0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 23.Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology. 2006;65:979–91. doi: 10.1016/j.theriogenology.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Sergerie M, Laforest G, Bujan L, Bissonnette F, Bleau G. Sperm DNA fragmentation: threshold value in male infertility. Hum Reprod. 2005;20:3446–51. doi: 10.1093/humrep/dei231. [DOI] [PubMed] [Google Scholar]
- 25.Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/S0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 26.Shen HM, Chia SE, Ong CN. Evaluation of oxidative DNA damage in human sperm and its association with male infertility. J Androl. 1999;20:718–23. [PubMed] [Google Scholar]
- 27.Oosterhuis GJE, Mulder AB, Kalsbeek-Batenburg E, Lambalk CB, Schoemaker J, Vermes I. Measuring apoptosis in human spermatozoa: a biological assay for semen quality? Fertil Steril. 2000;74:245–50. doi: 10.1016/S0015-0282(00)00623-3. [DOI] [PubMed] [Google Scholar]
- 28.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–8. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 29.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–7. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 30.Trisini AT, Singh NP, Duty SM, Hauser R. Relationship between human semen parameters and deoxyribonucleic acid damage assessed by the neutral comet assay. Fertil Steril. 2004;82:1623–32. doi: 10.1016/j.fertnstert.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 31.Ribas-Maynou J, Garcia-Peiro A, Fernandez-Encinas A, Abad C, Amengual MJ, Prada E, et al. Alkaline and neutral comet assay profiles of sperm DNA damage in clinical groups. Hum Reprod. 2012;27:652–8. doi: 10.1093/humrep/der461. [DOI] [PubMed] [Google Scholar]