Abstract
Purpose
According to the latest ART report for Europe, about 13 % of pregnancies after frozen embryo transfer are multiple. Our objective was to analyse the impact on the multiple pregnancy rate of two eSFET (elective single frozen embryo transfers) versus a DFET (double frozen embryo transfer) in women aged under 38 years, who had not achieved pregnancy in their fresh transfer and who had at least two vitrified embryos of A/B quality.
Methods
This study was conducted from January 2010 to June 2013 at a public hospital. The couples were divided into three groups. Group DFET: the first cryotransfer of two embryos (105 women); cSFET group: the only cryotransfer of a single vitrified embryo (60 women); eSFET group, individually vitrified embryos: 20 patients included in a clinical trial of single-embryo fresh and frozen transfer and 21 patients who chose to receive eSFET.
Results
The clinical pregnancy rate was 38.1 % in the DET group and the cumulative clinical pregnancy rate was 43.3 % in the eSFET group. There were no significant differences between the DFET and eSFET groups (30.0 vs 34.1 %) in cumulative live birth delivery rate. The rate of multiple pregnancies varied significantly between the DFET and eSFET groups (32.5 vs 0 %, p < 0.05).
Conclusions
For good-prognosis women aged under 38 years, taking embryo quality as a criterion for inclusion, an eSFET policy can be applied, achieving acceptable cumulative clinical pregnancy and live birth rates and reducing multiple pregnancy rates.
Keywords: Cumulative live birth rate, Elective single frozen embryo transfer, Multiple pregnancy rate, Vitrification
Introduction
The number of cryotransfer cycles practised has increased considerably in recent years for two main reasons: first, the increased effectiveness [1] and safety [2] of the process of embryo vitrification; second, the policy adopted of freezing all embryos in the stimulated cycle and transferring them in a natural or an artificial cycle, with the aim of reducing the risk of ovarian hyperstimulation [3] and of ectopic pregnancy [4]. This increase in cryotransfer cycles has been paralleled by increased rates of multiple pregnancy following cryotransfer, both in Spain and in other countries [5, 6].
In recently-published meta-analyses [7, 8] comparing elective single embryo transfer (eSET) vs. double embryo transfer (DET) in good-prognosis women, no significant differences were observed in cumulative pregnancy rates when cryopreserved embryo transfers were considered. Higher pregnancy rates in DET were only observed when fresh transfers alone were taken into account. However, the evident benefit provided by eSET with fresh transfers in terms of reducing rates of multiple pregnancy in good-prognosis patients, is partially lost with cryotransfer, because these cycles are usually followed by double frozen embryo transfer (DFET) [9, 10]. To our knowledge, no previous study has been made to compare the pregnancy rates obtained in DFET with those obtained with two sequential cycles of single frozen embryo transfers (eSFET).
In view of the above considerations, the aim of this study is to analyse the impact on the pregnancy rate per cryotransfer and on the rate of multiple pregnancies of the implementation of a programme of devitrified single-embryo cryotransfers. To do so, a retrospective comparison was made at our hospital of the results obtained from cryotransfers performed in good-prognosis women.
Material and methods
A retrospective study was carried out, for the period from January 2010 to June 2013, of cryotransfers carried out for 221 couples with good prognosis. Inclusion criteria were (in the corresponding fresh cycle) age less than 38 years, body mass index between 19 and 29 kg/m2, FSH below 15 mUl/ml on the third day of the cycle, first cycle IVF/ICSI or second cycle with previous pregnancy not carried to term. Exclusion criteria were more than five years of infertility, previous surgery (fibroids, endometriosis, hydrosalpinx), uterine malformations, repeated miscarriages and previous unsuccessful complete cycles of IVF/ICSI. The patients received ovarian stimulation for IVF treatment at the Human Reproduction Unit of the Virgen de las Nieves University Hospital (Granada, Spain).
We analysed the cryotransfer results of the women who had not achieved pregnancy in their fresh transfer and who had at least two vitrified embryos of A/B quality, a quality cleavage-stage embryos (7-9 cells and < 20 % fragmentation) [11]. The cryotransfers of three embryos (n = 3) still taking place in 2010 were excluded from the analysis.
The following study groups were established (Fig. 1): (i) DFET (double frozen embryo transfer): the first cryotransfer of two embryos (105 couples). (ii) cSFET (compulsory single frozen embryo transfer): the only cryotransfer of an embryo (60 patients who had only a vitrified embryo). (iii) eSFET (elective single frozen embryo transfer), of individually vitrified embryos: 21 patients who chose to receive eSFET and 20 patients included in a clinical trial of single-embryo fresh and frozen transfers (Clinical Trial Number: NCT01909570). This randomised clinical trial was approved by the Clinical Research Ethics Committee of the Virgen de las Nieves University Hospital (Granada, Spain). Institutional review board approval was not required for the present study owing to its retrospective nature. The data were collected from the medical records of couples. Written informed consent had been obtained from all patients in order to use the data for future scientific research.
Fig. 1.
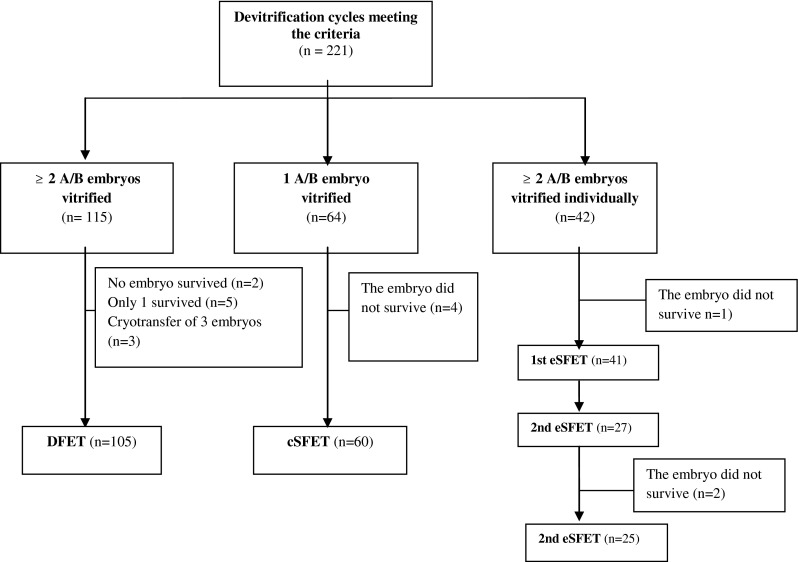
Flowchart of participants in the study
Protocols
All patients received ovulation stimulation treatment, in order to achieve multiple follicular development. GnRH agonists in the long analogue protocol or the antagonist protocol were used.
To ensure proper selection of the embryo or embryos to be transferred, with the best possible potential for implantation, they were assessed according to the criteria of the Spanish Association for the Study of Reproductive Biology (ASEBIR) revised in 2008 [11]. The non-transferred embryos of A/B quality were vitrified on the third day (D + 3), using commercial vitrification medium (Origio Vitrification, Denmark) containing ethylene glycol and 1,2-propanediol in HTF culture medium fluid in increasing concentrations, and the Cryoleaf (McGill Cryoleaf, Origio, Denmark) storage device. Cryotransfer protocol: the protocols used were natural cycle in 32 (13.9 %) cryotransfers and substitution cycle in 199 (86.1 %) cryotransfers. The day before cryotransfer, the embryos were devitrified using a devitrification kit (Origio Warming, Denmark) in accordance with the manufacturer’s instructions, evaluated for embryo quality and cryosurvival (≤50 % lysed cells) and left in culture until the next day. On the day of the cryotransfer (D + 4), embryo quality was again tested, with special attention to embryo cleavage.
Outcomes measured
Maternal age (years) was calculated at the time of oocyte collection. The thaw survival rate was calculated as embryos survived per total number of embryos warmed. The live birth rate was calculated by birth events per patient. The clinical miscarriage rate was calculated by foetal heart positive pregnancies that did not result in a live birth per foetal heart positive pregnancies. The multiple birth rate was calculated by the number of twin births per total birth events. Accumulative ongoing pregnancy per patient and accumulative live birth delivery per patient were calculated for the eSFET group.
Statistical analysis
The qualitative variables are described by their absolute value (n) and relative value (%) and compared using the chi square test. The quantitative variables are expressed as the mean, standard deviation and maximum and minimum values. The three groups were compared using analysis of variance and homogeneity of variances was analysed by the Levene test. In all cases, a significance level of 5 % was applied.
Results
Patients
The epidemiological characteristics of the patients, the stimulation cycle and the laboratory results in fresh cycles were similar in all three groups (Tables 1 and 2). In general, more total and metaphase II oocytes by follicular puncture were obtained in the DFET group than in the cSFET group, but the difference was not statistically significant.
Table 1.
Demographics parameters of patients
| DFET (n = 105) | cSFET (n = 60) | eSFET (n = 41) | |
|---|---|---|---|
| Woman’s age | |||
| Mean | 34.3 ± 4.1 | 34.4 ± 3.8 | 32.2 ± 3.6 |
| Range | (21–37) | (24–37) | (21–37) |
| Man’s age | |||
| Mean | 36.4 ± 4.2 | 36.3 ± 4.8 | 33.9 ± 4.2 |
| Range | (23–48) | (22–50) | (21–42) |
| Duration of infertility (years) | |||
| Mean | 3.2 ± 1.1 | 3.2 ± 1.0 | 3.1 ± 1.1 |
| Range | (1–5) | (1–5) | (1–5) |
| Cause of infertility, n (%) | |||
| Tubal factor | 10 (9.5) | 5 (8.4) | 4 (9.7) |
| Male factor | 47 (44.8) | 26 (43.3) | 18 (43.8) |
| Unknown | 38 (36.2) | 23 (38.3) | 15 (36.7) |
| Combined | 4 (3.8) | 3 (5.0) | 2 (4.9) |
| Other | 6 (5.7) | 3 (5.0) | 2 (4.9) |
Mean ± SD (min-max)
NS
Table 2.
Stimulated cycle results and laboratory characteristics for the fresh cycle
| DFET (n = 105) | cSFET (n = 60) | eSFET (n = 41) | |
|---|---|---|---|
| Days of stimulation | |||
| Mean ± SD | 10.7 ± 1.6 | 9 ± 2.2 | 10.5 ± 1.7 |
| Total dose of FSH (UI) | |||
| Mean ± SD | 1,938 ± 704 | 1,621 ± 607 | 1,864 ± 564 |
| E2 day hCG (pg/mL) | |||
| Mean ± SD | 2,390 ± 1,344 | 2,171 ± 1,564 | 2,355 ± 1,167 |
| Protocol, n (%) | |||
| Antagonist | 22 (29.4) | 14 (29.2) | 6 (28.6) |
| Long analogue | 53 (71.6) | 34 (70.8) | 15 (71.4) |
| Oocytes obtained | |||
| Mean | 12.5 ± 4.5 | 10.4 ± 4.2 | 11.1 ± 4.5 |
| Range | (4–16) | (3–16) | (3–18) |
| Oocytes MII | |||
| Mean | 11.2 ± 4.3 | 8.9 ± 4.0 | 9.0 ± 3.9 |
| Range | (4–16) | (1–16) | (2–18) |
| Oocytes MII rate (%) | 89.6 | 85.6 | 81.8 |
| Fertilised oocytes | |||
| Mean | 8.6 ± 3.5 | 6.2 ± 3.3 | 6.5 ± 3.3 |
| Range | (2–12) | (2–10) | (2–14) |
| Fertilisation rate (%) | 76.7 | 70.2 | 72.2 |
| Good-quality embryos vitrified (A/B) | |||
| Mean | 2.3 ± 2.3 | 1.0 ± 0 | 2.4 ± 1.9 |
| Range | (2–4) | (1) | (2–3) |
| Day of transfer, n (%) | |||
| D + 2 | 31 (29.5) | 18 (30.0) | 12 (29.3) |
| D + 3 | 74 (70.5) | 42 (70.0) | 29 (70.7) |
Mean ± SD (min–max)
NS
Table 3 shows the clinical results of the cryotransfers. The implantation rate was similar in all three groups (25.2, 21.7 and 22.5 % for DFET, cSFET and eSFET, respectively). The clinical pregnancy rate was 38.1 % (40/105) in the DET group and the cumulative clinical pregnancy rate was 43.3 % (18/41) in the eSFET group. Differences were not statistically significant (p = 0.52). However, there were significant differences in clinical pregnancy rates between the DFET and cSFET groups (38.1 vs 21.7 %, p = 0.03) and between the cSFET and eSFET groups (21.7 vs 43.3 %, p = 0.02). The overall rate of spontaneous miscarriage was similar in all three groups (22.5 % in DFET, 23.1 % in cSFET and 22.2 % in eSFET).
Table 3.
Clinical results of cryotransfers per patient
| DFET (n = 105) | cSFET (n = 60) | eSFET (n = 41) | |
|---|---|---|---|
| Implantation rate (%) | 25.2 | 21.7 | 22.5 |
| Clinical pregnancy/patient, n (%) | |||
| 1st Cryotransfer | 40 (38.1)a | 13 (21.7) | 11 (26.3) |
| Cumulative | 18 (43.3)a | ||
| Multiple gestation, n (%) | 13 (32.5)b | 0 (0) | 0 (0) |
| Miscarriage, n (%) | 9 (22.5) | 3 (23.1) | 4 (22.2) |
| Ongoing pregnancy/patient, n (%) | |||
| 1st Cryotransfer | 31 (30.0) | 10 (17.0) | 9 (21.8) |
| Cumulative | 14 (34.1)a | ||
| Live birth delivery rate/patient, n (%) | |||
| 1st Cryotransfer | 31 (30.0) | 10 (17.0) | 9 (21.8) |
| Cumulative | 14 (34.1)a | ||
| Multiple delivery, n (%) | 8 (26.0) | 0 (0) | 0 (0) |
a p < 0.05 vs cSFET; b p < 0.05 vs eSFET
With respect to the live birth delivery rate, there were no significant differences between the DFET and eSFET groups (30.0 vs 34.1 %). However, this rate was significant lower in the cSFET group (17.0 %) than in the eSFET group. The rate of multiple pregnancies varied significantly between the DFET and eSFET groups (32.5 vs 0 %, p = 0.04).
Results of embryo vitrification
Table 4 shows that the embryo survival rate was similar in the three groups (95.7 % DFET, 93.8 % cSFET, 95.2 % eSFET). There were no significant differences in the number of embryos divided after 24 h of post-vitrification culture (71.6 % DFET, 70.0 % cSFET, 71.2 % eSFET).
Table 4.
Laboratory characteristics for the cryotransfers
| DFET | SFET | ||
|---|---|---|---|
| cSFET | eSFET | ||
| Embryos devitrified (n) % | 224 | 64 | 69 |
| Embryos surviving (n) % (≥50 % of the blastomeres) | 215/224 (95.7) | 60/64 (93.8) | 66/69 (95.2) |
| Embryos divided (n) % | 154/215 (71.6) | 42/60 (70.0) | 47/66 (71.2) |
NS
Discussion
Our retrospective study shows that the multiple pregnancy rate following cryotransfer can be significantly reduced by implementing eSFET programmes within a selected population, without adversely affecting the success rate.
According to the latest ART report for Europe [6], about 30 % of the embryos obtained after IVF are cryopreserved, with embryo cryotransfers accounting for 22 % of IVF activity in assisted reproduction clinics in Europe and up to 31 % in Japan [12]. In Spain, almost 20 % of IVF pregnancies are achieved by cryotransfer [5]. Therefore, in evaluating the results achieved, we must consider not only the successes (live births per cycle) but also the complications arising from an IVF cycle. In our study, the multiple pregnancy rate for the DFET group was 32.5 %, considerably higher than the 16.0 % obtained elsewhere with the cryotransfer of two embryos [5]. These differences arise from the fact that our study was based on a population with good prognosis, for whom the risk of multiple pregnancy is higher.
Although the patients in our study were selected according to criteria of good prognosis, these criteria were broader than those described in the literature for other eSET studies [8]. Therefore, we believe that our results facilitate the implementation of eSFET programmes in daily clinical practice.
The high rates of cumulative clinical pregnancies (43.3 %) and of live births (34.1 %) with eSFET obtained in this study can be explained by the effectiveness of the vitrification technique. This would confirm the predictive model proposed by Roberts et al. [13], based on the results achieved at five centres in the UK, according to which it is possible to simultaneously predict the outcome of the transfer of two embryos (DET) and of one embryo (eSET). This model was applied to different cases using eSET, both in fresh cycles and in complete treatments including cryotransfers, and the authors concluded that the percentage of live births would be higher with eSET with these complete cycles, while the rate for live births with eSET plus cryotransfer would be very similar to that obtained by DET. These good results achieved with eSET, however, depend on there being a high rate of viability of the cryopreserved embryos. This condition was met in the present study.
Our study confirms the view proposed in the American Society for Reproductive Medicine (2012) [14] that with effective cryopreservation that provokes little or no damage to embryos, cumulative birth rates should, in theory, be higher when embryos are transferred individually. A recent study conducted in Japan [12] reported that the relative increase in the success rate of the entire cycle can reach 36.8 % in single embryo cryotransfer programmes.
The success of vitrification programmes can be influenced by many variables, especially the survival of embryos and their subsequent development in culture. The rates of embryo survival (91 %) and of cleavage after devitrification (70 %) observed in the different groups in our study are consistent with those described elsewhere for embryo vitrification programmes with own eggs, both for survival (85.5–94.2 %) [1, 15–17] and for cleavage (61.4–78 %) [18, 19]. These high rates are of crucial importance in determining the performance of cryotransfer programmes.
In our study, the rate of miscarriages (about 22.6 %) is higher than that reported in fresh eSET programmes, but the difference may be due to the fact that older women were included in our study. Moreover, our rate is similar to that obtained by Ishihara et al. (2013) in a programme of single embryo cryotransfers for women with similar ages to those in our study (26.6 %).
Women were included in the cSFET group when they had only one vitrified embryo, and so there was no option but to perform voluntary eSFET. The existence of fewer embryos to vitrify has been associated with a poorer reproductive prognosis [20], which to a certain extent is confirmed by the fact that this group presented lower rates of clinical pregnancy and of live birth delivery (17.0 %). The rates of implantation and of live birth in the cSFET group were similar to those obtained by Hyden-Granskog et al. (2005) [21]. However, the rates of implantation (28.6 %) and of live birth delivery (40.7 %) in the eSFET group were slightly higher than in our study. These differences with respect to the eSFET group may be due to the criteria used by these authors, who performed eSFET when two or three embryos had been cryopreserved in the same device and more than one embryo fulfilled the criteria for transfer after thawing; in all cases, an embryo with a blastomere survival rate of at least 75 % was selected for transfer, and if possible the remaining embryos were re-cryopreserved. The fact that our own study obtained similar results for the cSFET group, where it is not possible to apply these criteria, supports the above hypothesis.
Recent studies of re-cryopreservation have shown that with twice-frozen-thawed embryos, either with the vitrification method [22, 23] or with slow freezing [24, 25] similar rates of pregnancy and delivery rate are obtained, compared with once-frozen-thawed embryos. However, lower rates of embryo survival have been observed after the second thawing [22, 24]; these authors suggest the need for further studies to evaluate the long-term results and to confirm the real safety of transferring twice-frozen human embryos. In view of the data obtained, we believe that it is essential to plan, beforehand, the number of embryos to be vitrified by each device.
One limitation of our study is that after devitrification, no culture to blastocyst was performed, although this has been shown to improve pregnancy rates in cryotransfer programmes [26, 27]. In a future study, it would be useful to investigate the efficacy of sequential elective single frozen blastocyst transfer compared with cleavage stage embryo transfer.
Not all our patients achieved pregnancy in their fresh transfer, and for this reason our results cannot be generalised for FET when the cryopreservation of the entire embryo cohort is performed. At present, there is considerable controversy regarding the implantation of this strategy [28].
Although it was not the main aim of this study, we would also like to note some financial aspects concerning the implementation of eSET programmes. Many studies have examined the cost-effectiveness of SET [29–31], but only one has taken into consideration the results of cryotransfers [32], and none have analysed the cost-effectiveness of SET per single-embryo cryotransfer. Various models have been proposed to estimate the cost-effectiveness of this strategy [29, 33]. According to Dixon et al. (2008), the probability of eSET plus eSFET being cost-effective decreases with the woman’s age. Following the model proposed by De Sutter et al. (2002) and with the pregnancy rate per transfer of frozen-thawed embryo obtained in our study (26 %), the saving per child, using an eSET plus eSFET strategy rather than DET, would be around 13 %.
Taking into account these considerations, and in view of the important economic and social savings that may be obtained, countries like Norway, Denmark and Belgium only finance cycles if SET is performed. Furthermore, the results of our study have led our regional health administration to adopt policies promoting eSET: a third cycle is only offered to couples if, in one of the two previous cycles, eSET (fresh or cryotransfer) was chosen. Thus, Andalusia has taken the same line as the above European countries in adopting active public policies to reduce the unacceptably high rate of multiple pregnancies associated with assisted reproduction [34]. We believe that similar policies should be implemented throughout Spain.
In summary, our results demonstrate that eSFET should be incorporated into daily practice because, as with fresh transfers, it reduces rates of multiple pregnancies without affecting the rates of pregnancy or of cumulative live births in a selected population.
Acknowledgments
The authors wish to thank the doctors, embryologists, nurses and staff at the Virgen de las Nieves University Hospital in Granada for their enthusiastic help during all phases of this study. We also thank the infertile couples for their participation, to improve the results obtained in the future by infertile couples receiving assisted reproduction. This article is related to the Ph.D. doctoral thesis of M.L. López Regalado.
Conflict of interest
None declared.
Footnotes
Capsule An eSFET policy can be applied, achieving acceptable cumulative live birth rates and reducing multiple pregnancy rate, for good prognosis women who had not achieved pregnancy in their fresh transfer.
References
- 1.Cobo A, Santos MJ D l, Castellò D, Gámiz P, Campos P, Remohí J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. 2012;98:1138–46. doi: 10.1016/j.fertnstert.2012.07.1107. [DOI] [PubMed] [Google Scholar]
- 2.Liu SY, Teng B, Fu J, Li X, Zheng Y, Sun XX. Obstetric and neonatal outcomes after transfer of vitrified early cleavage embryos. Hum Reprod. 2013;28:2093–100. doi: 10.1093/humrep/det104. [DOI] [PubMed] [Google Scholar]
- 3.Absalan F, Ghannadi A, Kazerooni M. Reproductive outcome following thawed embryo transfer in management of ovarian hyperstimulation syndrome. J Reprod Infertil. 2013;14:133–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Decleer W, Osmanagaoglu K, Meganck G, Devroey P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization-embryo transfer cycles: a retrospective cohort study. Fertil Steril. 2014;101:163–5. doi: 10.1016/j.fertnstert.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Prados F, De los Santos MJ, Cabello Y, Buxaderas R, Segura A, Hernández J, et al. [Internet] Registro de la Sociedad Española de Fertilidad: Tecnicas de reproducción asistida (IA y FIV/ICSI). Año2.010. Available at: https://www.registrosef.com/public/Docs/sef2010_IAFIV.pdf
- 6.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, et al. The european ivf-monitoring (EIM) Consortium, for The european society of human reproduction and embryology (ESHRE) Hum Reprod. 2013;28:2318–31. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 7.Mc Lernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoilin JCM, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010:341. [DOI] [PMC free article] [PubMed]
- 8.Pandian Z, Bhattacharya S, Ozturk O, Serour G, Templeton A. Number of embryos for transfer following in-vitro fertilization or intra-cytoplasmic sperm injection. 2013;7. Cochrane Database Syst Rev. [DOI] [PMC free article] [PubMed]
- 9.Martikainen H, Tiitinen A, Tomás C, Tapanainen J, Orava M, Tuomivaara L, et al. One versus two embryo transfer after IVF and ICSI: a randomized study. Hum Reprod. 2001;16:1900–3. doi: 10.1093/humrep/16.9.1900. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez DB, Tur R, Mancini F, Parriego M, Rodríguez I, Barri PN, et al. Elective single embryo transfer and cumulative pregnancy rate: five-year experience in a Southern European Country. Gynecol Endocrinol. 2012;28:425–28. doi: 10.3109/09513590.2011.633662. [DOI] [PubMed] [Google Scholar]
- 11.Ardoy M, Calderón G, Cuadros J, Figueroa MJ, Herrer R, Moreno JM et al. II Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. 2ª Ed. Madrid: Asocicación para el Estudio de la Biología de la Reproducción (ASEBIR); 2008.
- 12.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson D. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2013;101:128–33. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Roberts SA, Fitzgerald CT, Brison D. Modelling the impact of single embryo transfer in a national health service IVF programme. Hum Reprod. 2011;24:122–31. doi: 10.1093/humrep/den355. [DOI] [PubMed] [Google Scholar]
- 14.American Society for Reproductive Medicine Elective single embryo transfer. Fertil Steril. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Rama GA, Haranath GB, Krishna KM, Prakash GJ, Madan K. Vitrification of human 8-cell embryos, a modified protocol for better pregnancy rates. Reprod BioMed Online. 2005;11:434–37. doi: 10.1016/S1472-6483(10)61135-2. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Chen ZJ, Yang HJ, Zhong WX, Ma SY, Li M. Comparison of vitrification and slow-freezing of human day 3 cleavage stage embryos: post-vitrification development and pregnancy outcomes. Zhonghua Fu Chan Ke Za Zhi. 2007;42:753–55. [PubMed] [Google Scholar]
- 17.Wang XL, Zhang X, Qin YQ, Hao DY, Shi HR. Outcomes of day 3 embryo transfer with vitrification using Cryoleaf: a 3-year follow-up study. J Assist Reprod Genet. 2012;29:883–9. doi: 10.1007/s10815-012-9814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XJ, Yang YZ, Lv Q, Wang Y, Cao XH, Guo C, et al. The impact of two different thaw protocols on outcomes of vitrified cleavage-stage embryos transfer. CryoLetters. 2012;33:411–7. [PubMed] [Google Scholar]
- 19.Desai N, Blackmon H, Szeptycki J, Goldfarb J. Cryoloop vitrification of human day 3 cleavage-stage embryos: post-vitrification development, pregnancy outcomes and live births. Reprod BioMed Online. 2007;14:208–13. doi: 10.1016/S1472-6483(10)60789-4. [DOI] [PubMed] [Google Scholar]
- 20.Mackenna A, Crosby J, Zegers-Hochschild F. Sibling embryo blastocyst development as a prognostic factor for the outcome of day-3 embryo transfer. Reprod Biomed Online. 2013;26:486–90. doi: 10.1016/j.rbmo.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Hydén-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod. 2005;20:2935–38. doi: 10.1093/humrep/dei133. [DOI] [PubMed] [Google Scholar]
- 22.Kumasako Y, Otsu E, Utsunomiya T, Araki Y. The efficacy of the transfer of twice frozen-thawed embryos with the vitrification method. Fertil Steril. 2009;91:383–6. doi: 10.1016/j.fertnstert.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 23.Taylor TH, Patrick JL, Gitlin SA, Michael Wilson J, Crain JL, Griffin DK. Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer. Reprod Biomed Online. 2014;29:59–64. doi: 10.1016/j.rbmo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Koch J, Costello M, Chapman M, Kilani S. Twice-frozen embryos are no detriment to pregnancy success: a retrospective comparative study. Fertil Steril. 2011;96:58–62. doi: 10.1016/j.fertnstert.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M, Egashira A, Murakami K, Araki Y, Kuramoto T. Perinatal outcome of twice-frozen-thawed embryo transfers: a clnical follow-up study. Fertil Steril. 2011;95:2648–50. doi: 10.1016/j.fertnstert.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99:389–92. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 27.Roy T, Bradley C, Bowman M, McArthur S. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101:1294–301. doi: 10.1016/j.fertnstert.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 29.De Sutter P, Gerris J, Dhont M. A health-economic decision-analytic model comparing double with single embryo transfer in IVF/ICSI. Hum Reprod. 2002;17:2891–96. doi: 10.1093/humrep/17.11.2891. [DOI] [PubMed] [Google Scholar]
- 30.Gerris J, De Sutter P, De Neubourg D, Va Royen E, Vander J, Mangelschots K, et al. A real-life prospective health economic study of elective single embryo transfer versus two-embryo transfer in first IVF/ICSI cycles. Hum Reprod. 2004;19:917–23. doi: 10.1093/humrep/deh188. [DOI] [PubMed] [Google Scholar]
- 31.Fiddelers A, Van Montfoort A, Dirksen C, Dumoulin J, Land J, Dunselman G, et al. Single versus double embryo transfer: cost-effectiveness analysis alongside a randomized clinical trial. Hum Reprod. 2006;21:2090–97. doi: 10.1093/humrep/del112. [DOI] [PubMed] [Google Scholar]
- 32.Fiddelers A, Severens J, Dirksen C, Dumoulin J, Land J, Evers J. Economic evaluations of single versus double-embryo transfer in IVF. Hum Reprod. 2007;13:5–13. doi: 10.1093/humupd/dml053. [DOI] [PubMed] [Google Scholar]
- 33.Dixon S, Faghih Nasiri F, Ledger W, Lenton E, Duenas A, Dutcliffe P, et al. Cost-effectiveness analysis of different embryo transfer strategies in England. BJOG. 2008;115:758–66. doi: 10.1111/j.1471-0528.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 34.Guía de reproducción humana asistida en el Sistema Sanitario Público de Andalucía. Sevilla: Consejería de Igualdad, Salud y Políticas sociales de Andalucía, 2013 [Access December 23. 2013]. Electronic text (pdf), 92 p. Available at: www.juntadeandalucia.es/servicioandaluzdesalud.


