Abstract
Purpose
To find a relationship between absence of annulus and asthenozoospermia in Iranian men.
Methods
In the present study, semen samples from 100 asthenozoospermic and 20 normozospermic patients were analyzed for sperm concentration and motility. Spermatozoa were immunostained for the two septin subunits Sept4 and Sept7. The absence of the annulus structure was confirmed by transmission electron microscopy and western blot analysis for septin 4. DNA sequencing for all coding exons of SEPT12 was performed for a patient using peripheral blood sample.
Results
Specific antibodies for SEPT4 and SEPT7 consistently labeled the annuli in spermatozoa from all of the 20 normozospermic men, while in one of 100 patients with asthenozoospermia, 75 % of sperms lacking septin 4 or septin 7 proteins at the annulus. It was shown that the structural defect in annulus formation is not caused by point mutation of SEPT12 gene.
Conclusions
In conclusion, the results of this study demonstrated that the frequency of the absence of annulus in asthenozoospermic sample of Iranian population has a low frequency and could not be assume as a diagnostic marker for classifying asthenozoospermic patients.
Keywords: Male infertility, Asthenozoospermia, Annulus, Septins
Introduction
Asthenozoospermia is one of the major causes of male infertility and can results from morphological and mechanical defects in spermatozoa [1]. The annulus is an electron-dense septin-based structure, which could function as a kind of physical barrier to lateral diffusion between the midpiece and principal piece regions of the sperm tail [2]. Thus, anomalous annuli that are secondarily disorganized by other genetic or nonhereditary etiologies could also accompany impaired motility [1].
Septins are polymerizing GTPases that can form hetero-ologomeric filaments required for a variety of cellular processes, such as cytokinesis, exocytosis, and vesicle traffickings [3–5]. Among the 14septin family members, Sept4 and Sept12 play important roles in the differentiation of postmeiotic germ cells. In addition, Sept1, 4, 7, 12 are the major constituents of the annulus [6, 7]. Previous reports showed that Septin4 null mice are viable; however, males are sterile because they produce immotile sperm with a defective annulus [8].
Analysis of a Japanese cohort of 15 infertile patients revealed annulus disorganization and septin 7 mislocalization in three cases of isolated asthenozoospermia and introduced septins as diagnostic markers for this pathology [7]. Another study on French population resulted to finding a case lacking annulus among 75 asthenozoospermic patients [8]. In the present study, we searched a large number of human asthenozoospermia samples and controls for absence of Sept4, Sept7 in annulus by performing an immunofluorescence detection assay on sperm smear preparations. We confirmed the absence of the annulus structure by transmission electron microscopy. Finally, sequence analysis of SEPT 12 gene was performed for defining the role of SEPT12 gene in the defect of annulus.
Materials and methods
Patients and samples
The samples in this study comprised 20 normozospermic and 100 male patients, showing asthenozoospermia, who visited for infertility at Royan institute. Asthenozoospermic samples were defined as those with <32 % motile sperm (grades a + b). Among the 100 samples that was displayed asthenozoospermia, 11 patients displayed oligo-asthenozoospermia (concentration <15 million/ml).
Semen analysis
All samples were prepared and evaluated following WHO standard criteria [9]. Semen samples were obtained by masturbation after 2–5 days of abstinence, and routine semen analysis was performed after liquefaction of the semen within 30 min at 37 °C.
Immunocytochemical analysis
Semen samples were centrifuged at 2,000 rpm for 5 min at 4 °C, the pellet was then resuspended in 1 ml of PBS. Aliquots of 20 μl of fresh sperm were spread on slides, dried and fixed for 10 min in cold paraformaldehyde (2 %), after permeabilization of the cells with 0.1 % TritonX-100 in phosphate buffered saline (PBS), the slides were blocked in 1 % bovine serum albumin in PBS for 1 h, and incubated with primary antibodies (rabbit anti- septin 4 or septin 7, Santa Cruz Biotechnology) for 2 h at room temperature (dilution1:100). After three washes of 5 min in PBS, the slides were incubated with goat anti-rabbit antibody conjugated with FITC, Santa Cruz Biotechnology, (dilution 1:1,000) for 1 h at room temperature. After three washes of 5 min in PBS, the slides were analyzed with a fluorescence microscope. PI (propidium iodide) was used for counterstaining.
Transmission electron microscopy analysis
Semen samples were washed in M16 medium (Sigma-Aldrich Co. Ltd, Irvine, UK), centrifuged at 300×g for 10 min and fixed in 0.1 M phosphate buffer pH 7 containing 3 % glutaraldehyde (Grade I; Sigma-Aldrich Co.) for 1.5 h at room temperature. The samples were then washed twice in PBS with 1 % sucrose and resuspended in 0.2 M sodium cacodylate buffer. Secondary fixation was performed using 1 % osmium tetra-oxide (Agar Scientific Ltd, Essex, UK), after which samples were dehydrated in graded alcohol and embedded in Epon resin (Polysciences Inc., Warrington, USA). Sections of approximately 90 nm were cut with a using a diamond knife, mounted on nickel grids, stained with uranyl acetate and lead citrate. Electron micrographs were taken using a Zeiss EM 900 transmission electron microscope (Zeiss, Germany).
Western blot analysis for Sept 4
Twenty milligrams of human sperm proteins were separated on 12 % SDS–PAGE gels. Proteins were then transferred to PVDF membranes employing a semi-dry transform cell (Bio-Rad), and membranes were blocked with 5 % BSA. Immunoblotted samples were then incubated with primary antibody at a suitable dilution in Tris-buffered saline, containing 5 % BSA and 0.1 % Tween 20, overnight at 4 °C. After three times washing with TBST, membranes were incubated with a goat-anti- rabbit secondary antibody conjugated with horseradish peroxidase (HRP) at a 1:2,000 dilution for 1 h at room temperature. Finally, proteins on the membranes were detected by the enhanced chemiluminescence’s system (ECL), following the manufacturer’s protocol.
Sequencing of the SEPT12 gene
We performed direct DNA sequencing for all coding exons of SEPT12, using peripheral blood sample of the patient who demonstrated defective annulus labeling. To analyze the DNA sequences, PCR-Sequencing technique was performed with an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 45 s and extension at 72 °C for 45 s. The PCR products were confirmed by running on 1.5 % agarose gel, and were applied for sequencing by an ABI 3730XL automated DNA sequencer (Macrogen, Seoul, Korea).
Results
To investigate sept4 and sept7 in cases of human asthenozoospermia, we screened asthenozoospermic samples for abnormal localization of sept4 and sept7. We used immunofluorescence detection assay on sperm smear preparations from 100 asthenozoospermic and 20 normozospermic subjects. Sept4 and Sept7 antibodies consistently labeled the annuli in the spermatozoa of the 20 normal samples and 99 patients with asthenozoospermia (Figs. 1 and 2). The patient with defective annuls was 29 years old at the time of the observation; he had been married for 24 months; he has three brothers which among them, one brother was infertile. The patient had no genito-urinary infections, nor previous. The results of two semen analysis have been shown in Table 1. There was no consanguinity in the patient’s history. The couple first consulted for infertility after 18 months of unsuccessful attempts to obtain a pregnancy.
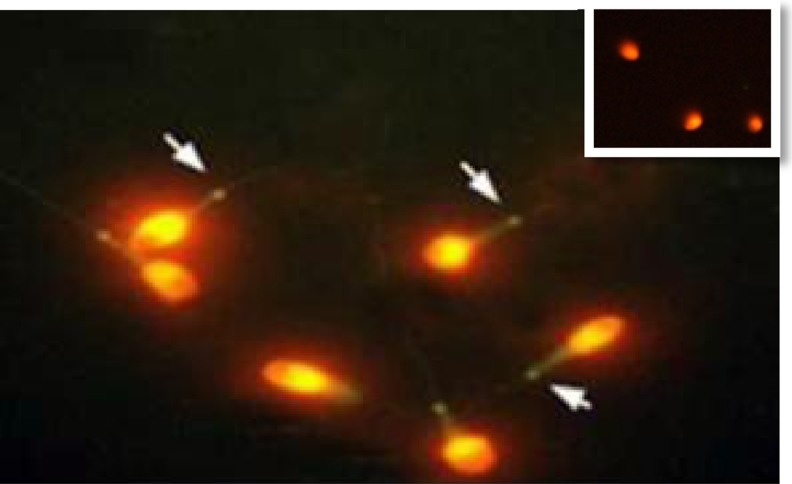
Fig. 1.
Immunofluorescence assay of Sept4 in the human sperm. Sept4 was expressed at the annulus of spermatozoa. Green, FITC; Red, PI. The negative control is on top right
Fig. 2.
Immunofluorescence assay of Sept7 in the human sperm. Sept7 (green) label the annulus region of spermatozoa. Green, FITC; Red, PI. The negative control is on top right
Table 1.
Two semen analysis of the patient without annulus
| First semen analysis | Second semen analysis (after two months) | |
|---|---|---|
| Volume (ml) | 2 | 4 |
| Concentration (×106 sperm/ ml) | 24 | 26 |
| Total motility (%) | 38.3 | 22.6 |
| Motility A + B (%) | 4.1 | 16.3 |
| Normal morphology (%) | 4 | 12 |
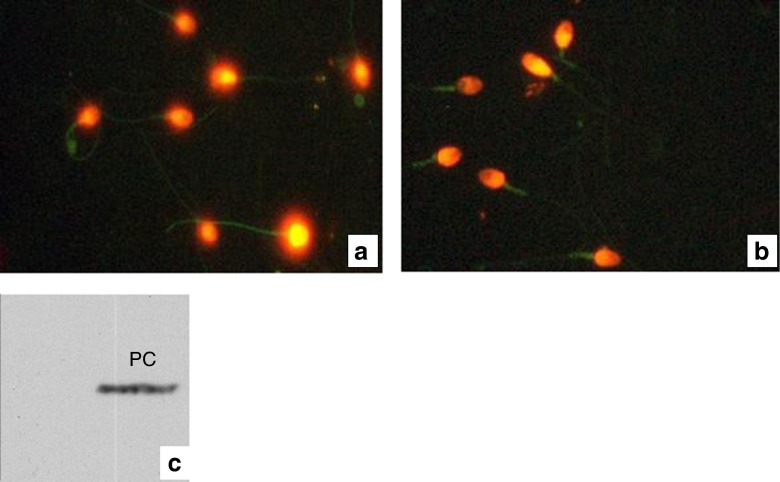
Immunocytochemical analysis of the sperm of this patient showed 75 % of the sperm population without septin 4 or septin 7 at the annulus (Fig. 3a & b). Absence of septin 4 was also confirmed by western blot analysis (Fig. 3c).
Fig. 3.
a & b Immunodetection of Sept4 and Sept7 in sperm smears from one (case) of the100 patients studied. The annulus structure was completely absent in all spermatozoa analyzed. c Western blot analysis for septin 4 which was not detected in the gel; PC positive control
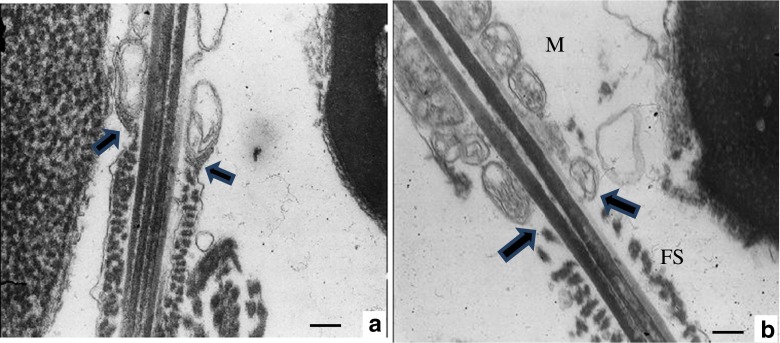
Moreover, the absence of the annulus structure was detected by transmission electron microscopy and observed that the annulus was not organized at many of sperms, in this sample (Fig. 4b).
Fig. 4.
Transmission electron microscopy analysis of spermatozoa. a Normal control spermatozoon showing the annulus at the junction of the MP and the PP. b Annulus was not organized in caudal end of middle piece in spermatozoa in infertile patient diagnosed with asthenozoospermia. Note that fibrous sheath (FS) is relatively disorganized. M mitochondrial sheath. Bars: 0.25 μm
For exploring the relationship between annulus defect and mutations in the SEPT12 gene, we analyzed peripheral blood DNA of the asthenozoospermic patient who had defective annulus on direct sequencing. It was shown that in the coding exons of SEPT12 there was not any nonsense or missense mutation comparing to the control sample.
Discussion
Annulus is a permanent septin-rich structure consisting of heteropolymers of septins 1,4,6,7 and 12 and it is required for sperm motility and sperm tail terminal differentiation. Septin proteins belong to the GTPase superclass of P‑loop NTPases [10–12]. Sept4 null mice show the reproductive phenotypes similar to a subset of asthenozoospermic patients [7, 8]. In addition, the expression pattern of SEPT7 suggested that this protein may be involved in the regulation of subcellular-compartment formation during spermiogenesis. The absence of a Sept7 signal correlated with multiple sperm defects [13].
Here, we used anti-sept4/sept7 to analyze a cohort of 100 asthenozoospermic and 20 normal samples and found only one patient showing moderate asthenozoospermia, with 75 % of sperm lacking septin 4 and/or septin 7 proteins at the annulus. To confirm this finding, we examined fresh ejaculated sperm samples by transmission electron microscopy. The annulus structure was completely absent from 75 % spermatozoa analyzed in this patient. Furthermore, we could not detect septin 4 or 7 by immunocytochemistry and immunoblotting.
A previous immunocytochemical study, in 2008 by Suginoa et al., in smaller Japanese asthenozoospermic cohort (n = 33) reported 10 cases of asthenozoospermia, who had defective SEPT4 and/or SEPT7 labeling. Their findings strongly indicated an association between the absence of annulus and human asthenozoospermia. Therefore, they introduced the septins as appropriate diagnostic markers for a subset of asthenozoospermia. In France, Lhuillier and colleagues [10] in a similar study analyzed 75 cases of asthenozoospermia including 28 cases of isolated asthenozoospermia, 13 cases of oligo-asthenozoospermia and 34 cases of terato-asthenozoospermia, but they found only one case of loss of annulus that is in accordance with our finding on a population with the same ethnicity. This discrepancy may be attributed to the origin of the populations screened (Japanese versus Caucasian), to the small sizes of the cohort studies or to other causes remains to be determined.
Recently, Miyakawa et al. reasoned septin 12 as a good candidate gene for male infertility [14]. In addition; Ying-Hung Lin et al. had found two missense mutations in men with infertility compared with controls located in the predicted GTP-binding domain of septin 12 [15]. Also, Kuo et al., reported a patient with a variation in SEPT12 (D197N) which had sperm with defective annulus with bent tail and loss of septin 12 from the annulus of abnormal sperm [16]. With mentioned to above articles, we hypothesized that genetic variations of SEPT12 gene may be associated with the absence of annulus but there was not any nonsense or missense mutation in the coding exons of SEPT12 in this patient comparing to control sample.
In conclusion, the results of this study demonstrated that the frequency of the absence of annulus in asthenozoospermic sample of Iranian population is similar to study conducted by Lhuillier in France and because of its low occasion, it could not be assume as a diagnostic marker for classifying asthenozoospermic patients in Caucasian population.
Footnotes
Capsule Absence of annulus has been reported in some of asthenozoospermic patients but its frequency in Iranian infertile men is low.
References
- 1.Sugino Y, Ichioka K, Soda T, Ihara M, Kinoshita M. Septins as diagnostic markers for a subset of human asthenozoospermia. Urology. 2008;180:2706–9. doi: 10.1016/j.juro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82(4):669–78. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Ding X, Yu W, Yang X, Shen S, Yu L. Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett. 2007;581(28):5526–32. doi: 10.1016/j.febslet.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT, Trimble WS. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol. 2010;191(4):741–9. doi: 10.1083/jcb.201006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt K, Nichols BJ. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 2004;5(1):43. doi: 10.1186/1471-2121-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, et al. The expression level of septin12 is critical for spermiogenesis. Am J Pathol. 2009;174(5):1857–68. doi: 10.2353/ajpath.2009.080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8(3):343–52. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8(3):353–64. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO laboratory manual for the examination and processing of human semen. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 10.Lhuillier P, Rode B, Escalier D, Lores P, Dirami T, Bienvenu T, et al. Absence of annulus in human asthenozoospermia: case report. Hum Reprod. 2009;24(6):1296–303. doi: 10.1093/humrep/dep020. [DOI] [PubMed] [Google Scholar]
- 11.Lin YH, Chou CK, Hung YC, Yu IS, Pan HA, Lin SW, et al. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil Steril. 2011;95(1):363–5. doi: 10.1016/j.fertnstert.2010.07.1064. [DOI] [PubMed] [Google Scholar]
- 12.Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9(6):478–89. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- 13.Chao HC, Lin YH, Kuo YC, Shen CJ, Pan HA, Kuo PL. The expression pattern of SEPT7 correlates with sperm morphology. J Assist Reprod Genet. 2010;27(6):299–307. doi: 10.1007/s10815-010-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyakawa H, Miyamoto T, Koh E, Tsujimura A, Miyagawa Y, Saijo Y et al. Single-nucleotide polymorphisms in the SEPTIN12 gene may be a genetic risk factor for Japanese patients with Sertoli cell-only syndrome. J Androl. doi:10.2164/jandrol.110.012146. [DOI] [PubMed]
- 15.Lin YH, Wang YY, Chen HI, Kuo YC, Chiou YW, Lin HH, et al. SEPTIN12 genetic variants confer susceptibility to teratozoospermia. PLoS One. 2012;7(3):e34011. doi: 10.1371/journal.pone.0034011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo YC, Lin YH, Chen HI, Wang YY, Chiou YW, Lin HH, et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum Mutat. 2012;33(4):710–9. doi: 10.1002/humu.22028. [DOI] [PubMed] [Google Scholar]