Abstract
Gleditsia triacanthos L. is a deciduous tree belonging to the family Fabaceae. It possesses important biological activities as anti-mutagenic, anticancer, cytotoxic and treating rheumatoid arthritis. The total ethanol extract (EtOHE) and successive extracts (petroleum ether, chloroform, ethyl acetate, and aqueous ethanol) were prepared from the leaves. Eight flavone glycosides and two flavone aglycones named vicenin-I (1), vitexin (2), isovitexin (3), orientin (4), isoorientin (5), luteolin-7-O-ß-glucopyranoside (6), luteolin-7-O-ß-galactopyranoside (7), apigenin-7-O-ß-glucopyranoside (8), luteolin (9) and apigenin (10) were isolated from the aqueous ethanol extract of G. triacanthos L. leaves. Potent cytotoxic activity of the EtOHE extract was observed against the liver (IC50 = 1.68 μg), breast (IC50 = 0.74 μg), cervix (IC50 = 1.28 μg), larynx (IC50 = 0.67 μg) and colon (IC50 = 2.50 μg) cancer cell lines. Cytotoxic activity of compounds 2, 4, 6 and 8 against, the liver, breast and colon cancer cell lines was also proved. Evaluation of the in-vivo antioxidant activity of the EtOHE and successive extracts revealed that the highest activity was exhibited by 100 mg of EtOHE (97.89% potency) as compared with vitamin E (100% potency). Compound 6 showed 91.8% free radical scavenging activity.
Keywords: Gleditsia triacanthos L., Cytotoxic activity, Cancer cell lines, Flavone glycosides
1. Introduction
Genus Gleditsia comprises 14 species of deciduous trees which can reach a height of 20–30 m (Huxley et al., 1992). Gleditsia species have been widely used in traditional Chinese medicine. The fruits and thorns are used for treating apoplexy, headache, productive cough, asthma and suppurative skin diseases (Zhong Yao Da Ci Dian, 1979). It was reported that Gleditsia triacanthos seed extracts can be used as a natural source of phenolic compounds and as antioxidants, also extracts of Gleditsia plant possess important pharmacological activities in treating rheumatoid arthritis, as anti-mutagenic, anticancer and they have significant cytotoxic activity against different cell lines (Miguel et al., 2010). Phytochemical studies were carried out on members of Gleditsia fruits that indicated the presence of triterpenoidal saponins which possess anti-inflammatory activity. (Yamahara et al., 1975; Shin and Kim, 2000; Ha et al., 2008, Dai and Hou, 2006; Hou et al., 2006). Triacanthosides saponin A1, G and C together with Gleditschosides A, B, C, D and E were reported to be isolated from Gleditsia fruits species (Badalbaeva et al., 1972a,b, 1973a,b). Triacanthoside C, oleanolic and echinocystic acids were reported to be isolated from the pericarp of G. triacanthos in addition to Gleditschosides A, B, C, D, and E (Badalbaeva et al., 1973a). Triacanthine alkaloid was isolated from the leaves of G. triacanthos (Panova et al.,1971; Masterova’ et al., 1977). Vitexin, luteolin, isovitexin, quercetin were reported to be isolated from G. triacanthos (Panova and Georgieva, 1972; Leibovici et al., 1986). Aim of the study is to prove the cytotoxic and antioxidant activities of the plant which were reported in folk medicine as well as isolation and identification of the flavonoid compounds of the leaves (see Fig. 1).
Figure 1.
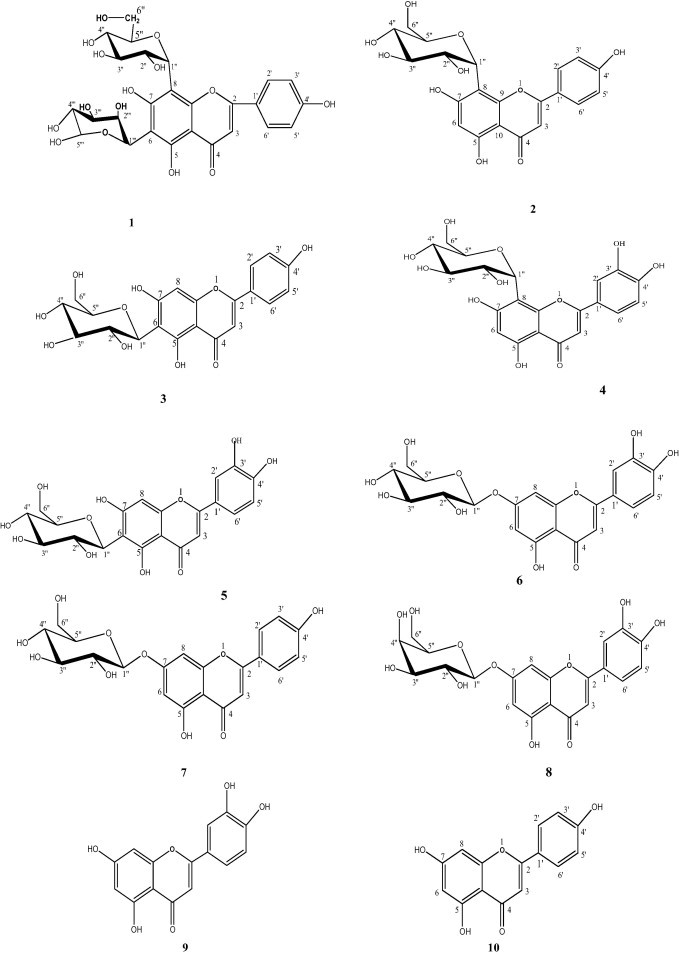
Chemical structure of compounds 1–10.
2. Materials
2.1. Plant material
Fresh leaves of G. triacanthos L. were obtained from the Zoo in May 2007. It was identified by Mrs. Terase Labib, plant taxonomist of Orman Garden, Giza, Egypt. A voucher specimen (M 96) was deposited by Dr. Mona Marzouk and kept in the herbarium of NRC (National Research Center).
2.2. Material for in-vitro cytotoxic activity
HepG2 (liver), MCF7 (breast), HeLa (cervix), HEp-2 (larynx) and HCT116 (colon) cancer cell lines were obtained from the American Type Culture Collection, University Boulevard, Manassas, USA.
2.3. Material for in-vitro antioxidant activity
Vitamin E (α-tocopheryl acetate) (Pharco Pharmaceutical Co.) is available in the form of gelatinous capsules each contains 400 g it was used as a reference antioxidant drug. Alloxane (Sigma Co.): was used for induction of diabetes in rats. DPPH (1,1-Diphenyl-2-picryl-hydrazil) is a relatively stable free radical.
3. Methods
3.1. Experimental
3.1.1. General procedure
The structure of the compounds was identified by spectroscopic methods including: UV/VIS (Ultraviolet and Visible Absorption Spectrometer, Labomed Inc.) for measuring UV spectral data of the isolated compounds, in the range of 200–500 nm in methanol and with different diagnostic shift reagents. NMR (Nuclear Magnetic Resonance Spectrophotometer, JEOL EX, 500 MHz for determination of 1H NMR and 125 MHz for determination of 13C NMR), ESI/MS (Electrospray Ionization Mass Spectrometer, Thermo Finnigan (ion trap)) were carried out for determination of molecular weight of compounds, CC was carried out on Polyamide 6S (Riedel-De-Haen AG, Seelze Haen AG, D-30926 Seelze Hanver, Germany) and Sephadex LH-20 (Pharmazia). PC (descending) Whatman No. 1 and 3 MM papers, using solvent systems 15% HOAc (H2O–HOAc 85:15), BAW (n-BuOH:HOAc:H2O 4:1:5, upper layer). Complete acid hydrolysis for O-glycosides (6, 7, 8) was carried out & followed by CO-chromatograph with authentic samples to identify the aglycone and sugar moieties, the sugar unit of (1, 2, 3, 4, 5) was determined using ferric chloride degradation (Mabry et al., 1970). Source of solvents used for plant extraction: SDFCL (Industrial Estate, 248 Worli Road, Mumbai-30, India).
3.2. Extraction and purification
Dried powdered leaves of G. triacanthos L (1 kg) were exhaustively extracted with solvents of increasing polarities, petroleum ether (Pet. ether 49 g), chloroform (Chlo. 21 g), ethyl acetate (EtOAc: 14 g) and 70% aqueous ethanol (AEtOH: 135 g) at room temperature, the previous extracts were dried under reduced pressure and subjected to in-vivo antioxidant assay. 80 g of AEtOH was dissolved in water then applied on the top of polyamide S6 column (120 × 5 cm) eluted by solvent systems of decreasing polarities starting from 100% water to 100% methanol. A total of 100 fractions were collected (250 ml each). Similar fractions were combined according to PC analysis (1MM) sheets using the following eluents: n-butanol:acetic acid:water (4:1:5), acetic acid:water (15:85). Components were detected under UV and by spraying with AlCl3 to give six main pooled fractions (A–F). Fraction A was chromatographed on cellulose column eluted by 10% MeOH-H2O to give one main subfraction, purification on Sephadex using MeOH-H2O (10%) yielded compounds 1 (10 mg). B was subjected to cellulose column eluted by saturated butanol to give one main subfraction which was purified on silica gel column eluted by CHCl3: MeOH 9:1 to give compounds 2 and 3 (20, and 18 mg respectively). C was chromatographed on PPC (3 MM) using acetic acid: water (15:85) followed by Sephadex and eluted by saturated butanol to give compounds 4 and 5 (20, and 10 mg). D was chromatographed on PPC (3 MM) using acetic acid:water (15:85) and purified on Sephadex with saturated butanol to give compound 6 and 7 (15, and 15 mg). E was purified on Sephadex using MeOH-H2O(10%) to give compound 8. F was subjected to Sephadex using MeOH-H2O (1:1) to yield compounds 9 (12 mg) and 10 (15 mg). Final purification of all compounds was achieved by Sephadex LH20 column using MeOH as eluent.
3.2.1. Compound 1 (vicenin-I)
Negative ESI-MS Molecular ion peak (M–H)− at m/z 563, the ESI-MS fragmentation pattern: 503, 473, 443, 383. UV: λ max (nm): MeOH: 272, 327; NaOMe: 282, 333 (sh), 390; AlCl3: 305, 345, 389; AlCl3/HCl: 305, 345, 489; NaOAc: 281, 307, 392; NaOAc/H3BO3: 279, 344. 1H NMR: (500 MHz, DMSO-d6, δ ppm): 6.30(1H, s, H-3), 7.83 (2H, d, J = 8.4 Hz, H-2′/H-6′), 6.78(2H, d, J = 8.4 Hz, H-3′/H-5′), 8-C-β-Glc: 4.35 (1H, d, J = 9.8 Hz, H-1′′′), 6-C-β-xylose: 3.8 (1H, d, J = 9.6 Hz H-1″, Xylose).
3.2.2. Compound 2: vitexin (apigenin 8-C-β-glucopyranoside)
UV: λ max (nm) MeOH: 270, 333; NaOMe: 285, 337, 391; AlCl3: 264sh, 278, 303, 352, 383; AlCl3/HCl: 262sh, 280, 302, 352, 383; NaOAc: 278, 299sh, 375; NaOAc/H3BO3: 272, 304sh, 355.
1H NMR (500 MHz, DMSO-d6, δ ppm): 6.59 (1H, s, H-3), 6.30 (1H, d, J = 2.3 Hz, H-6), 7.81 (2H, d, J = 8.4 Hz, H-2′/H-6′), 6.87 (2H, d, J = 8.4 Hz, H-3′/H-5′), 4.52 (1H, d, J = 9.8 Hz, H-1″), 3.2–3.9 (m, H-2″- H-4″, H-5″, H6″).
13C NMR (125 MHz DMSO-d6, ppm): 164.37 (C-2), 102.38 (C-3), 181.68 (C-4), 161.15 (C-5), 94.92 (C-6) 163.28 (C-7), 109.69 (C-8), 157.12 (C-9), 102.94 (C-10), 121.68 (C-1′), 128.69 (C-2′), 116.56 (C-3′), 161.83 (C-4′), 116.56 (C-5′), 128.69 (C-6′), 74.00 (C-1″), 70.72 (C-2″), 79.63 (C-3″), 70.94 (C-4″), 81.80 (C-5″), 61.73 (C-6″).
3.2.3. Compound 3: isovitexin (apigenin 6-C-β-glucopyranoside)
UV λ max (nm): MeOH: 270, 333; NaOMe: 279, 331, 396; AlCl3: 263sh, 278, 280, 303, 338sh, 380; AlCl3/HCl: 262sh, 279, 302, 341, 378; NaOAc: 280, 300, 379;
NaOAc/H3BO3: 271,320sh, 345.
1H NMR (500 MHz, DMSO-d6, δ ppm): 6.71 (1H, s, H-3), 6.50 (1H, d, J = 2.3 Hz, H-8), 7.86 (2H, d, J = 8.4 Hz, H-2′/H-6′), 6.89 (2H, d, J = 8.4 Hz, H-3′/H-5′), 4.54 (1H, d, J = 9.8 Hz, H-1″), 4.0 (1H, H-2″), 3.7–3.1 (m, H-3″, H-4″, H-5″, H-6′).
13C NMR (125 MHz DMSO-d6, ppm):163.00 (C-2), 102.62 (C-3), 181.78 (C-4), 160. 50 (C-5), 103.11 (C-6) 163.35 (C-7), 93.97 (C-8), 156.12 (C-9), 103.50 (C-10), 120.97 (C-1′), 128.30(C-2′), 115.90 (C-3′), 161.11 (C-4′), 115.90 (C-5′), 128.30 (C-6′), 72.94 (C-1″), 70.06 (C-2″), 78.86 (C-3″), 70.94 (C-4″), 81.43 (C-5″), 61.33 (C-6″).
3.2.4. Compound 4: orientin (luteolin 8-C-β-glucopyranoside)
UV λ max (nm): MeOH: 254, 269, 270sh, 350; NaOMe: 269, 337sh, 406; AlCl3: 275, 346, 422; AlCl3/HCl: 275, 298sh, 355, 341, 382; NaOAc: 277, 318sh, 399; NaOAc/H3BO3: 264, 376.
1H NMR (500 MHz, DMSO-d6, δ ppm).: 6.42 (1H, s, H-3), 5.97 (1H, d, J = 2.3 Hz, H-6), 7.33 (1H, d, J = 2.3 Hz, H-2′), 7.40 (1H, m, H-6′), 6.72, (1H, d, J = 8.4 Hz, H-5′), 4.68 (1H, d, J = 9.95, H-1″), 3.2–3.9 (m, H-2″, H-3″, H-4″,H-5″, H-6″).
3.2.5. Compound 5: isoorientin (luteolin-6-C-β-glucopyranoside)
UV λ max (nm): MeOH: 257, 270sh, 350; NaOMe: 278, 335sh, 410; AlCl3: 275, 303,425; AlCl3/HCl: 257sh, 278,300sh, 356; NaOAc: 271, 407; NaOAc /H3BO3: 268, 381.
1H NMR (500 MHz, DMSO-d6, δ ppm): 6.61(1H, s, H-3), 6.23 (1H, d, J = 2.3, H-8), 7.42 (1H, d, J = 2.3, H-2′), 6.82 (1H, d, J = 8.4, H-5′), 7.49 (m, H-6′), 4.65,(1H, d, J = 9.95, H-1″), 3.2–3.9(m,H-2″, H-3″, H-4″, H-5″, H-6″).
13C NMR (125 MHz DMSO-d6, ppm): 164.4 (C-2), 102.89 (C-3), 182.55 (C-4), 160. 88 (C-5), 105.00 (C-6) 164.62 (C-7), 98.63 (C-8), 156.51 (C-9), 104.48 (C-10), 122.46 (C-1′), 114.42 (C-2′), 146.33 (C-3′), 150.14 (C-4′), 116.17 (C-5′), 119.89 (C-6′), 73.86 (C-1″), 71.20 (C-2″), 79.20 (C-3″), 71.28 (C-4″), 82.43 (C-5″), 62.14 (C-6″).
3.2.6. Compound 6: (luteolin -7-O-β-glucopyranoside)
UV λ max (nm): MeOH: 253, 265sh, 348; NaOMe: 263, 300sh, 390; AlCl3: 273, 295,325,430; AlCl3/HCl: 273, 294,358, 377; NaOAc: 255, 266sh, 365sh, 400; NaOAc/H3BO3: 255, 375. 1H NMR (500 MHz, DMSO-d6, δ ppm). 6.61(1H, s, H-3), 6.42 (1H, d, J = 2.3 Hz, H-6), 6.90 (1H, d, J = 2.3 Hz, H-8), 7.43 (1H, d, J = 2.3 Hz, H-2′), 7.40(1H, dd, J = 2.3, 8.4 Hz, H-6‘), 6.92 (1H, J = 8.4 Hz, H-5′), 5.08(1H, d, J = 7.65 Hz, H-1″), 3.5–4.1 (mH-2″, H-3″, H-4″, H-5″, H-6″).
3.2.7. Compound 7: (luteolin -7-O-β-galactopyranoside)
UV λ max (nm) as in compound 6.
1H NMR(500 MHz, DMSO-d6, δ ppm).: 6.61, (1H, s, H-3), 6.43(1H, d, J = 2.3 H-6), 6.82 (1H, d, J = 2.3 H-8),7.44(1H, d, J = 2.3, H-2′), 6.92 (1H, d J = 8.4, H-5′) 7.40, (1H, dd, J = 2.3, 8.4, H-6′), 5.08 (1H, d, J = 7.65, H-1″), 3.4–4.1 (H-2″, H-3″, H-4″, H-5″, H-6″).
3.2.8. Compound 8: (apigenin-7-O-β-glucopyranoside)
UV λ max (nm) as in compound 2.
1H NMR (500 MHz, DMSO-d6, δ ppm).: 6.41 (1H, s, H-3), 6.61 (1H, d, J = 2.3 Hz, H-6), 6.77(1H, d, J = 2.3 Hz, H-8), 7.81 (2H, d, J = 8.4 Hz, H-2′/H-6′), 6.89 (2H, d, J = 8.4 Hz, H-3′/H-5′), 5.08(IH, d, J = 6.85 Hz, H-1″), 3.4–3.85 (H-2″, H-3″, H-4″, H-5″, H-6″).
Compound 9: (luteolin).
Compound 10:(apigenin).
3.3. Investigation of antioxidant activity
3.3.1. In-vivo antioxidant activity
Glutathione (GSH) is the body’s most abundant natural antioxidant. Diabetes is usually accompanied by increased production of free radicals and impaired antioxidant defenses. A simultaneous fall in blood GSH was observed following the injection of diabetogenic doses of alloxan into rats. Therefore, blood glutathione was estimated in alloxan-induced diabetic rats for evaluating the antioxidant activity (Oberley, 1988; Maritim et al., 2003). In-vivo antioxidant activity was carried out according to Eliasson and Samet, 1969. At the end of the experiment, blood glutathione was estimated using bio-diagnostic kits (Beutler et al., 1963). The results were expressed as mean ± S.E. The data were statistically analyzed using Student’s “t” test (Sendecor and Cohran, 1982). Results are considered statistically significant with P > 0.01.
3.3.2. In-vitro antioxidant activity (Free radical scavenging activity)
In-vitro antioxidant activity by DPPH is used to evaluate the free radical scavenging activity of compounds 2, 4, 6 and 8 following the method of Shimada et al (1992).
3.4. Investigation of cytotoxic activity
Cytotoxic activity of the EtOHE against five human tumor cell lines was tested using the method of Skehan et al. (1990). Compounds 2, 4, 6 and 8 were evaluated for their cytotoxic activities against three tumor cell lines by MTT assay (Mosmann, 1983). The method was carried out according to Thabrew et al. (1997).
3.4.1. Cytotoxic activity of the total ethanol extract (Skehan et al., 1990)
Cells were plated in 96-multi-well plate (104 cells/well) for 24 h before treatment with the total alcohol extract of the plant to allow attachment of the cell to the wall of the plate.
Different concentrations of the extract under test (0, 1, 2.5, 5 and 10 μg/mL) were added to the cell monolayer, triplicate wells being prepared for each individual dose.
Monolayer cells were incubated with the total alcohol extract of the plant for 48 h at 37 °C and in atmosphere of 5% CO2. After 48 h, cells were fixed, washed and stained with sulforhodamine B (SRB) stain (Sigma).
Excess stain was washed with acetic acid and attached stain was recovered with tris–EDTA buffer (Sigma). Color intensity was measured in an ELISA reader. The relation between surviving fraction and the plant extract concentration was plotted to get the survival curve of each tumor cell line after treatment. The potency was compared with reference (Cisplatin (Glaxo), DOX).
3.4.2. MTT assay (Mosmann, 1983)
Cell viability was assessed by the mitochondrial dependent reduction of the yellow MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to the purple formazan (Mosmann, 1983).The method was carried out according to Thabrew et al. (1997).
Cells were batch cultured for 10 days, then seeded at a concentration of 10 × 103 cells/well in fresh complete growth medium in 96-well microtiter plastic plates at 37 °C for 24 h under 5% CO2 using a water jacketed carbon dioxide incubator (Sheldon, TC2323, Cornelius, OR, USA).
Media were aspirated, fresh medium (without serum) was added and cells were incubated either alone (negative control) or with different concentrations of the samples to give a final concentration of 100–50-25–12.5–6.25–3.125–0.78 and 1.56 μg/ml.
Cells were suspended in RPMI 1640 medium[(for HepG2 and HT29 – DMEM for MCF 7)], 1% antibiotic–antimycotic mixture (10,000 U/ml potassium penicillin, 10,000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B) and 1% l-glutamine in 96-well flat bottom microplate at 37 °C under 5% CO2. After 48 h of incubation, the medium was aspirated, 40 μl MTT salt (2.5 μg/ml) was added to each well and incubation for further four hours at 37 °C under 5% CO2.
200 μL of 10% sodium dodecyl sulfate (SDS) in deionized water was added to each well and incubated overnight at 37 °C to stop the reaction and dissolve the formed crystals.
A positive control which composed of 100 μg/ml of Annona cherimolia extract was used as a known cytotoxic natural agent which gives 100% lethality under the same conditions. The absorbance was then measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595 nm and a reference wavelength of 620 nm. A statistical significance was tested between samples and negative control (cells with vehicle) using independent t-test by SPSS 11 program. DMSO is the vehicle used for dissolution of the plant extracts and its final concentration on the cells was less than 0.2%.
The percentage of change in viability was calculated according to the formula:
A probit analysis was carried out for IC50 and IC90 determination using SPSS 11 program. All the following procedures were done in a sterile area using a laminar flow cabinet biosafety class II level (Baker, SG403INT, Sanford, ME, USA).
4. Results and discussion
4.1. Identification of the isolated compounds
The phytochemical investigation of the AEtOH of G. triacanthos L leaves afforded ten flavonoids (1–10), they are vicenin-I (1), vitexin (2), isovitexin (3), orientin (4), isoorientin (5), luteolin-7-O-ß-glucopyranoside (6), luteolin-7-O-ß galactopyranoside (7), apigenin-7-O-ß-glucopyranoside (8), luteolin (9) and apigenin (10) their structure elucidation was carried out through Rf-values, color reactions, chemical investigations (complete acid hydrolysis and ferric acid degradation) and spectral investigations (UV, NMR and MS) (Mabry et al., 1970; Markham, 1982; Agrawal, 1989). Spectral data of the known flavonoids were in good accordance with those previously published (Agrawal, 1989; Markham and Geiger, 1994). Compounds 1, 4, 5, 6, 7, 8 and 10 were isolated for the first time from the plant under investigation.
4.2. Biological study
4.2.1. In-vivo antioxidant activity
The EtOHE of G. triacanthos leaves was subjected to LD50 determination it was found to be 6.6 g/kg body weight. In-vivo antioxidant activity of the EtOHE and successive extracts of G. triacanthos L. leaves (Table 1) were evaluated as indicated by the increase in glutathione level as compared with diabetic control. The result showed that EtOH at 100 mg showed the highest antioxidant activity (64.05% of change from diabetic control) followed by pet. ether (61.75%), ethyl acetate (61.21%), chloroform (58.989%) and AEtOH (57.14%) extract.
Table 1.
In vivo antioxidant activity of EtOH and successive extracts of G. triacanthos L. leaves.
| Group (dose in mg/kg b wt) | Blood glutathione (Mean ± SE) | % of change from diabetic control | Relative potency% |
|---|---|---|---|
| Diabetic (control) | 21.7 ± 0.3⁎ | – | – |
| Diabetic + EtOH (50) | 32.2 ± 0.8⁎ | 48.38 | 73.94 |
| Diabetic + EtOH (100) | 35.6 ± 1.2⁎ | 64.05 | 97.89 |
| Diabetic + Pet. ether (100) | 35.1 ± 1.1⁎ | 61.75 | 95.90 |
| Diabetic + Ch.(100) | 34.5 ± 0.8⁎ | 58.98 | 90.14 |
| Diabetic + EtOAc (100) | 35.2 ± 0.9⁎ | 61.21 | 95.07 |
| Diabetic + AEtOH (100) | 34.1 ± 0.6⁎ | 57.14 | 87.33 |
| Diabetic + Vit E (75) | 35.9 ± 0.9⁎ | 65.43 | 100 |
Significantly different from the diabetic control at p < 0.01.
4.2.2. In-vitro antioxidant activity (Free radical scavenging activity)
In-vitro antioxidant activity of compounds 2, 4, 8 showed no antioxidant activity while 6 showed 91.8% free radical scavenging activity.
4.2.3. 3.2.2.Cytotoxic activity
Potent cytotoxic activity (Table 2) of the AEtOH of G. triacanthos L. leaves was observed against, the liver (IC50 = 1.68 μg), breast (IC50 = 0.74 μg), cervix (IC50 = 1.28 μg), larynx (IC50 = 0.67 μg) and colon cancer cell lines (IC50 = 2.50 μg). Compounds 2, 4, 6 and 8 were screened for their cytotoxic activities on the breast, liver, and colon cell lines (Table 3) compound 2 caused 73.5%, 30.6%, 51.5% death of the breast, liver and colon cell lines respectively. Compound 4 caused 40.2% death of breast and has no cytotoxic activity against the liver or colon cell line. Compound 6 caused 36.1%, 0%, 15.6% death of the breast, liver and colon cell. Compound 8 caused 41.3% death of the breast while it showed no cytotoxic activity against the liver or colon cancer cell line. All these activities were obtained at100 μg/ml.
Table 2.
Cytotoxicitc activity of the EtOHE of G. triacanthos L. leaves.
| Concentration (μg/ml) | (% of Dead cells) |
||||
|---|---|---|---|---|---|
| Cell lines | |||||
| Hep G2 | MCF7 | HeLa | HEp-2 | HCT116 | |
| 1 | 37 | 61 | 41 | 64 | 25 |
| 2.5 | 62 | 72 | 72 | 67 | 50 |
| 5 | 89 | 80 | 80 | 72 | 65 |
| 10 | 91 | 80 | 89 | 76 | 79 |
| Cisplatin | |||||
| 1 | 40 | 89 | 10 | 80 | 75 |
| 2.5 | 41 | 87 | 58 | 77 | 70 |
| 5 | 48 | 87 | 83 | 69 | 67 |
| 10 | 48 | 86 | 94 | 73 | 63 |
Table 3.
Cytotoxicic activity of compounds 2, 4, 6 and 8.
| Cell line | Conc μg/ml | Doxorubicin | % of Dead cells |
|||
|---|---|---|---|---|---|---|
| Compounds | ||||||
| 2 | 4 | 6 | 8 | |||
| Hep G2 | 12.5 | 32.4 | 0 | 0 | 0 | 0 |
| 25 | 60.2 | 0 | 0 | 0 | 0 | |
| 50 | 97.2 | 9.4 | 0 | 0 | 0 | |
| 100 | 100 | 30.6 | 0 | 0 | 0 | |
| MCF7 | 12.5 | 32.7 | 31.7 | 0.7 | 10.9 | 3.6 |
| 25 | 57.6 | 47 | 14.1 | 16.7 | 22.1 | |
| 50 | 84.3 | 51.1 | 22.5 | 26.2 | 33.1 | |
| 100 | 100 | 73.5 | 40.2 | 36.1 | 41.3 | |
| HCT116 | 12.5 | 18.4 | 0 | 0 | 0 | 0 |
| 25 | 35.4 | 9.4 | 0 | 0 | 0 | |
| 50 | 65.2 | 23.4 | 0 | 3.5 | 0 | |
| 100 | 100 | 51.2 | 0 | 15.6 | 0 | |
5. Conclusion
Ten known flavonoids, seven of them were isolated for the first time from the dried leaves of G. triacanthos L. Their structures were based on apigenin and luteolin nucleolus. The EtOHE of the plant has potent cytotoxic activity against, the larynx, breast, cervix, liver and colon cancer cell lines this proves the uses of the plant in folk medicine as anticancer. EtOHE has a good antioxidant activity supporting the use of the plant as a natural source of phenolic compounds and as antioxidant.
This work was extracted from the MSC Thesis of Wedian El-Sayed Mohamed Ashour, Pharmacognosy Department, National Research Centre (Ashour, 2012).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
R.S. Mohammed, Email: reda_sayed2008@yahoo.com.
A.H. Abou Zeid, Email: abouzeida@yahoo.co.uk.
References
- Agrawal P.K. Elsevier Science Publishing Co. Inc.; New York: 1989. Carbone-13 NMR of flavonoids. [Google Scholar]
- Ashour, W.E., 2012. Phytochemical and Biological Studies on Gleditsia triacanthos L. Family Fabaceae. A Master thesis, Pharmacognosy Department Faculty of Pharmacy, Cairo University.
- Badalbaeva T.A., Kondratenko E.S., Mzhel’skaya L.G., Abubakirov N.K. Triterpene glycosides of Gleditschia triacanthos∗ II. Structure of triacanthosides C. Khim. Prir. Soedin. 1972;6:741–744. [Google Scholar]
- Badalbaeva T.A., Kondratenko E.S., Mzhel’skaya L.G., Abubakirov N.K. Triterpene glycosides of Gleditschia triacanthos III The Structure of triacanthosides G. Khim. Prir. Soedin. 1972;6:744–747. [Google Scholar]
- Badalbaeva T.A., Kondratenko E.S., Abubakirov N.K. Triterpene glycosides of Gleditschia triacanthos. V. Structure of triacanthosides C and the minor glycosides. Khim. Prir. Soedin. 1973;5:635–640. [Google Scholar]
- Badalbaeva T.A., Kondratenko E.S., Abubakirov N.K. Triterpene glycosides of Gleditschia triacanthos IV. Structure of triacanthosides A1 and G. Khim. Prir. Soedin. 1973;9:314–316. [Google Scholar]
- Beutler E., Duron O., Kelly B. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Dai Y., Hou L.F. Acta Pharmacol. Sin. 2006;27:277. doi: 10.1111/j.1745-7254.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Eliasson S.G., Samet T.M. Alloxan induced neuropathies lipid changes in nerve and root fragments. Life Sci. 1969;8(1):493–498. doi: 10.1016/0024-3205(69)90442-1. [DOI] [PubMed] [Google Scholar]
- Ha H.H., Park S.Y., KO W.S., Kim Y.H. J. Ethnophamacol. 2008;118:429–434. doi: 10.1016/j.jep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Hou L.F., Dai Y., Wang C., Xia Y.F. Pharm. Biol. 2006;44:651–656. [Google Scholar]
- Huxley A., Griffiths M., Levy M. Macmillan press; London: 1992. The New Royal Horticultural Society Dictionary of Gardening. 423–24. [Google Scholar]
- Leibovici B., Petrovanu M., Lazar M., Segal B. Determination of flavones components from Gleditschia triacanthos. Rev. Chim. 1986;37:81–82. [Google Scholar]
- Mabry T.J., Markham K.R., Thomas M.B. Springer-Verlag; New York: 1970. The Systematic Identification of Flavonoids. [Google Scholar]
- Markham, K.R., Geiger, H., 1994. The Flavonoids: Advances in Research since1986. London.
- Markham, K.R. (1982). Techniques of Flavonoid Identification. Academic Press, London Masterova, I., Grzna’r, K., Tomko, J. (1977). Monitoring the triacanthine content during development in the leaves of Gleditsia triacanthos L. cesk. Farm. 26: 305-307. [PubMed]
- Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Oberley L.W. Free radicals and diabetes. Free Radic. Biol. Med. 1988;5(2):113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Masterova’ I., Grzna’r K., Tomko J. Monitoring the triacanthine content during development in the leaves of Gleditsia triacanthos L. cesk. Farm. 1977;26:305–307. [PubMed] [Google Scholar]
- Miguel A., Cerqueira Bartolomeu W.S., Souza Joana T., Martins José.A., Teixeira António.A., Vicente Seed extracts of Gleditsia triacanthos: functional properties evaluation and incorporation into galactomannan films. Food Res. Int. 2010;43:2031–2038. [Google Scholar]
- Mosmann T. Rapid colorimetric assays for cellular growth and survival, application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Panova D.I., Georgieva E.S., Stanoeva E. Components of Gleditschia triacanthos. 2 isolation of triacanthine and detection of hydrocarbons and alcohols. Pharmazie. 1971;26:493–494. [PubMed] [Google Scholar]
- Panova D.I., Georgieva E.S. Study of the flavonoid composition of Gleditschia triacanthos leaves. Dokl Bolg Lkad. Nauk. 1972;25:71–74. [Google Scholar]
- Sendecor W.G., Cohran G.W. 10th ed. University Press; Iawa State: 1982. Statistical methods. [Google Scholar]
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the auto-oxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40(6):945–948. [Google Scholar]
- Shin T.Y., Kim D.K. Arch. Pharm. Res. 2000;23:401–406. doi: 10.1007/BF02975455. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colourimetric cytotoxicity assay for anticancer drug screening. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Thabrew M.I., Hughes R.D., McFarlane I.G. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J. Pharm. Pharmacol. 1997;49:1132–1135. doi: 10.1111/j.2042-7158.1997.tb06055.x. [DOI] [PubMed] [Google Scholar]
- Yamahara, J., Shintani, Y., Konoshima, T., Sawada, T., Fujimura, H., Zasshi, Yakugaku (1975). (95): 1179-82. [DOI] [PubMed]
- Zhong, Yao Da Ci Dian (encyclopedia of Chinese Materia Medica), Jiangsu New Medical College, Shanghai: Shanghai Scientific and Technological Press. (1979). 1144, 1145, 1147, 2198.



