Abstract
Ellagitannins are esters of glucose with hexahydroxydiphenic acid; when hydrolyzed, they yield ellagic acid (EA), the dilactone of hexahydroxydiphenic acid. EA has been receiving the most attention, because it has potent antioxidant activity, radical scavenging capacity, chemopreventive and antiapoptotic properties. Hepatocellular carcinoma (HCC) is the most frequent primary malignancy of liver, and accounts for as many as one million deaths worldwide in a year. The aim of the present study was to evaluate the antioxidant and chemopreventive efficiency of ellagic acid against N-nitrosodiethylamine (NDEA) induced hepatocarcinogenesis in rats. Rats were classified into four groups as follows: normal control group, group injected i.p. with a single dose (200 mg/kg b.wt.) of NDEA, third group daily administered orally EA with a dose of 50 mg/kg b.wt. for 7 days before and 14 days after NDEA administration, and fourth group received a similar dose of EA for 21 days after the dose of NDEA administration. The model of NDEA-injected hepatocellular carcinomic (HCC) rats elicited significant declines in liver antioxidant enzyme activities; glutathione peroxidase (GPX), gamma glutamyl transferase (γ-GT) and glutathione-S-transferase (GST), with a reduction in reduced glutathione (GSH) and serum total protein with concomitant significant elevations in tumor markers arginase and α-l-fucosidase, and liver enzymes; aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and glutathione-S-transferase (GST), glucose-6-phosphate dehydrogenase (G6PD), direct and total bilirubin. The oral administration of EA as a protective agent, produced significant increases in tested antioxidant enzyme activities and serum total protein concomitant with significant decreases in the levels of tumor markers arginase and α-l-fucosidase as well as liver enzymes, direct and total bilirubin. Similarly, the oral administration of EA, as a curative agent produced similar changes to those when EA was used as a protective agent, but to a lesser extent. In addition, it was noted that HCC rats exhibited a degree of DNA fragmentation; however, EA administration partially inhibited the DNA fragmentation. Therefore, EA has the ability to scavenge free radicals, prevent DNA fragmentation, reduce liver injury and protect against oxidative stress.
Keywords: Hepatocellular carcinoma, Ellagic acid, Tumor markers, Antioxidant enzymes, DNA fragmentation
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary malignancy of the liver and accounts for as many as one million deaths worldwide in a year. In some parts of the world, it is the most common form of internal malignancy and the most common cause of death from cancer (Caillot et al., 2009). Well-known risk factors of hepatocellular carcinoma include hepatitis B virus (HBV), hepatitis C virus (HCV), aflatoxins, alcohol and oral contraceptives (Gurtsevitch, 2008). One approach to control liver cancer is chemoprevention, when the disease is prevented, slowed or reversed substantially by the administration of one or more non-toxic naturally occurring or synthetic agents. In this regard, recently naturally occurring polyphenols are receiving increased attention because of their promising efficacy in several cancer models (Caillot et al., 2009).
Classically, markers are synthesized by the tumor and released into the circulation, but it may be produced by normal tissues in response to invasion by cancer cells (Befler and Bisceglie, 2002). A variety of substances, including enzymes, hormones, antigens, and proteins may be considered as tumor markers. Analysis of tumor markers can be used as an indicator of tumor response to therapy. Sensitive and specific liver cancer marker enzymes are used as indicators of liver injury. Analysis of these marker enzymes reflects mechanisms of cellular damage and subsequent release of proteins and extracellular turnover (Thirunavukkarasu and Sakthisekaran, 2003).
Ellagitannins are esters of glucose with hexahydroxydiphenic acid; when hydrolyzed, they yield EA, the dilactone of hexahydroxydiphenic acid. EA has been receiving the most attention, because it has potent antioxidant activity, radical scavenging capacity, chemopreventive (Turk et al., 2008) and antiapoptotic properties.
Ellagic acid (EA) (3,7,8-tetrahydroxy[1]-benzopyrano[5,4,3-benzopyran-5,10-dione) is a member of flavanoids. It is found in plants in the form of hydrolyzable tannins called ellagitannins. The most rich dietary sources include walnuts, pomegranates, strawberries, blackberries, cloudberries and raspberries (Bin et al., 2013).
It contains four hydroxyl groups and two lactone groups in which the hydroxyl group is known to increase antioxidant activity in lipid peroxidation and protect cells from oxidative damage (Pari and Sivasankari, 2008). Berries are the most EA rich fruits and they are highly consumed by humans worldwide. The presence of EA in seeds has been demonstrated by Bushman et al. (2004), who reported that red and black raspberry seeds contain 8.7 and 6.7 mg/g seed of EA, respectively. EA is a very stable compound and is readily absorbed through the gastrointestinal system in mammals, including humans (Falsaperla et al., 2005).
N-nitrosodiethylamine (NDEA) is a potent hepatocarcinogenic dialkyl nitrosamine present in tobacco smoke, cured and fried meats and in a number of beverages. It is the most characterized system of xenobiotic-induced hepatocarcinogenicity and is a common screening model to evaluate the hepatoprotective potential of drugs with antioxidant properties (Dakshayani et al., 2005).
Thus, the aim of the present study was to evaluate the chemopreventive efficiency of EA against NDEA-induced hepatocarcinogenesis in rats. The protective effect of EA on NDEA-induced liver carcinoma was assessed by evaluating the enzymatic and nonenzymatic antioxidants and DNA fragmentation, along with liver function tests.
2. Materials and methods
2.1. Chemicals
EA and NDEA were obtained from Sigma–Aldrich Chemical Co., St. Louis, MO, USA. Carbon tetrachloride (CCl4) was obtained from El-Gomhorya Company, Cairo, Egypt. Biochemical kits for serum analysis were purchased from Bio-Diagnostic Company for Chemicals, Dokki, Egypt.
2.2. Rats and diet
Thirty-six male adult Albino rats were supplied from the breeding unit of the Egyptian Organization for Biological Products and Vaccines (Helwan, Egypt), weighing 120–130 g.
Rats were randomly divided into 4 groups of 8 animals each. The groups were classified as follows:
Group 1: Normal control untreated rats.
Group 2: Rats were injected with NDEA and CCl4 in order to induce hepatocellular carcinoma.
Group 3: Rats were orally administered EA at 50 mg/kg/day for 7 days before, and 14 days after NDEA injection as a protective agent against liver injury induced by NDEA. [HCC-EA(P) group].
Group 4: Rats were orally administered EA at 50 mg/kg/day for 21 days after NDEA injection as a curative agent against liver injury induced by NDEA. [HCC-EA(C) group].
2.3. Induction of hepatocellular carcinoma
Rats were given a single intraperitoneal dose of NDEA (200 mg/kg b.wt.), followed by subcutaneous injection of carbon tetrachloride (CCl4) (200 mg/kg b.wt.) once weekly for 3 weeks to ensure induction of hepatocellular carcinoma as described by Sundaresan and Subramanian (2003).
2.4. Ellagic acid treatment
EA administered dose in this study was 50 mg/kg b.wt./day in dimethyl sulfoxide (DMSO); orally according to Buniatian (2003).
Clinical signs and general appearance were checked daily, at the end of the experimental period; animals were fasted overnight but allowed free access to water. Animals were sacrificed under anesthesia with diethyl ether, and then blood was collected. The serum was separated by allowing blood samples left for 15 min at temperature of 25 °C then centrifuged at 4000 rpm for 20 min, then kept in plastic vials at −20 °C until analysis. The abdomen was excised; then liver was removed immediately by dissection, washed in ice-cold isotonic saline and blotted between two filter papers. The livers were wrapped in aluminum foil and stored at −80 °C and kept for further determinations.
2.5. Determination of liver enzymatic and non-enzymatic antioxidant status
One portion of liver was used to prepare 10% homogenate in 1.15% KCl and 5% homogenate in 3% sulfosalicylic acid, centrifuged at 4000 rpm at 4 °C for 20 min and the supernatants were used to obtain the cytosolic fraction which was used for the assay of GPX (Arthur and Boyne, 1985), γ-GT (Szasz, 1969), GST (Habig et al., 1974) and GSH (Ellman, 1959).
2.6. Serum biochemical determinations
In serum the following parameters were estimated: arginase enzyme activity according to Patil et al. (1990), α-l-fucosidase activity according to Wang and Cao (2004) as tumor markers’ activities, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) according to Kaplan (1984), alkaline phosphatase (ALP) according to Belfield and Goldberg (1971), glucose-6-phosphate dehydrogenase (G6PD) according to Sies et al., (1967) serum total protein, total and direct bilirubin according to Lowry et al. (1951) and Martinek (1966).
2.7. DNA fragmentation analysis
Another portion of liver tissues was homogenized and incubated in 100 mM Tris–HCl (pH 8.0), 25 mM EDTA, 0.5% SDS, and 0.1 μg/ml proteinase K at 60 °C for 3 h. DNA was extracted with phenol/chloroform (1:1 v:v) and chloroform/isoamyl alcohol (1:24 v:v). The extracted DNA was precipitated and digested in 10 mM Tris–HCl (pH 5.0) containing 1 mM EDTA and 10 μg RNase for 1 h at 37 °C. Five micrograms of DNA per sample was electrophoretically separated on 1.5% agarose gel containing 0.5 μg/ml ethidium bromide. The DNA pattern was examined by an ultraviolet transilluminator (Sambrook et al., 1989).
2.8. Statistical analysis
The obtained results were expressed as mean ± SE. Data were evaluated statistically with computerized SPSS package program (SPSS 9.00 software for Windows) using one-way analysis of variance (ANOVA). Significant differences among means were estimated at p < 0.05 according to Snedecor and Cochran (1986).
3. Results
Table 1 shows that administration of NDEA elicited dramatic significant increase (p < 0.05) in the tumor markers arginase and α-l-fucosidase activities (+26% and +234.5%, respectively) in the untreated HCC group, compared to normal control rats. HCC-EA(P) pretreated rats showed significant reduction in arginase and α-l-fucosidase activities (−23.6% and −61.9%, respectively) as compared to HCC rats. However, rats in the HCC-EA(C) group elicited lower decreases (−7.5% and −22.6%) in arginase and α-l-fucosidase activities respectively as compared to HCC rats.
Table 1.
Effect of oral administration of ellagic acid on serum tumor markers: arginase and α-l-fucosidase activities in hepatocellular carcinomic rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | HCC rats | HCC-EA(C) | HCC-EA(P) | |
| Arginase enzyme activity (U/L) | 322.5 ± 5.8 | 406.0 ± 7.8 | 375.3 ± 10.9 | 310.0 ± 9.4 |
| α-l-Fucosidase activity (nmol/ml/h) | 62.6 ± 2.5 | 209.4 ± 4.3 | 112.0 ± 4.2 | 79.7 ± 5.3 |
NDEA-intoxication resulted in liver injury manifested by significant decreases in the activities of the liver antioxidant enzymes: GSH and GPX by −36% and −46.4% respectively, compared to normal controls. Oral administration of EA elicited dramatic increase in these enzymes, this increase was more pronounced in HCC-EA(P)-pretreated rats +53% and +83.9% than in HCC-EA(C) treated ones +21% and +47.8% for GSH and GPX respectively as compared to NDEA intoxicated rats. On the other hand, the increased activities of γ-GT and GST that were induced by NDEA in HCC rats (+55.7% and +54.4%, respectively) when compared to normal control rats. EA supplementation to HCC rats decreased the levels of γ-GT and GST activities compared to HCC rat group. These changes were more pronounced in HCC-EA(P)-pretreated rats than in HCC-EA(C)-treated ones. The levels of reduction of γ-GT and GST activities were −27.8% and −29.6% respectively, in HCC-EA(P)-pretreated rats, while they were −19.7% and −27.8% in HCC-EA(C)-treated rats when compared to HCC rats (Table 2).
Table 2.
Effect of oral administration of ellagic acid on hepatic concentrations of GSH, GPX, γ-GT and GST activities of hepatocellular carcinomic rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | HCC rats | HCC-EA(C) | HCC-EA(P) | |
| (GSH) (μmol/dl) | 40.21 ± 0.12 | 25.42 ± 0.25 | 30.85 ± 0.6 | 38.9 ± 0.25 |
| (GPX) (nmol/ml) | 93.2 ± 9.4 | 49.9 ± 3.4 | 73.8 ± 2.6 | 91.8 ± 0.6 |
| (γ-GT) (nmol/mg protein) | 98.1 ± 7.1 | 152.8 ± 10.6 | 122.6 ± 8.3 | 110.3 ± 4.3 |
| (GST) (nmol/mg protein/min) | 234.3 ± 12.4 | 361.8 ± 9.68 | 284.5 ± 17.3 | 254.7 ± 19.2 |
Table 3 shows that NDEA intoxication resulted in liver carcinoma which was manifested by significant increase in serum ALT, AST, ALP and G6PD activities by +87.7%, +98.1%, +47.5% and +70.19%, respectively when compared to normal control rats, thus indicating liver damage. Oral administration of EA to HCC rats significantly reduced the elevated levels of ALT, AST, and ALP activities along with more increasing activity of G6PD. HCC-EA(P) pretreated rats showed significant changes in the enzyme activities by −43.9%, −45.8%, −28.5% and +106.4%, respectively when compared to HCC rats. However, rats in the HCC-EA(C) rats elicited lower decreases in these enzyme activities −35.3%, −14.2%, −23.25 and +87.14%, respectively when compared to HCC rats.
Table 3.
Effect of oral administration of ellagic acid on serum activities of AST, ALT, ALP and G6PD of hepatocellular carcinomic rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | HCC rats | HCC-EA(C) | HCC-EA(P) | |
| (AST) (U/L) | 106.00 ± 8.91 | 199.00 ± 9.72 | 128.6 ± 9.98 | 111.5 ± 7.9 |
| (ALT) (U/L) | 76.70 ± 3.37 | 152.00 ± 3.33 | 130.3 ± 2.98 | 82.3 ± 3.5 |
| (ALP) (U/L) | 190.97 ± 4.48 | 281.09 ± 4.80 | 215.8 ± 3.78 | 200.7 ± 3.9 |
| G6PD (mol/mg protein/min) | 27.85 ± 2.29 | 47.4 ± 1.48 | 52.12 ± 4.04 | 57.50 ± 4.84 |
Table 4 shows that NDEA-intoxication resulted in a significant reduction in serum total protein level by −28.4% compared to normal controls (p < 0.05) this change was increased by +28.6% and +6.5% in HCC-EA(P) and HCC-EA(C) groups respectively, as compared to NDEA treated rats. On the other hand, the levels of direct and total bilirubin significantly reduced in HCC rats by −59.3% and −80%, respectively indicating liver injury. EA administration elicited dramatic increase in direct and total bilirubin levels by +15.9% and +35.8% in HCC-EA(P) pretreated rats and by +3.3% and +20.9% in HCC-EA(C) treated rats respectively, when compared to HCC rats.
Table 4.
Effect of oral administration of ellagic acid on serum concentrations of total protein, direct and total bilirubin of hepatocellular carcinomic rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | HCC rats | HCC-EA(C) | HCC-EA(P) | |
| Total protein (g/dl) | 6.82 ± 0.10 | 4.88 ± 0.43 | 5.2 ± 0.46 | 6.69 ± 0.45 |
| Direct bilirubin (mg/dl) | 0.59 ± 0.01 | 0.94 ± 0.02 | 0.61 ± 0.03 | 0.79 ± 0.06 |
| Total bilirubin (mg/dl) | 0.45 ± 0.01 | 0.81 ± 0.01 | 0.67 ± 0.03 | 0.52 ± 0.05 |
3.1. Liver DNA
NDEA-treated HCC rats showed a degree of hepatic DNA fragmentation which was nearly abolished in ellagic acid treated rats (Fig. 1).
Figure 1.
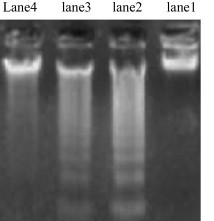
Influence of ellagic acid on NDEA-induced hepatic DNA fragmentation. Lane 1: no DNA fragmentation in normal control. Lane 2 depicts strong DNA fragmentation in NDEA-treated HCC rats. Lane 3 depicts weak DNA fragmentation in HCC-EA(C) rats. Lane 4 depicts no DNA fragmentation in rats administered with EA before and after NDEA injection, HCC-EA(P) rats.
4. Discussion
An understanding of how cancer may be prevented is one of the key objectives of the recent researches. This can be achieved to some extent by using chemopreventive agents, naturally occurring or synthetic, that can suppress or prevent the process of tumor development. Therefore, it is essential to identify agents as well as to evaluate their efficacy and to elucidate their mechanisms of action.
In the present study, serum obtained from tumor bearing rats showed significant increase in tumor markers arginase and fucosidase, AST, ALT, ALP, GST, G6PD, direct and total bilirubin along with significant decrease in serum total protein, GSH, GPX and γ-GT compared to control animals. The elevation of these enzyme activities was indicative of the toxic effect of NDEA on the liver tissue. It is known that N-nitroso compounds act as strong carcinogens in various mammals including primates (Swenberg et al., 1991). NDEA has been shown to be metabolized by cytochrome P-450 (CYP 2E1) to its active ethyl radical metabolite, which could interact with DNA causing mutation and carcinogenesis (Anis et al., 2001). Liver injuries induced by NDEA followed by CCl4 injection are the best-characterized system of the xenobiotic-induced hepatotoxicity and is a commonly used model for screening the antihepatotoxic/hepatoprotective activity of drugs (Lin et al., 2008). At a suitable dose, CCl4 causes extensive necrosis in the liver centrilobular regions around the central veins. CCl4 is biotransformed by the cytochrome P450 in the liver endoplasmic reticulum to the highly reactive trichloromethyl free radical (CCl3). This free radical in turn reacts with oxygen to form a trichloromethylperoxy radical, which leads to elicit lipid peroxidation, elevation of hepatic enzymes and finally results in cell death (Bun et al., 2006).
NDEA elicited dramatic significant increase in the tumor markers arginase and α-l-fucosidase activities compared to normal control rats.
Tumor markers are potential screening tools that are widely used for early diagnosis of tumors. Arginase (l-arginine ureohydrolase) is present in mammals and plants. In humans, arginase is expressed predominantly in the liver, and to lesser degrees in the breast, kidney, testes, salivary glands, epidermis and erythrocytes. Arginase catalyzes the conversion of arginine to ornithine and urea, completing the last step in the urea cycle. Arginase activity is a key diagnostic indicator so it has been reported that, some of the urea cycle enzymes leak rapidly from hepatocytes when liver cells are damaged (Merrick et al., 2006). Many studies have shown that increased stimulation of arginase expression in animal systems leads to production of polyamines that promote tumor cell proliferation and wound healing (Satriano, 2003) and (Cederbaum et al., 2004). α-l-Fucosidase enzyme is usually found as a soluble component of the lysosome and functions as an acid hydrolase in the degradation of a diverse group of naturally occurring fugoglycoconjugate. Increased level of this enzyme is an early indication of HCC. However, it has been proposed as a sensitive tumor marker (Othman et al., 2011).
A study by Sivaramakrishnan et al. (2007) found that the serum α-l-fucosidase activity level in patients with HCC is higher than patients with liver cirrhosis these findings suggest that an increase in serum α-l-fucosidase activity in patients with cirrhosis is the primary risk factor for developing HCC. Oral supplementation with EA reduced the serum enzyme levels when compared to the HCC rat group. The dose of EA (50 mg/kg b.w.) used in the study is safe because some toxicological evaluation revealed that blood coagulation may occur following intravenous injection of large doses (100 mg/kg b.wt.) (Sungwoo et al., 2010).
The antitumor activity of EA has proven an effective inhibitor of chemically induced cancer in the hepatic systems of mice (Han et al., 2006). Several mechanisms by which phytochemicals, such as ellagic acid, can alter carcinogenesis have been identified. Potential mechanisms include the inhibition of enzymes; modification of carcinogen detoxification through several pathways; antioxidation activities, including scavenging DNA reactive agents; suppressing abnormal proliferation of early preneoplastic lesions; and inhibiting certain properties of the cancer cell (Ceribas et al., 2010). Ellagic acid has been shown to have the potential to be a useful cancer preventive and/or chemotherapeutic agent. It decreases the rate of carcinogen metabolism by directly inhibiting the catalytic activity and frequency of gene expression (Yu et al., 2005). Another mechanism was included to reduce carcinogens by the effect of EA on quinone reductase (QR), it is a detoxification enzyme, functions to prevent the formation of superoxide radicals and detoxify a variety of foreign compounds. Carlsen et al. (2003) found that dietary ellagic acid significantly increased hepatic QR activity by 9- and 2-fold, respectively. In this way, ellagic acid effectively increases detoxification of carcinogens and reduces mutagenesis and tumorigenesis. Further studies of the structure–function relationship indicated an interaction between the lactones of EA and antioxidant responsive elements (Wedge et al., 2001). A study by Juranic et al. (2005) demonstrated that, EA possess the potential for antiproliferative action against human colon carcinoma cells in vitro.
Hepatocellular carcinoma was assessed by biochemical findings, serum GSH, GPX, GST and γ-GT. A study by Dakshayani et al. (2005) demonstrated that the oxidative stress may be the reason for the elevated lipid peroxidation level in the liver of NDEA injected rats. Reactive oxygen species (ROS) are produced during the metabolism of NDEA or during the process of carcinogenesis (Sundaresan and Subramanian, 2003). The higher mean activities of the antioxidant enzymes GSH and GPX seen in the ellagic acid treated group highlight the putative anti-radical and antioxidant effects of ellagic acid (Priyadarsini et al., 2002).
GSH is a tripeptide (l-γ-glutamylcysteinylglycine), it is critical for cellular protections such as detoxification of ROS conjugation and excretion of toxic molecules and control of inflammatory cytokine cascade .Depletion of GSH in tissues leads to impairment of the cellular defense against ROS, and may result in peroxidative injury, thus GSH is involved in many cellular processes including the detoxification of endogenous and exogenous compounds (Zaidi et al., 2005). Our findings are consistent with Pradeep et al. (2007) which showed that GSH concentration is decreased after NDEA injection.
The capacity of a tumor cell to maintain GST is determined by a number of interacting pathways. Many of the enzymes involved in these pathways have been targeted for therapeutic intervention by modulators of anticancer drug resistance. Increase in the expression of GST in multi-drug resistance cell lines has frequently been cited as a causal resistance mechanism, since glutathione is required to maintain the normal reduced state of cells and to counteract all the deleterious effects of oxidative stress (Tew, 1994). Ellagic acid has been shown to regulate intracellular GSH levels by induction of gamma-glutamylcysteine synthetase (Bansal et al., 2005), and to prevent n-nitrosodiethylamine induced tumorigenesis by enhancing the GSH-dependent protection. Similarly, Hassoun et al. (2004) observed a normalization in the enzyme activities GPX, GST and γ-GT in liver of ellagic acid-treated mice.
The scavenging action of EA on both oxygen and hydroxyl radicals, and inhibition of lipid peroxidation formation in vitro and in vivo, have also been documented by Iino et al. (2001). Turk et al. (2010) claimed that ellagic acid may act as a good lipophilic antioxidant, due to its solubility; it exhibits minimum solubility in water, however, its solubility increases in organic solvents such as methanol and dimethyl sulfoxide (DMSO). It has been shown that two lactone groups of EA can act as a hydrogen bond donor and acceptor, which might be involved in the free radical scavenging action of EA (Gil et al., 2000).
In HCC rats, it was observed that, the enzyme levels were significantly increased by NDEA injection. These results agreed with those obtained by Mittal et al. (2006) who found that activities of AST, ALT and ALP were increased significantly following nitroso compound treatment in rats due to substantial liver damage. Moreover, Vozarova et al. (2002) mentioned that the elevated activities of AST, ALT and ALP enzymes were signs of impaired liver functions in response to NDEA administration, the elevation of liver enzymes occurred due to their release from the cytoplasm into the blood circulation after rupture of the plasma membrane and cellular damage.
Treatment with EA reduced the activities of the elevated enzymes, a study by Leelavinothan and Ramasamy (2008) examined the ability of different concentrations of EA (30, 50, 60 and 90 mg/kg body weight) to decrease the activities of ALT and ALP in the plasma of liver injured rats. The study concluded that, administrations of EA at 50 mg/kg b.wt., significantly decreased the activities of hepatic marker enzymes compared with other doses of EA. This can be attributed to the antioxidant properties of EA. This result agreed with a study by Devipriya et al. (2007) which demonstrated the role of ellagic acid in reducing elevated enzyme levels and exerting preventive effects against chronic alcohol-induced liver damage in rats.
Glucose-6-phospahe dehydrogenase G6PD is an enzyme that catalyzes the first step in the hexose monophosphate pathway, produces ribose, which is incorporated into nucleotides and NADPH, the major cytoplasmic reducing compound. NADPH is a substrate for phase I and II detoxification enzymes. G6PD is elevated in response to external stimuli like toxic agents and oxidative stress. Frederiks et al. (2003) found that the activity of G6PD is up regulated by carcinogens and oxidative stress. EA administration to NDEA-treated rats showed elevated G6PD activity, indicating that increased amounts of NADPH are required for detoxification process.
Serum total protein is present in blood plasma abundantly (60%) and structurally well characterized. In the present study it was observed that, the administration of NDEA decreased the levels of serum total protein which is an evidence of existence of liver toxicity when compared to normal animals. Studies have shown that cellular proteins may be affected by free radical accumulation leading to the formation of carbonyl derivatives. The carbonyl derivatives of proteins may result from oxidative modification of amino acid side chains and reactive oxygen-mediated peptide cleavage (Afaq et al., 2004). Further, Bala et al. (2006) have reported that the primary target of the oxygen-radical attack, promoted by ethanol, is represented on cellular proteins.Treatment with EA significantly increased the levels of total protein because it would prevent the attack of free radicals on amino acids and thus diminish the production of the carbonyl group in EA-treated rats.
In this study, direct and total bilirubin concentrations were elevated in NDEA treated rats, this elevation may be due to decreased conjugation and decreased secretion from the liver or blockage of bile ducts (Bun et al., 2006). EA reduced the elevated levels of total and direct bilirubin in HCC rats. In a study by Talcott and Lee (2002), protective effects of 14 kinds of antioxidants on liver injury induced by carbon tetrachloride (CCl4) were investigated in terms of bilirubin concentration, consequently, the significant protective effects were found in ellagic acid, the biochemical restoration of bilirubin is due to the promoting effect of EA on bile glucoronidation.
NDEA followed by CCl4 injection was found to induce apoptosis as represented by DNA fragmentation. This result came in agreement with Castro et al. (1993) who reported that CCl4 induced necrosis and DNA fragmentation in Sprague–Dawley male rats. Nabeshima et al. (2006) proved that carbon tetrachloride poisoning induced DNA fragmentation, apoptosis and necrosis in rat liver by immuno-histochemical labeling of nuclear DNA fragmentation, flow cytometry and gel electrophoresis. Several reactive mutagenic and genotoxic lipid peroxidation products have been identified to bind to DNA and to damage it (Eder et al., 2006). Damage to DNA may lead to mutations, initiation of cancer cells and cancer progression (Walle et al., 2003).
Ellagic acid is an effective antimutagen and anticarcinogen phytotherapeutic agent, that prevents carcinogens binding to DNA and strengthens connective tissue and thus may keep cancer cells from spreading, inhibits cancer onset and tumor proliferation and protects healthy cells during chemotherapy (Turk et al., 2008).
One method by which cancer affects DNA is through covalent bonding of the carcinogen to the DNA molecule. Ellagic acid inhibits mutagenesis and carcinogenesis by forming adducts with DNA, thus masking binding sites to be occupied by the mutagen or carcinogen (Eder et al., 2006). Another mechanism by Nair et al. (2005) showed that, EA inhibits several different types of DNA modifying enzymes including topoisomerases I and II, gyrase and polymerase and hence inhibits the growth of cancerous cells.
When EA bind to DNA its molecules could be positioned in such a way, so it could effectively scavenge reactive intermediates that approach the critical sites on DNA. EA may also directly interact with the ultimate reactive metabolites of carcinogen by donating their electrons and rendering it inactive. There was a great relation between lipid peroxidation and DNA damage, existing evidence suggests that lipid peroxidation products of polyunsaturated fatty acids play a major role in genotoxicity of the cell (Kim et al., 2009). A study by Walle et al. (2003) aimed to evaluate the effect of polyphenols on DNA showed that; the binding of EA to DNA was as much as 500 times higher than for quercetin. This large difference may be due to the greater ability of EA than quercetin to intercalate with the double-stranded DNA molecule.
5. Conclusion
The data obtained from this study, indicate that oral administration of ellagic acid (which has potent free radical scavenging and antioxidant properties) to HCC rats, at least partially, alleviates NDEA-induced liver injury by preventing lipid peroxidation enzyme system, inhibiting DNA fragmentation, and increasing antioxidant enzyme activities. Moreover, the use of EA prior to NDEA treatment (as protection) is more effective than its curative effect (i.e., when ellagic acid administered after NDEA treatment) in preventing NDEA-induced liver injury. Therefore, EA administration seems to be a highly promising agent for protecting hepatic tissue against oxidative damage and in preventing hepatic injury and dysfunction.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afaq F., Malik A., Syed D., Maes D., Matsui M., Mukhtar H. Pomegranate fruit extract modulate UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes. Photochem. Photobiol. 2004;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- Anis K., Rajesh N., Kuttan R. Inhibition of chemical carcinogenesis by biberine in rats and mice. J. Pharm. Pharmacol. 2001;53:763–768. doi: 10.1211/0022357011775901. [DOI] [PubMed] [Google Scholar]
- Arthur J., Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985;36:1569–1575. doi: 10.1016/0024-3205(85)90381-9. [DOI] [PubMed] [Google Scholar]
- Bala I., Bhardwaj V., Hariharan S., Kumar M. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006;40:206–210. doi: 10.1016/j.jpba.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bansal A., Bansal M., Bhatnagar D. Protective role of vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005;156:101–111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Befler A., Bisceglie A. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- Belfield A., Goldberg D. Colorimetric determination of alkaline phosphatase activity. Enzyme. 1971;12:561–566. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- Bin L., Kim H., Lindsay W., Lynne S., Kevin J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr. Polym. 2013;92:1443–1450. doi: 10.1016/j.carbpol.2012.10.051. [DOI] [PubMed] [Google Scholar]
- Bun S., Bun H., Guedon D., Rosier C., Ollivier E. Effect of green tea extracts on liver functions in Wistar rats. Food Chem. Toxicol. 2006;44:1108–1113. doi: 10.1016/j.fct.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Buniatian G. Stages of activation of hepatic stellate cells: effects of ellagic acid, an inhibiter of liver fibrosis, on their differentiation in culture. Cell Prolif. 2003;36:307–319. doi: 10.1046/j.1365-2184.2003.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman B., Phillips B., Isbell T., Ou B., Crane J., Knapp S. Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004;52:7982–7987. doi: 10.1021/jf049149a. [DOI] [PubMed] [Google Scholar]
- Caillot F., Derambure C., Bioulac-Sage P. Transient and etiology related transcription regulation in Cirrhosis prior to hepatocellular carcinoma occurrence. World J. Gastroenterol. 2009;15:300–309. doi: 10.3748/wjg.15.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen H., Myhrstad M., Thoresen M., Moskaug J., Blomhoff R. Berry intake increases the activity of the gamma-glutamylcysteine synthetase promoter in transgenic reporter mice. J. Nutr. 2003;133:2137–2140. doi: 10.1093/jn/133.7.2137. [DOI] [PubMed] [Google Scholar]
- Cederbaum S., Yu H., Grody W. Arginase I and II: do their functions overlap? Med. Genet. Metab. 2004;81:138–144. doi: 10.1016/j.ymgme.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Ceribas A., Turk G., Sonmez M., Sakin F., Ates A. Toxic effect of cyclophosphamide on sperm morphology, testicular histology and blood oxidant–antioxidant balance, and protective roles of lycopene and ellagic acid. Basic Clin. Pharmacol. Toxicol. 2010;107:730–736. doi: 10.1111/j.1742-7843.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- Dakshayani K., Subramanian P., Manivasagam T., Mohamed M., Manoharan S. Melatonin modulates the oxidant–antioxidant imbalance during N-nitrosodiethylamine induced hepatocarcinogenesis in rats. J. Pharm. Pharm. Sci. 2005;8:316–321. [PubMed] [Google Scholar]
- Devipriya N., Srinivasan M., Sudheer A., Menon V. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: a drug dose-dependent study. Singapore Med. J. 2007;48:311–318. [PubMed] [Google Scholar]
- Eder E., Wacker M., Lutz U., Nair J., Fang X., Batsch H., Beland F., Schlatter J., Lutz W. Oxidative stress related DNA adducts in the liver of female rats fed with sunflower, rapeseed, olive or coconut oil supplemented diets. Chem. Biol. Interact. 2006;159:81–89. doi: 10.1016/j.cbi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Ellman G. Tissue sulfahydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Falsaperla M., Morgia G., Tartarone A., Ardito R., Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur. Urol. 2005;47:449–455. doi: 10.1016/j.eururo.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Frederiks W., Bosch K., De Jong J., Van N. Post-translational regulation of glucose-6-phosphate dehydrogenase activity in preneoplastic lesions in rat liver. J. Histochem. Cytochem. 2003;51:105–112. doi: 10.1177/002215540305100112. [DOI] [PubMed] [Google Scholar]
- Gil M., Francisco A., Barberan T., Pierce B., Holcroft D., Kader A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Gurtsevitch V. Human oncogenic viruses: hepatitis B and hepatitis C viruses and their role in hepatocarcinogenesis. Biochemistry. 2008;73:504–513. doi: 10.1134/s0006297908050039. [DOI] [PubMed] [Google Scholar]
- Habig W., Pabst M., Jakoby W. Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Han D., Lee M., Kim J. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26:3601–3606. [PubMed] [Google Scholar]
- Hassoun E., Vodhanel J., Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J. Biochem. Mol. Toxicol. 2004;18:196–203. doi: 10.1002/jbt.20030. [DOI] [PubMed] [Google Scholar]
- Iino T., Nakahara K., Miki W., Kiso Y., Ogawa Y., Kato S., Takeuchi K. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion. 2001;64:214–221. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- Juranic Z., Zizak Z., Tasic S., Petrovic S., Nidzovic S., Leposavic A. Antiproliferative action of water extracts of seeds or pulp of five different raspberry cultivars. Food Chem. 2005;93:39–45. [Google Scholar]
- Kaplan, A., 1984. Clin Chem. The C.V. Mosby Co., St Louis, Toronto, Princeton, pp. 1088–1273.
- Kim S., Gaber M., Zawaski J., Zhang F., Richardson M., Zhang X. The inhibition of glioma growth in vitro and in vivo by a chitosan/ellagic acid composite biomaterial. Biomaterials. 2009;30:4743–4751. doi: 10.1016/j.biomaterials.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Leelavinothan P., Ramasamy S. Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundam. Clin. Pharmacol. 2008;22:395–401. doi: 10.1111/j.1472-8206.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- Lin H., Tseng H., Wang C., Lin J., Lo C., Chou F. Hepatoprotective effects of Solanum nigrum Linn. extract against CCl4-induced oxidative damage in rats. Chemico-Biol. Interact. 2008;171:283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lowry O., Rosenbrough N., Farr A., Randall R. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Martinek R. Improved micro-method for determination of serum bilirubin. Clin. Chim. Acta. 1966;13:61–170. doi: 10.1016/0009-8981(66)90290-7. [DOI] [PubMed] [Google Scholar]
- Merrick B., Bruno E., Madwnspacher B., Wetmore J., Foley R., Pieper R., Zhano M., Makusky A., McGrath A. Alterations in the rat serum proteome during liver injury from acetaminophen. J. Pharmacol. Exp. Ther. 2006;318:792–802. doi: 10.1124/jpet.106.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal G., Brar A., Soni G. Impact of hypercholesterolemia on toxicity of N-nitrosodiethylamine: biochemical and histopathological effects. Pharmacol. Rep. 2006;58:413–419. [PubMed] [Google Scholar]
- Nabeshima Y., Tazuma S., Kanno K., Hyogo H., Iwai M., Horiuchi M. Antifibrogenic function of angiotensin II type 2 receptor in CCl4-induced liver fibrosis. Biochem. Biophys. Res. Commun. 2006;346:658–664. doi: 10.1016/j.bbrc.2006.05.183. [DOI] [PubMed] [Google Scholar]
- Nair J., Furstenberger G., Burger F., Marks F., Bartsch H. Pro-mutagenic etheno-DNA adducts in multistage mouse skin carcinogenesis: correlation with lipoxygenase-catalysed arachidonic acid metabolism. Chem. Res. Toxicol. 2005;13:703–709. doi: 10.1021/tx000045d. [DOI] [PubMed] [Google Scholar]
- Othman A., El-Houseini M., El-Sofy M., Aboul-Enein H.Y. Potentiometric determination of α-l-fucosidase enzyme by using 2-chloro-4-nitrophenol-rhodamine B ion pair chemical recognition in PVC membrane sensor. Anal. Bioanal. Chem. 2011;400:787–795. doi: 10.1007/s00216-011-4774-0. [DOI] [PubMed] [Google Scholar]
- Pari L., Sivasankari R. Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundam. Clin. Pharmacol. 2008;22:395–401. doi: 10.1111/j.1472-8206.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- Patil B., Somvanshi S., Kothari R. A simple and rapid high recovery protocol for the purification of arginase. Biotechnol. Tech. 1990;4:133–136. [Google Scholar]
- Pradeep K., Mohan C., Gobianand K., Karthikeyan S. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Priyadarsini K., Khopde S., Kumar S., Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002;50:2200–2206. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch F., Maniatis T. CSHL Press; NY: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Satriano J. At the crossroads of the arginine pathways. Ann. N.Y. Acad. Sci. 2003;1009:34–43. doi: 10.1196/annals.1304.004. [DOI] [PubMed] [Google Scholar]
- Sies M.H., Le Bon A., Canivenc-Lavier M., Suschetet M. Modification of hepatic drug-metabolizing enzymes in rats treated with alkyl sulfides. Cancer Lett. 1967;120:195–201. doi: 10.1016/s0304-3835(97)00309-1. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan V., Shilpa P., Praveen V., Niranjali D. Attenuation of N-nitrosodiethylamine induced hepatocellular carcinogenesis by a novel flavonol-Morin. Chem. Biol. Interact. 2007;171(79–8):8. doi: 10.1016/j.cbi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Snedecor G., Cochran W. fourth ed. Iowa State University Press; Ames, Iowa, USA: 1986. Statistical Methods. 91. [Google Scholar]
- Sundaresan S., Subramanian P. S-allylcysteine inhibits circulatory lipid peroxides and promotes in N-nitrosodiethylamine induced carcinogenesis. Polish J. Pharmacol. 2003;55:37–42. [PubMed] [Google Scholar]
- Sungwoo K., Satoru K., Joel D., Warren O., Haggard M., Waleed G., Yunzhi Y. A chitosan/b-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31:4157–4166. doi: 10.1016/j.biomaterials.2010.01.139. [DOI] [PubMed] [Google Scholar]
- Swenberg J., Hoel D., Magee P. Mechanistic and statistical insight into the large carcinogenesis bioassays on N-nitrosodiethylamine and N-nitrosodimethylamine. Cancer Res. 1991;51:6409–6414. [PubMed] [Google Scholar]
- Szasz G. Kinetic determination of serum gamma glutamyle transferase. Clin. Chem. 1969;15:124–126. [PubMed] [Google Scholar]
- Talcott S., Lee J. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J. Agric. Food Chem. 2002;50:3186–3192. doi: 10.1021/jf011500u. [DOI] [PubMed] [Google Scholar]
- Tew K. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- Thirunavukkarasu C., Sakthisekaran D. Sodium selenite modulates tumour marker indices in N-nitrosodiethylamine initiated and phenobarbital-promoted rat liver carcinogenesis. Cell Biochem. Funct. 2003;21:147–153. doi: 10.1002/cbf.1011. [DOI] [PubMed] [Google Scholar]
- Turk G., Ates S., Ahin A., Sonmez M., Eribas A., Yuce A. Measurement of cisplatin-induced injuries to sperm quality, the oxidant–antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 2008;89:1474–1481. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- Turk G., Eribas A., Sakin F., Sonmez M., Ates S., Ahin A. Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide-induced testicular lipid peroxidation and apoptosis. Reprod. Fertil. Dev. 2010;22:587–596. doi: 10.1071/RD09078. [DOI] [PubMed] [Google Scholar]
- Vozarova B., Stefan N., Lindsay S., Saremi A., Pratley E., Bogardus C., Tatarnni A. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type2 diabetes. Diabetes. 2002;51(1889–1):895. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- Walle T., Vincent T., Walle U. Evidence of covalent binding of the dietary flavonoid quercetin to DNA and protein in human intestinal and hepatic cells. Biochem. Pharmacol. 2003;65:1603–1610. doi: 10.1016/s0006-2952(03)00151-5. [DOI] [PubMed] [Google Scholar]
- Wang J.J., Cao E.H. Rapid kinetic rate assay of the serum alpha-l-fucosidase in patients with hepatocellular carcinoma by using a novel substrate. Clin. Chim. Acta. 2004;347:103–109. doi: 10.1016/j.cccn.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wedge D., Meepagala K., Magee J., Smith S., Huang G., Larcom L. Anticarcino-genic activity of strawberry, blueberry and raspberry extracts to breast and cervical cancer cells. J. Med. Food. 2001;4:49–51. doi: 10.1089/10966200152053703. [DOI] [PubMed] [Google Scholar]
- Yu Y., Chang W., Wu C., Chiang S. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J. Nutr. Biochem. 2005;16:675–681. doi: 10.1016/j.jnutbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zaidi S., Kashif R., Al-Qirim M., Tariq N., Banu D. Effects of antioxidant vitamins on glutathione depletion and lipid peroxidation induced by restraint stress in the rat liver. Drugs. 2005;6:157–165. doi: 10.2165/00126839-200506030-00004. [DOI] [PubMed] [Google Scholar]



