Abstract
Purpose
Vascular endothelial growth factor polymorphism (VEGF-634G/C, rs 2010963) has been considered a risk factor for the development of retinopathy of prematurity (ROP). However, the results remain controversial. Therefore, the aim of the present meta-analysis was to determine the association between VEGF-634G/C polymorphism and ROP risk.
Methods
Published literature from PubMed and other databases were retrieved. All studies evaluating the association between VEGF-634G/C polymorphism and ROP risk were included. Pooled odds ratio (OR) and 95% confidence interval (CI) were calculated using random or fixed effects model. A total of six case-control studies including 355 cases and 471 controls were included.
Results
By pooling all the studies, we found that VEGF-634G/C polymorphism was not associated with ROP risk at co-dominant and allele levels and no association was also found in dominant and recessive models. While stratifying on ethnicity level no association was observed in Caucasian and Asian population.
Discussion
This meta-analysis suggests that VEGF-634G/C polymorphism may not be associated with ROP risk, the association between single VEGF-634G/C polymorphism and ROP risk awaits further investigation.
Keywords: VEGF, ROP, Polymorphism, Meta-analysis
Introduction
Retinopathy of prematurity (ROP) is a developmental disorder that occurs in the incompletely vascularized retina of premature infants and is an important cause of blindness and impaired vision among children in both the developed and the developing countries.1,2 Vision impairment induced by ROP is an important problem, especially in middle-income countries such as the European and Latin American countries. In these regions, ROP is the most common cause of childhood blindness.3,4
The pathogenesis of advanced ROP and its etiology is currently not known. In the past, many causative factors such as length of time exposed to supplemental oxygen, excessive light exposure, and hypoxia have been suggested, but evidence for these as independent risk factors in recent years is not compelling.5,6 It has been suggested that vascular endothelial growth factor (VEGF) may play a causative role in the development of ROP,7 and it is known that VEGF is important in physiological growth of retinal vessels. However, VEGF is also found to be present in human subjects with non proliferative diabetic retinopathy.8
The human VEGF gene (OMIM 192240) is located on chromosome 6p12. Many polymorphisms of the VEGF gene have been described, although most are relatively rare. Single nucleotide polymorphisms (SNPs) in the VEGF 5′ or 3′ untranslated region (UTR) have been reported to be associated with ROP in different populations. A common polymorphism, VEGF-634G/C (rs 2010963, previously denoted +405G/C, position relative to transcription start site) is located in the 5′ UTR.9 Two studies have found association of VEGF-634G/C polymorphism with ROP risk10,11 but other studies show no association between VEGF-634G/C polymorphism and risk of ROP.12–15 These studies revealed an inconsistent conclusion, probably due to the relatively small size of subjects, since individual studies are usually underpowered in detecting the effect of low penetrance genes; therefore, in this study we conducted a meta-analysis to investigate the association between VEGF-634G/C polymorphism and the risk for ROP.
Materials and methods
Identification and eligibility of relevant studies
To identify all articles that examined the association of VEGF-634G/C polymorphism with ROP, we conducted a literature search in the PubMed, EMBASE, Cochrane Library, Google, dogpile and CBM database, up to December 2013 using the following terms and keywords: “VEGF”, “vascular endothelial growth factor”, “-634G/C polymorphism” “rs 2010963” and “retinopathy of prematurity”. Additional studies were identified by a manual search from other sources (e.g., Web of Knowledge), references of original studies or review articles on this topic.
Inclusion and exclusion criteria
To minimize heterogeneity and facilitate the interpretation of our results, studies included in the current meta-analysis had to meet all the following criteria: (1) evaluation of the VEGF-634G/C and ROP risk, (2) use of a case-control design, (3) recruitment of confirmed ROP patients and disease-free controls, (4) have an available genotype frequency, and (5) an unrelated case-control study, if studies had partly overlapped subjects, only the one with a larger sample size was selected, (6) sufficient published data for estimating an odds ratio (OR) with 95% confidence interval (CI), (7) papers published in english and (8) all the studies included were according to tenets of the Declaration of Helsinki. The major reasons for exclusion of studies were (1) overlapping data and (2) case-only studies, (3) all three genotype frequency missing, (4) family based studies, and (5) review articles.
Data extraction
Four investigators independently assessed the articles for inclusion/exclusion and extracted data, and reached a consensus on all of the items. For each study, the following information was extracted: name of the first author; publication year; ethnicity (country); sample size (numbers of cases and controls); types of disease; sources of samples; genotyping methods; and the minor allele frequency in the controls with the Hardy–Weinberg Equilibrium (HWE) p-value, respectively.
Statistical analysis
The association between VEGF-634G/C polymorphism and ROP was estimated by calculating pooled ORs and 95% CIs. The significance of the pooled OR was determined by Z test (p < 0.05 was considered statistically significant). The risk of VEGF-634G/C CC genotype on retinopathy of prematurity was evaluated by comparing with their reference wild type homozygote and then evaluated the risks of (GC + CC vs. GG) and (GG + GC vs. CC) on retinopathy of prematurity, assuming dominant and recessive effects of the variant C allele, respectively. A random-effects or fixed-effects model was used to calculate pooled effect estimates in the presence (p < 0.10) or absence (p > 0.10) of heterogeneity. Publication bias was tested for the overall pooled analysis of VEGF-634G/C CC genotypes, dominant and recessive model. Additionally, Begg’s funnel plot was drawn. Asymmetry of the funnel plot means a potential publication bias. Hardy Weinberg Equilibrium was calculated by chi-square (χ2) test. All meta-analysis tests were performed through comprehensive meta-analysis version 2.0.16
Results
Ten studies were preliminarily retrieved. Two studies were excluded after reading the titles and abstracts. According to inclusion and exclusion standards, six studies were finally included,10–15the publication years of the literatures were between 2004 and 2013. The general features of the included studies are shown in Table 1.
Table 1.
Characteristics of studies included in the VEGF-634G/C meta-analysis.
| S no. | Study | Year | Country | Ethnicity | Genotyping method | Cases | Controls | p-Value | HWE | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cooke et al.10 | 2004 | United Kingdom | Caucasian | PCR, SSCP, RFLP | 91 | 97 | 0.03 | 0.202 | PMID: 15161830 |
| 2 | Vannay et al.11 | 2005 | Hungary | Caucasian | Real-time PCR | 86 | 115 | 0.007 | 0.212 | PMID: 15635051 |
| 3 | Shastry et al.12 | 2007 | USA | Caucasian | PCR-RFLP, Sequencing | 61 | 61 | 0.89 | 0.797 | PMID: 17119993 |
| 4 | Kwinta et al.13 | 2008 | America | Caucasian | PCR-RFLP | 60 | 101 | 0.41 | 0.266 | PMID: 18546007 |
| 5 | Kaya et al.14 | 2013 | Turkey | Asian | PCR-RFLP | 42 | 31 | 0.77 | 0.144 | PMID: 23094709 |
| 6 | Kalmeh et al.15 | 2013 | Iran | Asian | PCR-RFLP | 15 | 66 | 0.88 | 0.854 | PMID: 23644986 |
HWE = Hardy–Weinberg Equilibrium.
Potential publication bias and sensitivity analysis
The influence of CC genotype at -634G/C locus on ROP was taken as the analysis index and inverted funnel plot was drawn. Due to the small amount of the included research and unobvious distribution trends, the inverted funnel plot showed trend symmetry, indicating that the publication bias was not big (Fig. 1).
Figure 1.
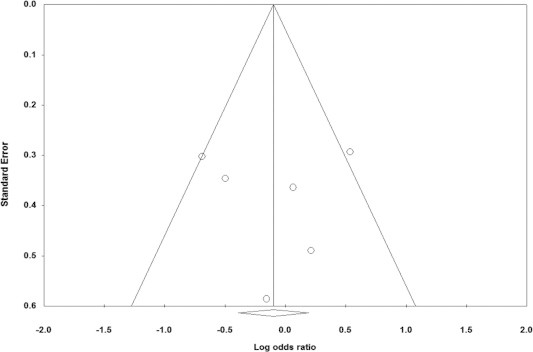
Funnel plots showed symmetric distribution. Log OR is plotted against the standard error of log OR for studies on VEGF-634G/C.
Meta-analysis database
In an attempt to obtain a common estimate of the VEGF-634G/C polymorphism effect on risk for ROP and provide compelling evidence for association, we performed a meta-analysis combining all available genotype and allele frequency data from six published studies. The detailed characteristics of each study included in the meta-analysis are presented in Table 1, and the VEGF-634G/C polymorphism genotype distributions from each study are presented in Table 2. By pooling all the studies, VEGF-634G/C polymorphism was not associated with ROP risk (Fig. 2). In subgroup analysis, we found that VEGF polymorphism was not significantly correlated with increased ROP risk at allele level (Fig. 3). When stratified by dominant and recessive models, no association was found between VEGF-634G/C polymorphism and risk of ROP in all models by pooling all six studies (Figs. 4 and 5). We also examined the association between VEGF-634G/C polymorphism and ROP risk at ethnicity level; the overall result showed that VEGF-634G/C polymorphism was not associated with ROP risk in Caucasians and Asians (Fig. 6).
Table 2.
VEGF-634G/C polymorphism genotype distribution of each study included in the meta-analysis.
| Author/year | Genotype frequency |
Allele frequency |
Dominant model |
Recessive model |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
Cases |
Controls |
Cases |
Controls |
|||||||||||
| GG | GC | CC | GG | GC | CC | G | C | G | C | GG | GC + CC | GG | GC + CC | CC | GG + GC | CC | GG + GC | |
| Cooke et al.10 | 44 | 39 | 8 | 31 | 53 | 13 | 127 | 55 | 115 | 79 | 44 | 47 | 31 | 66 | 8 | 83 | 13 | 84 |
| Vannay A et al.11 | 30 | 41 | 15 | 55 | 53 | 7 | 101 | 71 | 163 | 67 | 30 | 56 | 55 | 60 | 15 | 71 | 7 | 108 |
| Shastry et al.12 | 27 | 27 | 7 | 28 | 26 | 7 | 81 | 41 | 82 | 40 | 27 | 34 | 28 | 33 | 7 | 54 | 7 | 54 |
| Kwinta et al.13 | 32 | 18 | 10 | 55 | 36 | 10 | 82 | 38 | 146 | 56 | 32 | 28 | 32 | 46 | 10 | 50 | 10 | 91 |
| Kaya et al.14 | 25 | 11 | 6 | 20 | 8 | 3 | 61 | 23 | 48 | 14 | 25 | 17 | 20 | 11 | 6 | 36 | 3 | 28 |
| Kalmeh et al.15 | 6 | 6 | 3 | 24 | 31 | 11 | 18 | 12 | 79 | 53 | 6 | 9 | 24 | 42 | 3 | 12 | 11 | 55 |
Figure 2.
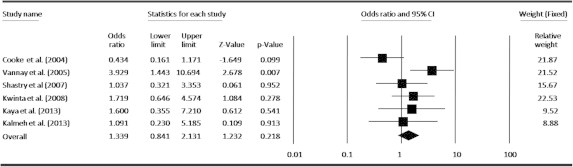
Forest plots of the association between VEGF-634G/C (co-dominant model; GG vs. CC) polymorphism and ROP risk. The squares and horizontal lines correspond to OR and 95% CI of specific study, and the area of squares reflects study weight (inverse of the variance). The diamond represents the pooled OR and its 95% CI.
Figure 3.
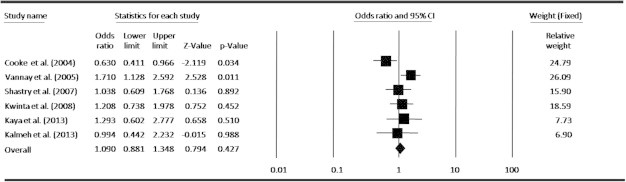
Meta-analysis under allelic model (G vs. C) for the association between ROP risk and the VEGF-634G/C polymorphism.
Figure 4.
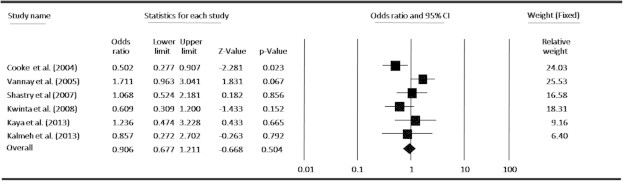
Meta-analysis under dominant model (GC + CC vs GG) for the association between ROP risk and the VEGF-634G/C polymorphism.
Figure 5.
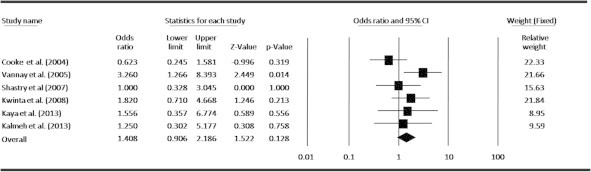
Meta-analysis under recessive model (GG + GC vs. CC) for the association between ROP risk and the VEGF-634G/C polymorphism.
Figure 6.
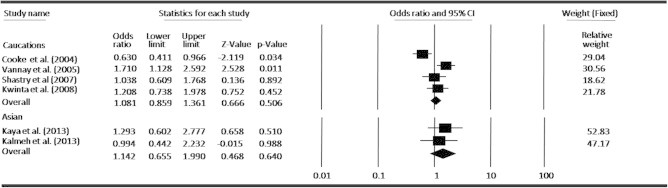
Meta-analysis under ethnicity level for the association between ROP risk and the VEGF-634G/C polymorphism.
Discussion
In the present study, we systemically reviewed all available published studies and performed a meta-analysis to examine the association between the VEGF-634G/C polymorphism and susceptibility to ROP. This is the first study where we have examined all the possible models (co-dominant, dominant, recessive and allele level models) to validate our results. The association of VEGF-634G/C polymorphism with ROP risk was also assessed at ethnicity level. Our meta-analysis showed that VEGF-634G/C polymorphism was not associated with ROP risk. These results are in agreement with the independent reports12,15 but in direct contradiction to the previously published data10,11 in which a significant difference in the G/G genotype has been reported. By pooling all the studies, we did not find an association between VEGF-634G/C polymorphism and ROP, suggesting that previous controversial data may be due to small size of population.
The deviation most likely indicates a genotyping assay problem with an erroneous gain/loss of homozygous genotypes. The commonly used polymerase chain reaction-restriction fragment length polymorphism analysis for genotyping is reported to have poor accuracy and reproducibility17 and may underlie this finding. Conversely, effects of sample selection and differences in biological and environmental complexity between samples could also hinder efforts to replicate association in most of the studies which are statistically underpowered. The meta-analysis helps researchers to deal with the diversity of the published data but in general cannot do justice to complex human diseases, which involve multiple genetic and environmental determinants.18 However, in the total combined data, no evidence for association between the VEGF-634G/C polymorphism and risk of ROP was observed. Therefore, the different results across studies may result from small sample size and/or genotyping technique rather than ethnic differences. Since the studies included were very limited, it is necessary to validate the association between VEGF-634G/C polymorphism and ROP risk in future studies. A well-designed meta-analysis can provide valuable information for researchers, policymakers, and clinicians.
In conclusion, the present meta-analysis suggested that VEGF-634G/C polymorphism may not be associated with risk for ROP. More epidemiologic studies are suggested to further ascertain the relationship between VEGF-634G/C polymorphism and genetic predisposition to ROP.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Haines L., Fielder A.R., Baker H., Wilkinson A.R. UK population based study of severe retinopathy of prematurity: screening, treatment, and outcome. Arch Dis Child Fetal Neonatal Ed. 2005;90:240–244. doi: 10.1136/adc.2004.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser R.S., Trese M.T., Williams G.A., Cox M.S., Jr. Adult retinopathy of prematurity: outcomes of rhegmatogenous retinal detachments and retinal tears. Ophthalmology. 2001;108:1647–1653. doi: 10.1016/s0161-6420(01)00660-1. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C., Fielder A., Gordillo L., Quinn G., Semiglia R., Visintin P. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:518–525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 4.Tompkins C. A sudden rise in the prevalence of retinopathy of prematurity blindness? Pediatrics. 2001;108:526. [PubMed] [Google Scholar]
- 5.Niranjan H.S., Benakappa N., Reddy K.B., Nanda S., Kamath M.V. Retinopathy of prematurity promising newer modalities of treatment. Indian Pediatr. 2012;49:139–143. doi: 10.1007/s13312-012-0028-2. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds J.D., Hardy R.J., Kennedy K.A., Spencer R., van Heuven W.A., Fielder A.R. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light reduction in retinopathy of prematurity (LIGHT-ROP) cooperative group. N Engl J Med. 1998;338:1572–1576. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 7.Aiello L.P. Vascular endothelial growth factor and the eye. Past, present and future. Arch Ophthalmol. 1996;114:1252–1254. doi: 10.1001/archopht.1996.01100140452016. [DOI] [PubMed] [Google Scholar]
- 8.Amin R.H., Frank R.N., Kennedy A., Eliott D., Puklin J.E., Abrams G.W. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:36–47. [PubMed] [Google Scholar]
- 9.Watson C.J., Webb N.J., Bottomley M.J., Brenchley P.E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine. 2000;12:1232–1235. doi: 10.1006/cyto.2000.0692. [DOI] [PubMed] [Google Scholar]
- 10.Cooke R.W., Drury J.A., Mountford R., Clark D. Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2004;45:1712–1715. doi: 10.1167/iovs.03-1303. [DOI] [PubMed] [Google Scholar]
- 11.Vannay A., Dunai G., Bányász I., Szabó M., Vámos R., Treszl A. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res. 2005;57:396–398. doi: 10.1203/01.PDR.0000153867.80238.E0. [DOI] [PubMed] [Google Scholar]
- 12.Shastry B.S., Qu X. Lack of association of the VEGF gene promoter (-634 G→C and -460 C→T) polymorphism and the risk of advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007;245:741–743. doi: 10.1007/s00417-006-0480-6. [DOI] [PubMed] [Google Scholar]
- 13.Kwinta P., Bik-Multanowski M., Mitkowska Z., Tomasik T., Pietrzyk J.J. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1467–1475. doi: 10.1007/s00417-008-0865-9. [DOI] [PubMed] [Google Scholar]
- 14.Kaya M., Çokakli M., Berk A.T., Yaman A., Yesilirmak D., Kumral A. Associations of VEGF/VEGF-receptor and HGF/c-Met promoter polymorphisms with progression/regression of retinopathy of prematurity. Curr Eye Res. 2013;38:137–142. doi: 10.3109/02713683.2012.731550. [DOI] [PubMed] [Google Scholar]
- 15.Kalmeh Z.A., Azarpira N., Mosallaei M., Hosseini H., Malekpour Z. Genetic polymorphisms of vascular endothelial growth factor and risk for retinopathy of prematurity in South of Iran. Mol Biol Rep. 2013;40:4613–4618. doi: 10.1007/s11033-013-2554-y. [DOI] [PubMed] [Google Scholar]
- 16.Comprehensive Meta-analysis version 2.0. <http://www.meta-analysis.com>.
- 17.Ding C., Cantor C.R. A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS. Proc Natl Acad Sci USA. 2003;100:3059–3064. doi: 10.1073/pnas.0630494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins J.A. Clinical research evidence and clinical practice. Hum Reprod. 1997;12:1847–1850. doi: 10.1093/humrep/12.9.1847. [DOI] [PubMed] [Google Scholar]


