Abstract
Chronic methamphetamine (MA) use is associated with moderate deficits in learning and memory, but the extent to which MA users are aware of such memory deficits (i.e., metamemory) is not known. In the current study, 195 participants with lifetime MA use diagnoses (MA+) and 195 non-MA-using comparison subjects (MA−) underwent comprehensive neuropsychiatric research assessments, including performance-based and self-report measures of episodic memory. MA use disorders, major depressive disorder (MDD), and their interaction were uniquely associated with metamemory functioning, such that MDD increased the likelihood of a metamemory deficit among the MA+ participants. Within the MA group, individuals who over-estimated their memory abilities demonstrated greater executive dysfunction and lower cognitive reserve. Chronic MA use is associated with reduced awareness of objective deficits in memory acquisition and recall, which is particularly exacerbated by the presence of major depression. Efforts to enhance metamemory accuracy and deployment of compensatory mnemonic strategies may benefit substance abuse treatment outcomes.
Keywords: Neuropsychology, Memory, Drug/Substance Abuse
Introduction
Methamphetamine (MA) has a preferential neurotoxic effect on the frontostriatal systems (Earnst, et al., 2000) that contributes to both emotion dysregulation (London, et al., 2004) and neurocognitive impairment (Scott, et al., 2007). MA-related neurocognitive deficits most commonly include episodic memory and executive functions (Woods et al., 2005) and are associated with poorer functioning in daily activities (Henry, Minassian, Perry, 2010), including unemployment (Weber, et al., 2012). One mechanism by which such MA-related neurocognitive deficits may impact daily functioning is via poor awareness of the nature and extent of one’s impairment. For example, a MA dependent individual who is unaware of a memory deficit would be much less likely to use a compensatory strategy during daily tasks (e.g., using a calendar or alarm to help them remember to take a medication), and is therefore more vulnerable to experience critical memory failures in real life (e.g., medication nonadherence).
According to Nelson and Narens (2007), awareness of memory abilities (i.e., metamemory) may be disrupted at several time points during the acquisition, retention, and/or retrieval of new information. Specifically, the overall correspondence between an individual’s perceived memory abilities and his actual memory capacity is postulated to be influenced by how well the material has been learned (e.g., do I need to continue studying?), initiation and termination of recall search strategies, and recall selection choices (e.g., how confident am I that this is the memory I am searching for?); all of which require internal self-regulation. Such processes utilize prefrontal systems, and the combination of executive and memory dysfunction appears to confer greater metamemory inaccuracy (Pannu & Kaszniak, 2005). These same systems are commonly disrupted among MA users suggesting a potential vulnerability to metamemory dysfunction in this population (Ersche et al., 2013). Yet only two studies to date have examined metacognition in chronic MA users; Cattie and colleagues (2012) found that self-reported symptoms of executive dysfunction in daily life were not related to objective laboratory measures of executive dysfunction among individuals with MA dependence, while Kirkpatrick et al. (2008) illustrated that increasing doses of intranasal methamphetamine administration among MA users disrupted accuracy of metacognitive judgments.
Given the prefrontal and striatal predilection of MA-associated neural disruption, it is not surprising that mood dysregulation, such as major depression, is a highly comorbid (Conway et al., 2006) and functionally impactful condition among chronic MA users (Glasner-Edwards, et al., 2009). As postulated in the Nelson and Narens model of metamemory (2007), an individual’s belief regarding the difficulty level of the information to be learned (i.e., self-efficacy) in combination with his motivation to learn, directly influence his memory behaviors (e.g., to study the information or not). Given that depressive symptoms directly impact self-efficacy and motivation and are related to increased prevalence of memory symptoms in the general population (Ponds & Jolles, 1996), major depressive disorder (MDD) may moderate metamemory accuracy by lowering self-perceived memory abilities and motivation to learn. Therefore, it may not be surprising that memory self-efficacy is consistently a stronger indicator of memory complaints than actual memory test performance (Ponds & Jolles, 1996; Dellefield & McDougall, 1996). In fact, depression has been consistently associated with inaccurate underestimation of actual memory abilities in the general population (Kalska et al., 1999). For example, depression demonstrated a stronger relationship with reported memory symptoms than memory test performance among a cohort of older healthy adults (Bolla, Lindgren, Bonaccorsy, Bleecker, 1991), and increasing levels of depression are consistently associated with greater number of memory symptoms among both younger and older adults, regardless of actual memory capacities (Bolla, Lindgren, Bonaccorsy, Bleecker, 1991; Niederehe & Yoder, 1989; Kalska, Punamaki, Makinen-Pelli, Saarinen, 1999).
Considering the frontostriatal systems that are disrupted in MA use and depression and the role of such systems in metamemory, MA+ individuals may be particularly susceptible to inaccurate perception of their memory abilities; yet, no studies to date have examined this construct among substance users. Therefore, we aim to determine the independent and additive impact of MA use and MDD on metamemory, as well as explore other factors that may affect metamemory processes within the MA+ cohort (e.g., executive dysfunction).
Materials and Methods
Participants
The sample was composed of 390 participants who participated in National Institute of Drug Abuse (NIDA) funded research studies conducted from 1999–2012 that were approved by the University of California, San Diego’s Human Research Protections Program. All participants provided written informed consent prior to study participation. MA use disorder diagnoses were determined via the Composite International Diagnostic Interview Version 2.1 (CIDI; WHO, 1997) or the Structured Clinical Interview for the DSM-IV-TR (SCID; First, Spitzer, Gibbon, 1991), which follow the Diagnostic and Statistical Manual version IV-text revised (DSM-IV-TR; APA, 2000) criteria; participants who met criteria for lifetime MA dependence and MA abuse or dependence within the past 18 months were included in the MA+ group (n = 195). One hundred ninety five participants who did not meet DSM-IV-TR criteria for MA abuse or dependence currently or in the past, and also did not meet DSM-IV-TR criteria for other drug or alcohol abuse or dependence within the past 18 months were included as a MA− comparison sample.
For both groups, participants were not enrolled in the study if they presented with a positive urine toxicology result for all illicit substances (excluding marijuana) on the day of testing, if they qualified for dependence of alcohol or other drugs (excluding marijuana and prescribed medications) within the past year, or, specific to marijuana, participants were excluded if they reported use the morning before testing (i.e., acutely intoxicated). Additionally, participants were excluded if they had histories of primary psychotic disorders (e.g., schizophrenia; substance-induced psychosis was allowed), severe medical problems (e.g., seizures, TBI), or if they scored < 70 on the Wide Range Achievement Test – reading subtest (WRAT-3 Reading; Wilkinson, 1993).
Psychiatric Assessment
Mood disorders and other substance use diagnoses (e.g., alcohol) were determined based on the CIDI or the SCID. Participants were classified as having lifetime Major Depressive Disorder (MDD) if they ever met DSM-IV-TR criteria for MDD (including currently). A semi-structured timeline follow-back interview (Rippeth et al., 2004) was used to determine MA use characteristics (e.g., frequency, quantity).
Neuromedical evaluation
All participants completed a medical evaluation including a standard medical history interview, structured neurological and medical examination, and laboratory testing of blood and urine samples (Heaton et al., 2011).
Neurobehavioral Assessment
A comprehensive neuropsychological battery, designed to capture the primary cognitive domains affected by MA dependence (Scott et al., 2007) was administered to all participants. This battery included measures of episodic memory, executive functions, psychomotor speed, attention/working memory, verbal fluency, and motor functioning (Rippeth et al., 2004).
Episodic Memory Assessment
Learning and memory were specifically evaluated using the Brief Visuospatial Memory Test (BVMT-R; Benedict, 1997) and Hopkins Verbal Learning Test (HVLT-R; Benedict et al., 1998). Learning (i.e., trials 1–3) and delayed recall scores on each measure were calculated and converted into scaled scores (M=10, SD=3) and averaged across the two measures to create summary learning and delayed recall scores. Participants were classified as “memory impaired” if they received a scaled score of < 7 on learning and/or delayed recall. Given that the primary aim of this study was to determine accuracy of one’s memory appraisal (i.e., one’s actual memory abilities in everyday life), we used demographically-uncorrected scaled scores in order to best represent absolute memory functioning (versus memory functioning expected for one’s age, education, gender, and ethnicity). However, group differences in demographic (i.e., education, ethnicity, gender) and neuropsychiatric functioning (i.e., WRAT-3 reading, lifetime MDD, lifetime alcohol or other substance use disorders) were covaried in all between-group analyses.
Perceived Memory Functioning
Perceived memory ability in everyday life was assessed using the memory domain (first 10 items) of the Patient’s Assessment of Own Functioning (PAOFI; Chelune, Heaton, Lehman, 1986). On the PAOFI, each item (e.g., “How often do you forget people whom you met in the last day or two?”) is rated from 1 (“almost always”) to 6 (“almost never”), with lower scores indicating higher symptom severity. “Significant” memory symptoms (i.e., items endorsed as 1 “Almost Always”, 2 “Very Often”, or 3 “Fairly Often”) were then summed into a total memory symptom score. Scores on the memory domain were used to classify participants as either having significant symptoms (memory domain score≥1) or no symptoms (memory domain score=0) of memory functioning.
Metamemory
All participants were then compared across objective memory performance (impaired vs. intact) and memory symptoms (reported symptoms vs. no symptoms) to operationalize four classifications: 1) under-estimators (intact performance, but symptoms; n=147), 2) over-estimators (impaired performance, but no symptoms; n=37), 3) accurate/normal (intact performance and no symptoms; n=170), and 4) accurate/impaired (impaired performance and symptoms; n=37).
Statistical Analysis
In order to determine the impact of MA+ on objective episodic memory performance and perceived memory functioning, we conducted a series of multivariable linear regression models co-varying (i.e., controlling) for important demographic factors (i.e., age, education, ethnicity, gender, WRAT-3 reading) and neuropsychiatric MA group differences in (i.e., lifetime MDD, lifetime alcohol use disorder, and lifetime non-alcohol or MA use disorders). Three parallel models were conducted predicting learning performance (HVLT-R and BVMT-R learning), delayed recall performance (HVLT-R and BVMT-R recall), and reported memory symptoms (PAOFI memory scale; see Table 2).
Table 2.
Multiple regression analyses showing the adverse effect of methamphetamine (MA) use disorders on objective episodic memory performance, daily memory symptoms, and metamemory.
| Multiple Linear Regression | Adjusted R2 | F | b | p-value |
|---|---|---|---|---|
| Learning Test Performance | 0.18 | 11.7 | <0.001 | |
| Age | −0.07 | <0.001 | ||
| Education | 0.20 | <0.001 | ||
| Ethnicity | −0.67 | 0.003 | ||
| Gender | 0.66 | 0.007 | ||
| LT MDD | 0.21 | 0.38 | ||
| LT Alcohol Use Dx | −0.33 | 0.20 | ||
| LT Other Substance Use Dx | 0.21 | 0.41 | ||
| LT MA Use Dx | 0.63 | 0.011 | ||
| Recall Test Performance | 0.18 | 11.5 | <0.001 | |
| Age | −0.08 | <0.001 | ||
| Education | 0.25 | <0.001 | ||
| Ethnicity | −0.37 | 0.003 | ||
| Gender | −0.80 | 0.003 | ||
| LT MDD | −0.14 | 0.26 | ||
| LT Alcohol Use Dx | −0.21 | 0.12 | ||
| LT Other Substance Use Dx | 0.09 | 0.51 | ||
| LT MA Use Dx | 0.30 | 0.03 | ||
| Daily Memory Symptoms | 0.06 | 4.2 | <0.001 | |
| Age | 0.001 | 0.92 | ||
| Education | −0.03 | 0.65 | ||
| Ethnicity | 0.43 | 0.13 | ||
| Gender | 0.39 | 0.19 | ||
| LT MDD | −0.58 | 0.046 | ||
| LT Alcohol Use Dx | 0.43 | 0.17 | ||
| LT Other Substance Use Dx | −0.53 | 0.09 | ||
| LT Methamphetamine Use Dx | −1.1 | <0.001 | ||
|
| ||||
| Logistic Regression | X2 | p-value | ||
|
| ||||
| Metamemory | 88.4 | <0.001 | ||
| Age | 12.7 | 0.005 | ||
| Education | 11.2 | 0.011 | ||
| Ethnicity | 6.25 | 0.10 | ||
| Gender | 9.07 | 0.03 | ||
| LT MDD | 9.59 | 0.02 | ||
| LT Alcohol Use Dx | 2.21 | 0.53 | ||
| LT Other Substance Use Dx | 6.65 | 0.08 | ||
| LT MA Use Dx | 8.03 | 0.045 | ||
| LT MDD * LT MA Use Dx | 11.9 | 0.008 | ||
Note. LT = Lifetime; MDD = Major Depressive Disorder; Dx = Disorder; MA = methamphetamine.
Next, a multivariable logistic regression model was conducted including the same independent variables as the previous models and including an interaction term between MA+ and lifetime MDD predicting metamemory status. Finally, to examine which neurocognitive factors may be independently driving differences on metamemory accuracy within the MA+ group, we ran a multivariable logistic regression model controlling for demographics (i.e., age, education, ethnicity, gender) and lifetime MDD with neurocognitive abilities as the independent variables and metamemory as the dependent variable (see Figure 2).
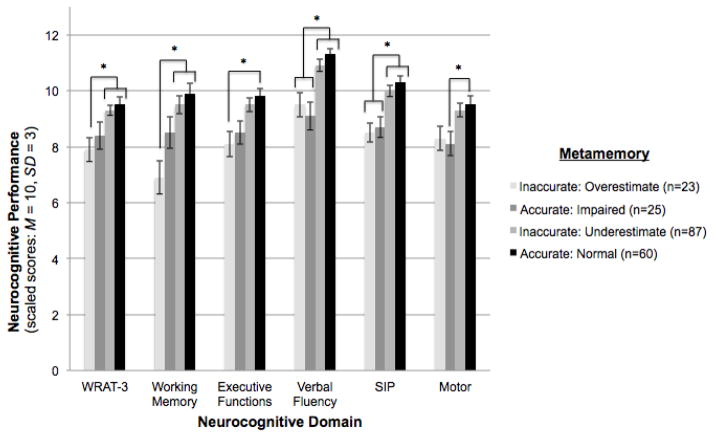
Figure 2. Executive and working memory dysfunction, and lower cognitive reserve (i.e., WRAT-3) are independently associated with overestimating memory abilities among methamphetamine (MA+) users (n = 195).
Displayed in the graph below, the bars represent the metamemory groups among the MA+ users, while neurocognitive domain (i.e., ability area) is represented on the x-axis and average neurocognitive performance in scaled score units is represented on the y-axis. For scaled scores, higher scores indicate better neurocognitive abilities; average scaled score = 10, standard deviation = 3. The brackets indicate the metamemory group(s) that performed significantly different from one another on each neurocognitive domain. *p<0.05.
Note. WRAT-3 = Wide Range Achievement Test, 3rd edition; SIP=Speed of Information Processing.
Results
Demographic and medical disease characteristics are represented in Table 1. Significant (ps<0.01) multivariable linear regression models indicated that MA+ participants demonstrated lower scores on objective measures of learning (i.e., BVMT-R and HVLT-R learning; F(8,381)=27.6, p=0.011) and recall (BVMT-R and HVLT-R delayed recall; F(8,381)=24.3, p=0.028) and higher scores on perceived memory symptoms (PAOFI; F(8,381)=13.2, p<0.001) than MA− participants.
Table 1.
Clinical characteristics of study samples.
| MA+ (n = 195) | MA− (n = 195) | p-value | |
|---|---|---|---|
| Demographic | |||
| Age (years) (mean, SD) | 38.4 (9.2) | 37.9 (12.3) | 0.67 |
| Education (years) (mean, SD) | 12.0 (2.2) | 13.3 (2.3) | <0.001 |
| Gender (% Male) | 80.0% | 65.6% | 0.001 |
| Ethnicity (% White) | 71.3% | 60.5% | 0.025 |
| Hepatitis C (%) | 24.6% | 18.5% | 0.14 |
| WRAT-3 | 96.8 | 101.2 | <0.001 |
| LT Major Depressive Disorder (%) | 38.5% | 21.5% | <0.001 |
| Current Major Depressive Disorder (%) | 10.8% | 3.6% | 0.006 |
| Beck Depression Inventory (mean, SD) | 13.9 (11.1) | 6.3 (7.6) | <0.001 |
| Employed (%) | 55.2% | 39.5% | 0.002 |
| Methamphetamine Use Parameters | |||
| First MA use (age)a | 20.0 (16, 26) | ----- | ----- |
| Last MA use (months)a | 3.0 (1.5, 7.1) | ----- | ----- |
| Duration of MA use (months)a | 123.2 (68, 192) | ----- | ----- |
| Quantity of MA use (grams)a | 3045.0 (1060, 6693) | ----- | ----- |
| Injection MA use ever (%) | 38% | ----- | ----- |
| LT Alcohol Use Disorder (%) | 70.8% | 28.2% | <0.001 |
| LT Cannabis Use Disorder (%) | 54.4% | 19.0% | <0.001 |
| LT Opioid Use Disorder (%) | 11.8% | 4.1% | 0.005 |
| LT Cocaine use Disorder (%) | 34.4% | 7.2% | <0.001 |
| LT SUD (non-MA/non-alcohol) (%) | 68.2% | 25.6% | <0.001 |
Note. MA = methamphetamine; WRAT-3 = Wide Range Achievement Test, version 3; LT = lifetime; SUD = substance use disorder.
Median (IQR)
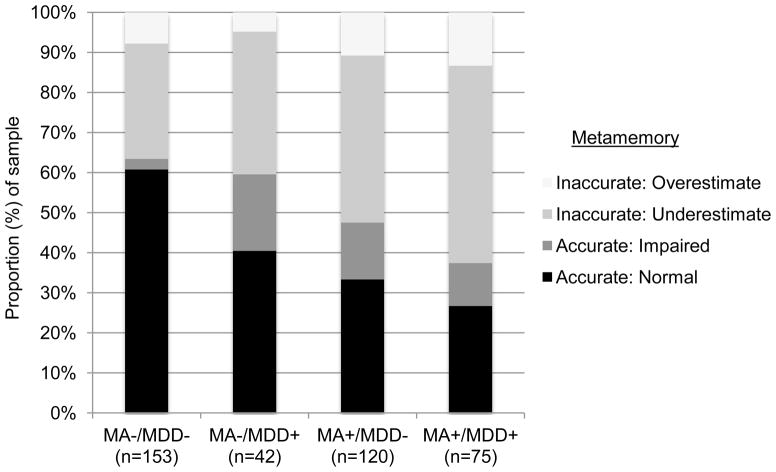
As detailed in the methods, metamemory was operationalized as a four-level variable across all study subjects: under-estimate memory abilities, over-estimate memory abilities, accurately estimate impaired memory, or accurately estimate normal memory. In a multivariable logistic regression model similarly controlling for demographics and those variables that differed between the MA groups, we found independent main effects for MA status (χ2=10.0, p=0.02) and lifetime MDD (χ2=8.3, p=0.04), as well as an interaction between these two variables (MA x lifetime MDD: χ2=11.7, p=0.008; Overall model: χ2=107.8, p<0.001). At the univariate level, the MA x lifetime MDD interaction demonstrated a stair step effect such that each risk factor contributed to increasingly greater proportions of inaccurate metamemory (i.e., increased overestimators and under-estimators), with the highest prevalence of both inaccurate under- and over-estimators observed in MA+ participants with a history of MDD (χ2=42.2, p< 0.001). These data are displayed in Figure 1. Examining the main effect of MA use on metamemory abilities demonstrated that up to 56% of MA+ users exhibited some sort of metamemory inaccuracy (44% under- and 12% over-estimating), compared to only 37% inaccuracy rate among the MA− comparison participants (30% under- and 7% over-estimating).
Figure 1.
Stacked bar chart illustrating that methamphetamine dependence (MA+) and lifetime major depressive disorder (MDD+) demonstrate an additive, adverse impact on metamemory accuracy.
Next, we examined clinical factors that differentiated the four metamemory group classifications within the MA+ cohort. Using multivariable logistic regression models controlling for demographics (i.e., age, education, gender, ethnicity) and lifetime MDD, we found that WRAT-3 (over-estimators: b=−8.2, p<0.001), executive functions (over-estimators: b=−1.5, p=0.005), and working memory (over-estimators: b=−2.9, p<0.001) were each independently associated with metamemory group membership. Specifically, MA+ individuals who overestimated their memory abilities performed the poorest across these measures, as compared to those who accurately appraised their memory to be within normal limits or under-estimated their memory abilities (see Figure 2). On the other hand, metamemory group differences in verbal fluency (over-estimators: b=−1.8, p<0.001; accurately impaired: b=−2.04, p<0.001), speed of information processing (over-estimators: b=−1.7, p<0.001; accurately impaired: b =−1.3, p=0.003) and fine motor skills (accurately impaired: b =−1.4, p=0.009) were driven by a memory impairment effect, such that only those MA+ individuals who accurately reported their memory as impaired and those who over-estimated their memory abilities (i.e., were objectively memory impaired but not reporting it) performed the poorest on these measures. No MA use parameters (see Table 1), or other substance use disorders were associated with metamemory group membership among the MA+ users (ps>0.05).
Discussion
Deficits in episodic memory are prevalent and impactful in chronic MA users. This study examined the degree, nature, and correlates of metamemory (i.e., awareness of memory deficits) in a large cohort of persons with histories of MA use disorders. Consistent with prior studies (Scott et al., 2007), we found that MA+ participants demonstrated mild-to-moderate deficits on objective tests of learning and recall and greater reported symptoms of memory problems in daily life versus a MA− comparison group. However, a disconnect existed between the objective and self-report indicators of memory functioning, with less than half of the MA+ individuals accurately assessing their memory abilities (44% under-estimating and 12% overestimating) compared to about two-thirds in the MA− group (30% under-estimating and 7% overestimating).
Among MA+ users, risk for under-estimating memory ability appeared to be exacerbated in those with histories of MDD. Specifically, we found an additive, stair-step effect of MA use and MDD on metamemory accuracy, such that individuals with no risk factors evidenced the lowest prevalence of metamemory inaccuracy (MA−/MDD−: 37%), those with one risk factor showed intermediate levels of inaccuracy (MA−/MDD+: 41%; MA+/MDD−: 53%), and those with both risk factors evidenced the highest prevalence of metamemory inaccuracy (MA+/MDD+: 63%). Consistent with prior studies of metacognition in the context of mood disorders (Kalska et al., 1999), MA+/MDD+ individuals showed the greatest proportion of underestimation (i.e., inaccurately reported memory symptoms), as well as the lowest proportion of individuals accurately assessing their memory to be impaired (i.e., accurate memory symptoms); in other words, clinically, memory symptoms do not appear to show good specificity as an indicator of actual memory abilities among MA users with co-occurring mood dysregulation. Given the penchant for frontostriatal disruption in both MA use and MDD (Earnst et al., 2000), and the importance of such prefrontal systems for metamemory processes (Pannu & Kaszniak, 2005), these findings may demonstrate behavioral evidence of increased frontal system burden with each condition. On the other hand, MA+/MDD+ individuals also evidenced the largest proportion of over-estimators, indicating that not all individuals with mood dysregulation will complain of such neurocognitive problems. Therefore, careful clinical interviewing and referral for objective neurocognitive testing are especially warranted in order to obtain an accurate picture of memory capacity in this substance abusing population.
The metamemory deficits observed among individuals with MA use disorders may have important real life implications for these individuals. For instance, the nearly doubled prevalence of MA+ participants over-estimating their memory abilities (as compared to MA− participants) may indicate a significant subset of individuals who are especially likely to engage in tasks that they do not have the capacity to successfully complete without appropriate compensatory support, thereby increasing the incidence of daily functioning errors. On the other hand, the large proportion of MA+ participants under-estimating their memory abilities is problematic in that this group of individuals may not be living up to their “true” capacity, and therefore, perhaps unnecessarily limiting themselves from engaging in the full range of daily tasks that they are capable of successfully completing. This may, in turn, lead to a greater burden on caregivers or healthcare systems for management of daily activities that may otherwise be accomplished by the patient. Although our study begins to delineate the nature of how metamemory deficits manifest among MA+ users, future studies are warranted to extend these findings to real world functioning; that is, determine how such MA-related metamemory impairments truly impact the daily lives of these individuals (e.g., vocational functioning, medication adherence).
Further supporting possible implications for daily functioning, within the MA+ cohort, individuals who over-estimated their memory abilities evidenced a unique pattern of greater executive dysfunction (i.e., working memory, cognitive flexibility, and novel problem solving) and lower reading scores compared to those MA+ participants who accurately appraised their memory abilities. The sensitivity of greater executive dysfunction, including working memory, in the over-estimator group is consistent with previous literature demonstrating that frontal systems disruption is strongly linked to changes in metacognitive accuracy (Stuss, 2011). That is, frontally-mediated executive dysfunction interferes with accurate appraisal of one’s cognitive abilities, possibly due to the fact that appreciating one’s true memory capacity requires integration of subjective experiences with objective evidence of problems and failures. These processes likely rely upon deliberative and strategic abilities, which are supported by executive functions.
Additionally, lower reading performance among MA+ individuals who over-estimated their memory abilities may be indicative of lower “cognitive reserve,” or reduced capacity to counteract the effects of brain injury through enhanced cognitive networks (Stern, 2002). On one hand, the pre-existing lower levels of cognitive reserve may simultaneously increase risk for MA-associated cognitive effects while also reducing the ability to detect the presence of such deficits (e.g., due to the reliance of metacognitive accuracy on executive functions). An alternate, though not necessarily mutually exclusive, explanation is that the dearth of compensatory cognitive networks is itself the very reason that the cognitive problems are not detected. That is, not having the ability to engage cognitive networks to compensate for memory problems means that those difficulties are not detected. This is consistent with a recent study demonstrating a relationship between higher education levels (another proxy for cognitive reserve) and greater metamemory accuracy in Alzheimer’s Disease (Szajer & Murphy, 2013). Additionally of note, both cognitive reserve and metacognitive accuracy have been positively associated with external compensatory strategy use (Garrett, Grady, Hasher, 2010). Therefore, there may be some interplay between cognitive reserve levels and metacognitive processes that leads to successful compensatory strategy application in real life. Future studies further dissecting these relationships would be critical to determine mechanisms by which compensatory strategy use occurs, and therefore, identifying potential points of intervention to improve compensation approaches.
There are also several limitations to the current study that should be considered when interpreting our data. First, our measure of metamemory examined memory symptoms in daily life as compared to performance on neuropsychological memory tests in the laboratory. Such laboratory-based memory tests, therefore, do not take into account the use of compensatory strategies in daily life (e.g., writing notes), which may impact the severity of reported memory symptoms (e.g., perhaps an individual uses memory-based compensatory strategies in daily life and therefore does not have symptoms, but does indeed show a memory deficit when these strategies are not employed). Future studies examining the impact of metamemory accuracy on compensatory strategy deployment, however, are needed in order to better delineate this effect. Additionally, our retrospective paradigm examined metamemory from a broad approach (i.e., global correspondence between everyday memory symptoms and memory test performance); other experimental metamemory models that include direct comparisons between predictions of memory abilities as applied to a specific test at hand (e.g., predict how many words you will remember on a list learning task) may be additionally informative to indicate the level at which memory perceptions become dissociated among MA+ users.
Our study represents the first evidence of a metamemory deficit among individuals with MA use disorders, and begins to elucidate the nuanced nature of that deficit. MA users who both under- and over-estimate their memory abilities (i.e., over half of the cohort) are of important public health concern given that such inaccurate metamemory processes may results in potential over-utilization of unnecessary services (e.g., caregiver burden) or risk of daily errors (e.g., medication nonadherence), respectively. The additive, adverse role of depression on awareness of memory processes is also a critical domain to consider when presented with a substance use client with memory symptoms. Future studies that target metamemory accuracy as a vehicle for neurorehabilitation, especially via successful deployment of compensatory strategies, are needed in the substance use literature.
Highlights.
We compared awareness of memory deficits (i.e., metamemory) in methamphetamine (MA) users vs. non-users (MA−)
MA users were more impaired, but less aware of their memory impairment than MA−
MA and depression interacted, such that MA users with depression had the most metamemory inaccuracies
Metamemory inaccuracy was associated with executive dysfunction and lower cognitive reserve in MA users
Acknowledgments
This research was supported by National Institutes of Health grants P01-DA12065, T32-DA31098, L30-DA032120, F31-DA035708, F31-DA034510, and P30-MH62512. This study was also supported (in part) by a Foundation for Rehabilitation Psychology Dissertation Award. The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50-DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D., Virawudh Soontornniyomkij, M.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Chief), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D., Jared Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
Footnotes
The authors report no conflicts of interest.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test–Revised. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test–retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory Complaints in Older Adults: Fact or Fiction? Arch Neurol. 1991;48(1):61–64. doi: 10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Iudicello JE, et al. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. J Neuropsychiatry Clin Neurosci. 2012;24(3):331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RA. Advances in Clinical Neuropsychology. Springer US; 1986. Neuropsychological and personality correlates of patients’ complaints of disability; pp. 95–126. [Google Scholar]
- Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006 doi: 10.4088/jcp.v67n0211. [DOI] [PubMed]
- Dellefield KS, McDougall GJ. Increasing metamemory in older adults. Nursing Research. 1996;45(5):284–290. doi: 10.1097/00006199-199609000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnst T, Chang L, Leonido-Yee M, et al. Evidence for long-term neurotoxicity associated with methamphetamine abuse. Neurology. 2000;54(4):1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, et al. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IVTR Axis I Disorders, Research Version, Patient Edition. New York, NY: Biometrics Research; 1991. [Google Scholar]
- Garrett DD, Grady CL, Hasher L. Everyday memory compensation: The impact of cognitive reserve, subjective memory, and stress. Psychol Aging. 2010;25(1):74–83. doi: 10.1037/a0017726. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, et al. Depression among methamphetamine users: association with outcomes from the Methamphetamine Treatment Project at 3-year follow-up. J Nerv Ment Dis. 2009;197(4):225. doi: 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalska H, Punamaki RL, Makinen-Pelli T, et al. Memory and metamemory functioning among depressed patients. Applied Neuropsychology. 1999;6(2):96–107. doi: 10.1207/s15324826an0602_5. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Metcalfe J, Greene MJ, et al. Effects of intranasal methamphetamine on metacognition of agency. Psychopharmacology. 2008;197(1):137–144. doi: 10.1007/s00213-007-1018-2. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. The Psychology of Learning and Motivation. 1990;26:125–141. [Google Scholar]
- Niederehe G, Yoder C. Metamemory perceptions in depressions of young and older adults. The Journal of nervous and mental disease. 1989;177(1):4–14. doi: 10.1097/00005053-198901000-00002. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: A review. Neuropsychol Rev. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Ponds RW, Jolles J. Memory complaints in elderly people: The role of memory abilities, metamemory, depression, and personality. Educational Gerontology: An International Quarterly. 1996;22(4):341–357. [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: Relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Szajer J, Murphy C. Education level predicts retrospective metamemory accuracy in healthy aging and Alzheimer’s disease. J Clin Exp Neuropsychol. 2013;35(9):971–982. doi: 10.1080/13803395.2013.844771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Iudicello JE, et al. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1):146–153. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test (WRAT-3): Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- Woods SP, Rippeth JD, Conover E, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19(1):35. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview, version 2.1. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]