Abstract
Nur77 is an orphan nuclear receptor that belongs to the nuclear receptor 4A (NR4A) subfamily, which has been implicated in a variety of biological events, such as cell apoptosis, proliferation, inflammation, and metabolism. Activation of Nur77 has recently been shown to be beneficial for the treatment of cardiovascular and metabolic diseases. The purpose of this study is to identify novel natural Nur77 activators and investigate their roles in preventing vascular diseases. By measuring Nur77 expression using quantitative RT-PCR, we screened active ingredients extracted from Chinese herb medicines with beneficial cardiovascular effects. Hyperoside (quercetin 3-D-galactoside) was identified as one of the potent activators for inducing Nur77 expression and activating its transcriptional activity in vascular smooth muscle cells (VSMCs). We demonstrated that hyperoside, in a time and dose dependent manner, markedly increased the expression of Nur77 in rat VSMCs, with an EC50 of ∼0.83μM. Mechanistically, we found that hyperoside significantly increased the phosphorylation of ERK1/2 MAP kinase and its downstream target cAMP response element-binding protein (CREB), both of which contributed to the hyperoside-induced Nur77 expression in rat VSMCs. Moreover, through activation of Nur77 receptor, hyperoside markedly inhibited both vascular smooth muscle cell proliferation in vitro and the carotid artery ligation–induced neointimal formation in vivo. These findings demonstrate that hyperoside is a potent natural activator of Nur77 receptor, which can be potentially used for prevention and treatment of occlusive vascular diseases.
Keywords: Hyperoside, Nur77, vascular smooth muscle cells, proliferation, neointimal formation
1. Introduction
NR4A receptors are immediate-early genes that are regulated by many physiological stimuli including growth factors, hormones, and inflammatory signals and are involved in a wide array of important biological processes, including cell apoptosis, brain development, glucose metabolism, and vascular remodeling[1, 2]. The NR4A subfamily consists of 3 well-conserved members, Nur77 (NR4A1), Nurr1 (NR4A2), and NOR-1 (NR4A3), respectively[1]. So far, no ligands have been identified for these receptors and therefore they are classified as orphan receptors. Like other nuclear receptors, NR4A nuclear receptors consist of an N-terminal transcriptional activation function-1 (AF-1), a central 2-zinc-finger DNA-binding domain (DBD), and a C-terminal ligand-binding domain (LBD) and ligand-dependent transcriptional activation function 2 or AF-2[3]. Accumulating evidence suggests that the NR4A receptors are constitutive active and their expression levels and post-translational modifications, such as phosphorylation and sumoylation, primarily regulate their transcriptional activities[4-7]. In the nucleus, all three NR4A receptors bind to the response elements of the target genes either as monomers or homodimers. In contrast to NOR-1, Nur77 and Nurr1 can heterodimerize with retinoid X receptor[8-10]. In addition, NR4A receptors influence gene transcription also through recruiting corepressor complexes[11]. For example, Nur77 exhibits a direct, inhibitory interaction with the p65 subunit of NF-kB[12].
Recently, there has been much attention paid to the function of these receptors in cardiovascular system[13]. For example, in vascular endothelial cells, NR4A nuclear receptors are induced by several stimuli, such as hypoxia and vascular endothelial growth factor, and modulate EC growth, survival, and angiogenesis[14-16]. Recently, our study showed that Nur77 expression is induced by inflammatory cytokines TNF-α and IL-1β and that overexpression of Nur77 suppresses cytokine-induced expression of VCAM-1 and ICAM-1, as well as monocyte adhesion via induction of IκBα expression in human ECs[17]. Accordingly, Nur77 deficient mice exhibit an increased formation of atherosclerosis[13, 18]. Indeed, in vascular smooth muscle cells, the expression of Nur77 and NOR-1 was significantly induced by atherogenic stimuli, such as platelet-derived growth factor-BB, epidermal growth factor, and α-thrombin[19, 20]. Overexpression of Nur77 has been shown to inhibit cell proliferation and attenuate vascular injury-induced neointimal formation and atherogenesis in vivo[21, 22]. Together, these studies further highlight the importance of NR4A receptors in regulating vascular function and bring up the possibility that modulating the expression and/or transcriptional activity of Nur77 may provide pharmacological applications for the treatment of certain cardiovascular diseases, such as atherosclerosis and restenosis.
Thus far, the most extensively investigated small molecular agonist of Nur77 is 6-mercaptopurine (6-MP)[15, 21, 23], which is an active metabolite of immunosuppressive prodrug azathioprine. Indeed, activation of Nur77 by 6-MP has been shown to induce angiogenesis[21], attenuate atherosclerosis and neointimal formation[21, 24]. However, the immunosuppressive effects of 6-MP hinder its potential use for the treatment of cardiovascular diseases. Therefore, further identification of potent and selective Nur77 agonists with low toxicity will not only provide tool compounds for further understanding the biological functions of Nur77, but also help lay the foundation for the discovery of therapeutic agents for preventing and treating cardiovascular and metabolic disorders. In the present study, we sought to identify Nur77 activators by screening active ingredients extracted from Chinese herb medicines with cardiovascular beneficial effects. Consequently, hyperoside has been identified as a potent natural agonist for inducing Nur77 expression in vascular smooth muscle cells (VSMCs).
2. Materials and methods
2.1 Reagents
Hyperoside (quercetin 3-D-galactoside), 6-Mercaptopurine monohydrate, and Cytosporone-B were purchased from Sigma-Aldrich (St. Louis, MO, USA). U0126, SB203580 and SP600125 were purchased from Cell Signaling Technology (Cambridge, MA, USA). Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin solution, and 0.25% trypsin–EDTA were obtained from GIBCO (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT, USA). All other chemical, unless otherwise specified, were from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Cell Culture
Rat aortic vascular smooth cells (VSMCs) were isolated and cultured as described previously [25]. Briefly, adult Sprague–Dawley rats (12 to 14 weeks) were killed instantly, and their thoracic aortas were excised. After adherent fat and connective tissue were removed and washed by PBS, the aortas were cut longitudinally, and the endothelial cells were removed by gentle scraping with fine forceps. The aortas were then minced into small pieces and attached to the culture disk bottom. Culture dishes were turned the bottom upside and then incubate at 37°C for 4 hr without medium. The culture disk was turned over and Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal calf serum (FBS) was added carefully and incubated for 72 hr and then change medium every 48 hr. The harvested cells were then seeded at a density of 3 to 5×105/cm2 and cultured at 37°C in 95% air /5% CO2. Cells from passages 5 to 8 were used in all experiments. The phenotype of the cultured VSMCs was determined by staining the cells for α-SM actin (Santa Cruz, CA, USA).
2.3 Determination of mRNA Expression Levels
Total RNA was extracted using the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. 1μg total RNA was used to perform the reverse transcription with High Capacity cDNA Archive Kit (Applied Biosystem, Foster City, CA, USA). Real-time quantitative polymerase chain reaction (qPCR) analysis for Nur77, Nor1, and Nurr1 was performed using TaqMan gene expression assays and the ΔΔCt method with housekeeping gene 18S as the endogenous control[26].
2.4 Western Blot
Total proteins were extracted from RVSMCs and the protein concentration was measured by BCA kit (Hyclone, Logan, USA). Equal amounts of protein lysates were separated by 10% SDS-PAGE, transferred to Nitrocellulose membrane (Bio-Rad) and then immunoblotted with antibodies against ERK1/2 (1:1000 dilution; Cell Signaling Technology, Cambridge, MA, USA), phospho-ERK1/2 (1:1000 dilution; Cell Signaling Technology, Cambridge, MA, USA), CREB (1:1000 dilution; Cell Signaling Technology, Cambridge, MA, USA), phospho-CREB (1:500 dilution; Cell Signaling Technology, Cambridge, MA, USA), Nur77 (1:500 dilution, Santa Cruz, CA, USA), and GAPDH (1:1000 dilution; Santa Cruz, CA, USA), followed by incubating with either IRDye700 or 800 secondary antibodies and visualized using Odyssey Infrared Imaging System software (Li-Cor, Nebraska, USA) as described previously [27]. Quantitative analysis of the band intensity was carried out by image J software.
2.5 Immunofluorescent Staining
Starved rat VSMCs were treatment with 5 μmol/L hyperoside for 12 hr and fixed in 4% formaldehyde in PBS. Fixed VMSCs were incubated with anti-Nur77 antibody (1:100 dilution; Santa Cruz, CA, USA), followed by fluorescein-5-isothiocyanate (FITC)-conjugated secondary antibodies (1:250 dilution, Invitrogen, Carlsbad, CA, USA). Cell nuclei were stained with DRAQ5. Images were visualized using an Olympus confocal microscope (Olympus, Tokyo Japan).
2.6 VSMC Proliferation Assay in Vitro
VSMC proliferation in vitro was determined by cell number counting and MTT assays, as we previously described[27]. Briefly, VSMCs were plated at a density of 5 × 104 cells/ml in 96-well plates in a 37 °C, 5% CO2 When grown to 70–80% confluence, cells were synchronized in DMEM containing 0.1% FBS for 24 h and then pretreated with hyperoside for 1 h, followed by stimulation with 10% FBS. After 24 h of FBS stimulation, 20 μl MTT (5 mg/ml) was added to each well, and the cells were further incubated for an additional 4 h. The supernatant was removed and the formation of formazan was resolved with 150 μl/well of DMSO. The optical density was measured at 570 nm on a microplate reader.
2.7 Determination of Cell Viability
To exclude the possibility that the experimental agents at maximal concentration used in the study may exert cytotoxic effects, cell viability was determined by trypan blue exclusion.
2.8 Chromatin Immunoprecipitation (CHIP) Assay
CHIP assays were performed with a CHIP kit according to the manufacturer's instructions (Millipore, Billerica, USA). Briefly, cells were formaldehyde cross-linked. Crosslinking reaction was stopped by adding glycine solution. Cross-linked chromatin was sheared by enzymatic digestion for 15 min at 37 °C. Debris was removed by centrifugation and supernatants were collected. The purified chromatin was immunoprecipitated with antibodies against CREB (Santa Cruz Biotechnology) and normal rabbit IgG (Active Motif). The DNA/Protein complexes were then collected by using Protein G-argrose beads. Beads were then washed and bound DNA was eluted. After reverse cross-linking reaction and proteinase K digestion, the eluted DNA was used in 35 cycles of PCR amplification with the CREB binding site-specific primers (forward: GGGTCTGGAAGCTGCTATATTT; Reverse: AGCTTTGGCCATACAAGGG).
2.9 Luciferase Reporter Assay
Rat VSMCs were transfected with 500 ng pNBRE-Luc and 50 ng of Renilla luciferase reporter plasmid pRL-RSV40 (Promega, Madison, WI, USA) using FuGene 6 transfection reagent (Roche, Indianapolis, USA). 36 hours after transfection, cell lysates (20 μL) were assayed for luciferase activity with a dual-luciferase reporter assay system (Promega, Madison, WI, USA).
2.10 Knockdown of Nur77 Expression
Knockdown of Nur77 expression was performed by using Nur77 siRNAs, with AllStars negative siRNAs (QIAGEN, Valencia, CA) as a control, as described previously [28]. 2 hrs after seeded into the 6 well plates, cells were transfected using HiPerFect Transfection Reagent (QIAGEN, Valencia, CA) according to the manufacture's protocol. Transfection medium was replaced by regular cell culture medium after 24 hrs of transfection.
2.11 Carotid Artery Ligation Injury
Carotid artery ligation in mice was performed as we described previously[27]. Briefly, male C57BL/6N mice (2 months old, 20 to 28 g, Jackson Laboratory, Bar Harbor, ME, USA) were anesthetized with an intraperitoneal injection of avertin (400 mg/kg). The left common carotid artery was ligated with a 6-0 silk suture so that the common carotid artery blood flow was completely disrupted. The animals were intraperitoneal injection of Hyperoside (50 mg/kg) or vehicle one day before surgery and every two days after surgery. Carotid arteries were harvested 2 weeks after ligation. Animals were anesthetized and perfused with 0.9% NaCl, fixed with 4% paraformaldehyde, and embedded in paraffinum. Tissue was sectioned at 6 μm and stained with hematoxylin and eosin and was examined by a light microscope (Nikon); the neointimal area was measured by the computer program ImageJ2x. This study was reviewed and approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
2.12 Statistical Analysis
Data were expressed as mean ± SD and analyzed for statistical significance by the unpaired Student t test or ANOVA using SPSS software (version 18.0) (SPSS Inc., USA). P<0.05 was considered statistically significant in all experiments.
3. Results
3.1 Hyperoside Induces Expression of NR4A Receptors in RVSMCs
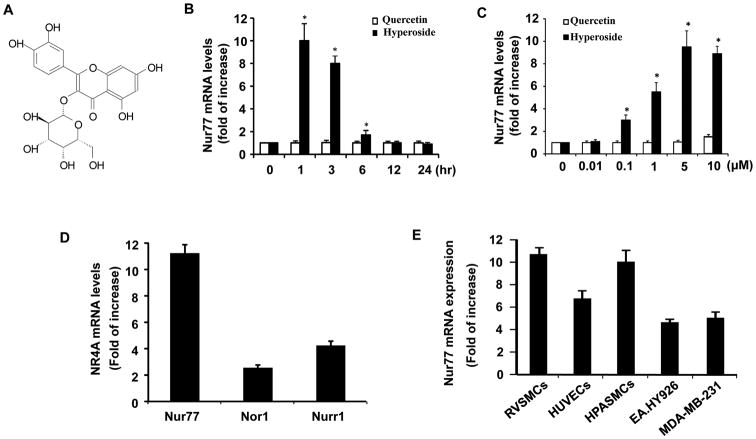
Activation of Nur77 exhibits beneficial effects in cardiovascular system through inhibiting vascular SMC proliferation[29]. Therefore, to identify novel naturally occurring agonists of Nur77, we screened the natural compounds extracted from Chinese herb medicines with beneficial cardiovascular effects by using qRT-PCR. We found that hyperoside (quercetin 3-D-galactoside) (Figure 1A), a major constituent of Chinese herbal medicine Prunella vulgaris L, is one of the most potent agonist for inducing Nur77 expression in RVSMCs. Hyperoside increases Nur77 expression in a time and dose dependent manner (Figure 1B and 1C). In contrast, quercetin, an analog of hyperoside, had no effect on Nur77 expression in rat VSMCs (Figure 1B and 1C). Treatment of RVSMCs with 5 μmol/L hyperoside increased Nur77 expression by approximately 10-fold, while the expression of NOR-1 and Nurr1 increased by approximately 3-fold and 4-fold, respectively (Figure 1D). In addition, hyperoside significantly increased Nur77 expression in a range of 4 to 10-fold in human umbilical vein endothelial cells (HUVECs), human endothelial cell line (EA.Hy926), human pulmonary artery smooth muscle cells (HPASMCs), and MDA-MB-231 breast cancer cells.
Figure 1.
Expression of NR4A Family members in RVSMCs. (A) Structure of Hyperoside. (B) Time-dependent effect of either hyperoside (5 μmol/L) or quercetin (5 μmol/L) on Nur77 expression in RVSMCs as determined by qRT-PCR (n=5, *P<0.05 vs time at time 0). (C) Dose-dependent effects of hyperoside or quercetin on Nur77 expression in RVSMCs, as determined by qRT-PCR (n=5, *P<0.05 vs vehicle). (D) RVSMCs were treated with hyperoside (5 μmol/L) for 1 hr. The expression of NR4A receptors was determined by qRT-PCR (n=5). E, Different types of cells were treated with hyperoside (5 μmol/L) for 1 hr. The expression of Nur77 was determined by qRT-PCR (n=5).
To compare the potency of hyperoside with the commercially available Nur77 activators, such as 6-mercaptopurine (6-MP) and Cytosporone-B (CSN-B)[30, 31], we treated rat VSMCs for 1 hr with different concentrations of hyperoside, 6-MP, and CSN-B. As shown in Table I, the maximal induction of Nur77 expression by hyperoside, 6-MP, and CSN-B after 1 hr treatment is about 10, 4, and 12-fold, respectively. The EC50 value of hyperoside is 0.81 μmol/L, which is much lower compared with the EC50 values of 6-MP and CSN-B, suggesting that hyperoside is a potent natural compound for the induction of Nur77 expression in VSMCs. Furthermore, treatment of rat VSMCs with either hyperoside, 6-MP or CSN-B for 1hr had no effect on cell viability at all tested concentrations, as determined by trypan blue exclusion (data not shown).
Table 1. EC50 values of different Nur77 activators in RASMCs (n=5).
| Name | Maximal Induction (fold increase vs DMSO) | EC50 value (μM) |
|---|---|---|
| Hyperoside | 10.23±0.86 | 0.83±0.06 |
| 6-Mercaptopurine (6-MP) | 4.17±0.41 | 15.14±1.22 |
| Cytosporone B (CSN-B) | 12.02±0.98 | 16.18±1.15 |
3.2 Hyperoside Increases Transcriptional Activity of Nur77 in RVSMCs
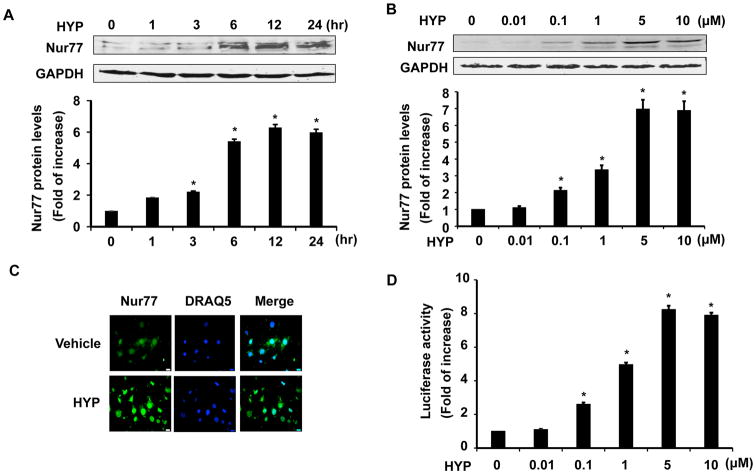
To corroborate hyperoside-induced Nur77 mRNA expression, we performed western blot to determine the protein levels of Nur77 in response to hyperoside treatment. As shown in Figure 2A and 2B, Hyperoside increased Nur77 protein expression in a time and dose dependent manner. After 6 hr treatment with 5 μmol/L hyperoside, the Nur77 expression was maximally induced by approximately 6-fold, and the increased expression of Nur77 mainly occurred in the nucleus of RVSMCs, as determined by immunofluorescent staining (Figure 2C). Furthermore, treatment of RVSMCs with 5 μmol/L hyperoside for 24 hr markedly increased Nur77 transcriptional activity, as determined by measuring the NGFI-B response element (NBRE)-dependent luciferase activity (Figure 2D). Together, these results suggest that hyperoside potently augments the transcriptional activity of Nur77 through increasing its expression in the nucleus of RVSMCs.
Figure 2.
Hyperoside Induces Nur77 dependent transcriptional activation in RVSMCs. (A) RVSMCs were treated with hyperoside (HYP) (5 μmol/L) for indicated time points. The expression of Nur77 was then determined by Western blot analysis (n=4, *P<0.05 vs time at 0). (B) RVSMCs were treated with increasing concentrations of hyperoside (HYP) for 12 hrs and the expression of Nur77 was determined by Western blot analysis (n=4. *P<0.05 vs treatment with vehicle). (C) RVSMCs were treated with either vehicle or hyperoside (HYP) (5μmol/L) for 12 hrs. The localization of Nur77 was determined by immunofluorescent staining. (D) RVSMCs were transfected with NBRE-Luc reporter plasmid. 48 hrs after transfection, RVSMCs were stimulated with different doses of hyperoside (HYP) for 12 hr and the luciferase activity was then determined (n=5, *P<0.05 vs treatment with vehicle).
3.3 Hyperoside Induces Nur77 Expression through the MEK1/2/CREB Pathway
To investigate which the molecular signaling pathway responsible for the Hyperoside-induced Nur77 expression, RVSMCs were pretreated with various MAP Kinase inhibitors for 1 hr before hyperoside stimulation. As shown in Figure 3A, the expression of Nur77 induced by hyperoside was significantly inhibited by MEK1/2 inhibitor U0126, but not by p38 inhibitor SB203580 and JNK inhibitor SP600125. Accordingly, hyperoside markedly induced the phosphorylation of MAPK ERK1/2 (Fig. 3B), which was inhibited by the MEK1/2 inhibitor U0126 (Fig. 3B and 3C) in RVSMCs, while hyperoside treatment barely affected the phosphorylation of JNK and p38 (data not shown).
Figure 3.
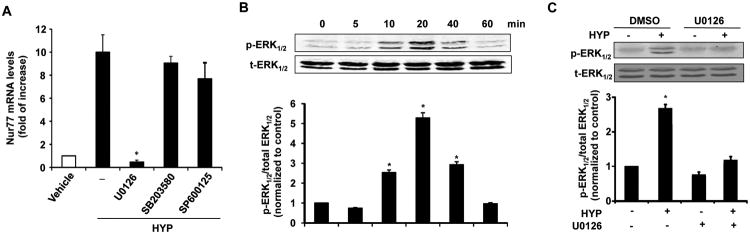
Hyperoside induces Nur77 expression through MEK pathway in RVSMCs. (A) RVSMCs were pretreated with vehicle, MEK1/2 inhibitor U0126 (20 μmol/L), the p38 MAP kinase inhibitor SB203580 (10 μmol/L), and c-Jun N-terminal kinase inhibitor SP600125 (50 μmol/L) for 1 hr, and then stimulated with hyperoside (HYP) (5 μmol/L) for 1 hr. The expression of Nur77 was then measured by qRT-PCR (n=5, *P<0.05 vs DMSO plus hyperoside (HYP) treatment). (B) RVSMCs were stimulated with hyperoside (HYP) (5 μmol/L) at different time points and the levels of phosphorylated ERK1/2 and total ERK1/2 were determined by Western blot analysis (n=4, *P<0.05 vs HYP at 0 min). (C) RVSMCs were pretreated with either vehicle or the MEK1/2 inhibitor U0126 for 1 hr, and then stimulated with hyperoside (HYP) (5 μmol/L) for 20 min, the levels of phosphorylated ERK1/2 and total ERK1/2 were then determined by Western blot analysis (n=4, *P<0.05 vs either DMSO or HYP plus U0126 treatment).
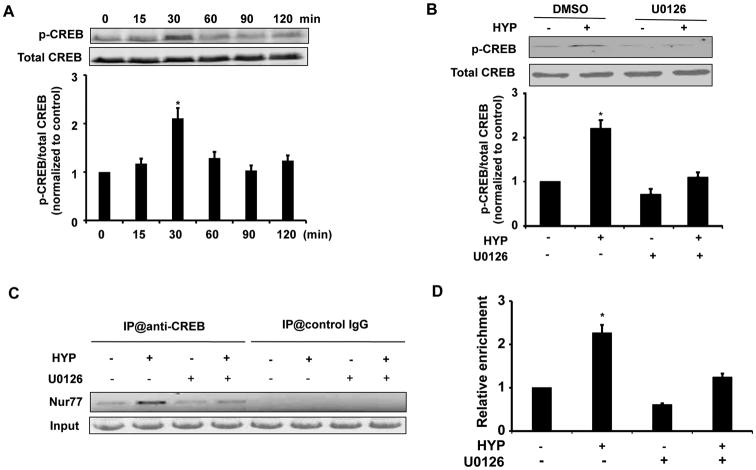
The transcription factor CREB (cAMP Responsive Element Binding Protein) has been reported to regulate Nur77 expression in various cell types[32-34]. To determine the role of CREB in hyperoside-induced Nur77 expression in rat VSMCs, we first examined CREB phosphorylation in response to hyperoside stimulation. As shown in Figure 4A, hyperoside-indeed increased Ser-133 phosphorylation of CREB by approximately 2.5-fold 30 min after hyperoside stimulation. Inhibition of MEK1/2 by a specific inhibitor U0126 markedly attenuated the hyperoside-induced phosphorylation of CREB (Figure 4B), indicating that CREB is a downstream target of the MEK1/2 pathway.
Figure 4.
CREB is involved in hyperoside-induced Nur77 expression in RVSMCs. (A) RVSMCs were stimulated with hyperoside (5 μmol/L) at different time points and the levels of phosphorylated CREB and total CREB were determined by Western blot analysis. (n=4, *P<0.05 vs hyperoside (HYP) at 0 min). (B) RVSMCs were pretreated with MEK1/2 inhibitor U0126 (20 μmol/L) for 1 hr and then stimulated with hyperoside (HYP) (5 μmol/L) for 30 min and the levels of phosphorylated CREB and total CREB were determined by Western blot analysis (n=4, *P<0.05 vs either DMSO or HYP plus U0126 treatment). (C) RVSMCs were treated or untreated with hyperoside (HYP) (5μmol/L) in the absence or presence of MEK1/2 inhibitor U0126 (20 μmol/L). PCR analysis of sheared DNA from control and hyperoside treated cells before immunoprecipitation (input) and after Chromatin immunoprecipitation (ChIP) with antibody directed against CREB or control IgG. (D) Quantitative analysis of ChiP results from three independent experiments as shown in panel C (n=4, *P<0.05 vs either DMSO or HYP plus U0126 treatment).
To further determine the binding of CREB to the promoter region of Nur77, we performed chromatin immunoprecipitation (ChIP) assays. As shown in Figure 4C, hyperoside stimulation markedly increased the binding of CREB to the Nur77 promoter, which was attenuated by MEK1/2 inhibitor U0126 (Figure 4C). Together, these results suggested that the MEK1/2/CREB pathway is mainly responsible for the hyperoside-induced Nur77 expression in RVSMCs.
3.4 Hyperoside Attenuates VSMC Proliferation through Induction of Nur77
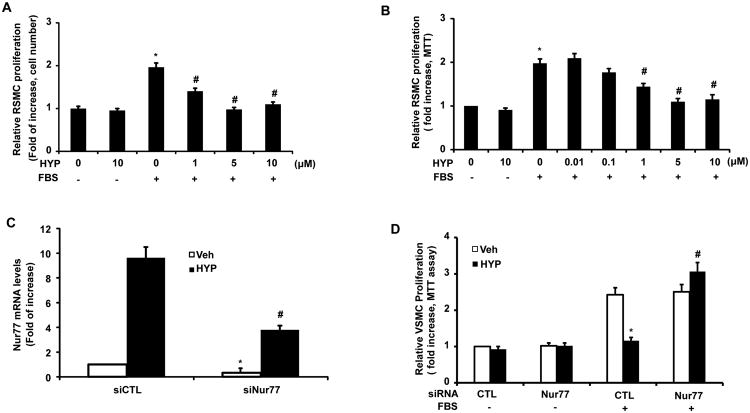
Accumulating evidence suggests that Nur77 is critically involved in VSMC proliferation[21, 33, 35]. To evaluate the functional significance of hyperoside-induced Nur77 in VSMCs, we investigated whether hyperoside could affect VSMC proliferation. As shown in Figure 5, hyperoside dose-dependently inhibited FBS-induced rat VSMC proliferation with an IC50 approximately 1 μM, as determined by both cell counting (Figure 5A) and MTT assays (Figure 5B). The maximal inhibition of hyperoside on FBS-induced rat VSMC proliferation is about 90% in both assays. To further substantiate the role of Nur77 in hyperoside-mediated inhibition of VSMC proliferation, we performed a loss-of-function study by using Nur77 siRNA. As shown in Figure 5C, in Nur77 siRNA transfected cells, both basal and hyperoside-induced Nur77 expression were substantially reduced by approximately 70%. Accordingly, the inhibitory effect of hyperoside on FBS-induced RVSMC proliferation, as determined by MTT assays, was significantly attenuated (Figure 5D). Taken together, these results suggest that hyperoside suppresses RVSMC proliferation, at least in part, through inducing Nur77 expression.
Figure 5.
Inhibition of VSMC proliferation by hyperoside is Nur77 dependent. RVSMCs were treated with different doses of hyperoside (HYP) for 48 hours in the presence and absence of 10% FBS. Hyperoside significantly inhibited FBS-induced RVSMCs proliferation, as determined by cells number counting (A) and MTT (B) (n=5. *P<0.05 vs vehicle; #P<0.05 vs vehicle plus 10% FBS). (C) RVSMCs were transfected with either control siRNAs or Nur77 specific siRNAs. 72 hr after transfection, RVSMCs were stimulated with hyperoside (HYP) (5 μmol/L) for 1 hr, and the expression of Nur77 mRNA was measured by qRT-PCR (n=5, *P<0.05 vs control siRNA without HYP treatment; #P<0.05 vs conrol (CTL) siRNA with HYP treatment). (D) RVSMCs were transfected with either control siRNAs or Nur77 specific siRNAs. 48 hrs after transfection, RVSMCs were treated with or without hyperoside (HYP) (5 μmol/L) in the absence or presence of 10% FBS for 48 hr. RVSMC proliferation was then determined by MTT assays (n=5, *P<0.05 vs CTL siRNA plus vehicle; #P<0.05 vs CTL siRNA plus HYP treatment).
3.5 Hyperoside Inhibits Carotid Artery Ligation-Induced Neointimal Formation in Mice
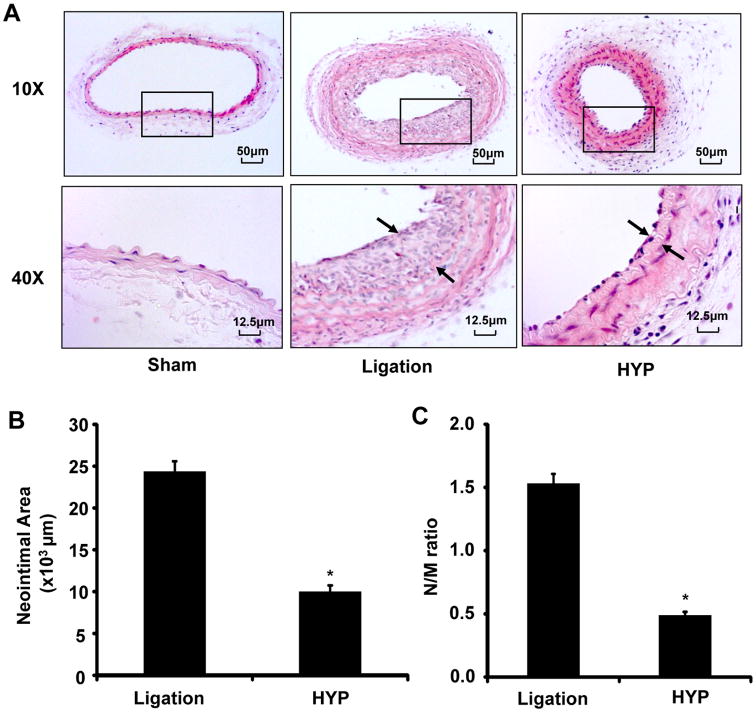
To determine whether hyperoside attenuates vascular lesion formation in vivo, a mouse carotid artery ligation model was employed and the neointimal formation was determined 14 days after vascular injury, as we described previously[27]. Hyperoside (40 mg/kg) or vehicle was given to mice every 2 days by intraperitoneal injections. The dose of 40 mg/kg hyperoside was chosen based on a previous study [36], and did not show any signs of toxicity or weight loss during the entire study. 14 days after surgery, no neointimal formation was observed in the unligated left common carotid arteries. In contrast, flow cessation led to a substantial increase in neointimal formation in carotid arteries of mice injected with vehicle (Figure 6A). Treatment of the mice with hyperoside markedly inhibited the carotid artery ligation–induced neointimal formation by approximately 50%, which is associated with decreases in both neointimal area (Figure 6B) and the intima-to-media ratio (Figure 6C) in hyperoside–treated arteries.
Figure 6.
Hyperoside attenuates neointimal formation in a mouse model of carotid artery ligation. (A) Hyperoside significantly reduced neointimal formation in vivo. Representative hematoxylin and eosin (H&E)-stained carotid artery slices from mouse treated with or without hyperoside (HYP) (40 mg/kg) at 14 days after carotid artery ligation. (B) and (C), The effect of hyperoside on vascular neointimal lesion formation in mouse carotid arteries at 14 days after ligation injury as quantitated by neointimal area and neointima/media (N/M) ratio. *P<0.05 vs ligation group.
4. Discussion
Hyperoside is a major pharmacologically active component from Prunella vulgaris L. [37] and Hypericum perforatum,[38] that exerts a wide variety of biological activities, including antioxidant[39], antihyperglycemic[40], anticancer[41], anti-inflammatory[42, 43], and cardiovascular protective effects[44, 45]. However, the underlying molecular mechanisms remain largely elusive. In the present study, we identified hyperoside as a potent natural agonist for activating Nur77 in vascular smooth cells. We demonstrated that hyperoside markedly increased Nur77 expression in vascular SMCs through the MEK1/2/CREB pathway, with an EC50 of 0.81 μmol/L, which is much more potent than commercially available Nur77 agonists, such as 6-MP and CSN-B. Furthermore, induction of Nur77 by hyperoside significantly inhibited FBS-induced VSMC proliferation and vascular injury-induced neointimal formation in vivo. In this regard, our results provide significant evidence indicating that hyperoside may elicit the cardiovascular protective effects at least in part through activating the orphan nuclear receptor Nur77 in cardiovascular cells.
Accumulating evidence indicates that NR4A receptors play critical roles in the development of cardiovascular diseases, such as atherosclerosis, restenosis and angiogenesis[46]. For instance, Nur77 is highly expressed in the neointimal and advanced atherosclerotic lesions[47]. In vascular SMCs, expression of Nur77 is significantly induced by pathological stimuli, such as PDGF-BB, FBS, ox-LDL, and inflammatory cytokines[47]. Moreover, ectopic expression or pharmacological activation of Nur77 has been shown to inhibit VSMC proliferation in vitro and neointimal formation in vivo[22, 48, 49], suggesting potential therapeutic values of Nur77 activators for the treatment of occlusive vascular diseases, such as atherosclerosis and restenosis [20, 28, 33]. Therefore, identification of potent Nur77 agonist with lower toxicity is of great scientific and therapeutic interests. In our current study, we found that hyperoside causes a rapid and remarkable induction of Nur77 in rat VSMCs, in a dose and time dependent manner. Induction of Nur77 by hyperoside mainly occurs at transcriptional levels, since hyperoside treatment induces a rapid phosphorylation of transcription factor CREB at serine 133, and subsequently increases its binding to Nur77 promoter and nur77 expression. Accordingly, the Nur77 transcriptional activity is significantly increased in response to hyperoside stimulation. In this regard, our study unveiled a ligand-independent mechanism underlying the hyperoside-induced activation of Nur77 in vascular SMCs. Indeed, the crystal structure of the LBD of Nurr1 and Nur77 shows that the LBD exists in a closed confirmation with the ligand-binding cleft inaccessible[50]. Therefore, in most circumstances, expression of Nur77 protein is thought to be the major mechanism regulating its transcriptional activity without the need for ligand stimulation[51]. However, this mechanism of action is recently challenged by Cytosporone B (Csn-B), which is the first identified naturally occurring agonist for Nur77. Indeed, Csn-B has been shown to specifically bind to the LBD of Nur77 and stimulate the Nur77-dependent transcriptional activity, thus constituting a positive feedback loop for inducing Nur77 expression[30]. At this point, whether hyperoside-mediated activation of Nur77 partially involves the direct binding to Nur77 is unknown, but certainly warrants further investigation.
Vascular inflammation plays important roles in the development of vascular diseases, such as atherosclerosis and diabetic vascular complications[17]. Recently, hyperoside has been shown to exert a potent anti-inflammatory effect[42, 43]. For instance, hyperoside has been to shown to inhibit high glucose-induced vascular permeability, monocyte adhesion, expression of cell adhesion molecules (CAMs), and activation of nuclear factor NF-κB in vascular endothelial cells[43]. In addition, hyperoside has been shown to attenuate production of tumor necrosis factor, interleukin-6, and nitric oxide in lipopolysaccharide-stimulated mouse peritoneal macrophages through inhibiting NF-κB activation[42], although the mechanism underlying inhibition of NF-κB activation by hyperoside remains elusive. Interestingly, in endothelial cells, our recent studies demonstrate that Nur77 transcriptionally up-regulates the expression of IκBα via directly binding to the promoter region of IκBα, which eventually leads to a suppression of the activation of the nuclear factor (NF)-κB pathway in ECs under inflammatory conditions[17]. Furthermore, overexpression of Nur77 has been shown to inhibit ox-LDL induced expression of TNF-alpha and MCP-1 in murine microphages[47, 52]. Thus, we attempt to speculate that induction of Nur77 by hyperoside might be responsible for the hyperoside-induced anti-inflammatory effects in both ECs and microphages. Ongoing studies are currently testing these hypotheses.
In summary, the data reported herein provide the evidence that hyperoside is a potent naturally existing agonist for Nur77, with a lower toxicity in vivo. Induction of Nur77 by hyperoside markedly inhibits VSMC proliferation in vitro and neointimal formation in vivo. Given the critical roles of Nur77 not only in cardiovascular disease, but also in metabolic disorders and cancer progression[53, 54], identification of hyperoside as a potent Nur77 agonist has important therapeutic implications for pharmacological treatment of these diseases. Further structure-activity relationship studies are required to develop more potent and selective Nur77 agonist to improve its therapeutic efficiency in vivo.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (HL103869) and grants from the Chinese Natural Science Foundation No. 381170114 and No. 81370418 to JS and Shanghai Committee of Science and Technology, China (Grant No. 14ZR1432200) to YH.
Abbreviations
- VSMCs
vascular smooth muscle cells
- NR4A
nuclear receptor 4A family
- ChiP
Chromatin immunoprecipitation
- CREB
cAMP response element-binding
- HUVECs
human umbilical vein endothelial cells
- MEK
MAP Kinase Kinase
- qRT-PCR
quantitative reverse transcriptase PCR
- ERK
extracellular-signal-regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- siRNA
small interfering RNA
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NBRE-Luc
Nur77-binding response element driven luciferase
Footnotes
Conflict of Interest: None of the authors has any real or potential conflicts of interest related to this research
Authors' Contributions: YH: Study conception and design, acquisition, analysis and interpretation of data. BY: Acquisition, analysis and interpretation of data. MC: Acquisition, analysis and interpretation of data. NW: Acquisition, analysis and interpretation of data. PC: Acquisition, analysis and interpretation of data. CG: Acquisition, analysis and interpretation of data. JS: Study design, analysis and interpretation of data; writing manuscript; final approval and overall responsibility for the published work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hsu HC, Zhou T, Mountz JD. Nur77 family of nuclear hormone receptors. Curr Drug Targets Inflamm Allergy. 2004;3:413–23. doi: 10.2174/1568010042634523. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/Nur77 in colon cancer cell apoptosis. Cancer research. 2003;63:5401–7. [PubMed] [Google Scholar]
- 3.Davis IJ, Hazel TG, Chen RH, Blenis J, Lau LF. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993;7:953–64. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 4.Wingate AD, Campbell DG, Peggie M, Arthur JS. Nur77 is phosphorylated in cells by RSK in response to mitogenic stimulation. Biochem J. 2006;393:715–24. doi: 10.1042/BJ20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson J, Burger ML, Whang H, Winoto A. Protein kinase C regulates mitochondrial targeting of Nur77 and its family member Nor-1 in thymocytes undergoing apoptosis. Eur J Immunol. 2010;40:2041–9. doi: 10.1002/eji.200940231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Nie Y, Li Y, Wan YJ. ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells. Biochem Pharmacol. 2011;81:910–6. doi: 10.1016/j.bcp.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredondo C, Orellana M, Vecchiola A, Pereira LA, Galdames L, Andres ME. PIASgamma enhanced SUMO-2 modification of Nurr1 activation-function-1 domain limits Nurr1 transcriptional synergy. PloS one. 2013;8:e55035. doi: 10.1371/journal.pone.0055035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang HJ, Song MR, Lee SK, Shin EC, Choi YH, Kim SJ, et al. Retinoic acid and its receptors repress the expression and transactivation functions of Nur77: a possible mechanism for the inhibition of apoptosis by retinoic acid. Experimental cell research. 2000;256:545–54. doi: 10.1006/excr.2000.4832. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Li Y, Liu R, Agadir A, Lee MO, Liu Y, et al. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J. 1997;16:1656–69. doi: 10.1093/emboj/16.7.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallen-Mackenzie A, Mata de Urquiza A, Petersson S, Rodriguez FJ, Friling S, Wagner J, et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes & development. 2003;17:3036–47. doi: 10.1101/gad.276003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Kim BY, Soh JW, Cho EJ, Liu JO, Youn HD. A novel function of Nur77: physical and functional association with protein kinase C. Biochem Biophys Res Commun. 2006;348:950–6. doi: 10.1016/j.bbrc.2006.07.167. [DOI] [PubMed] [Google Scholar]
- 12.Harant H, Lindley IJ. Negative cross-talk between the human orphan nuclear receptor Nur77/NAK-1/TR3 and nuclear factor-kappaB. Nucleic acids research. 2004;32:5280–90. doi: 10.1093/nar/gkh856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonta PI, Pols TW, de Vries CJ. NR4A nuclear receptors in atherosclerosis and vein-graft disease. Trends Cardiovasc Med. 2007;17:105–11. doi: 10.1016/j.tcm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Jia H, Holmes DI, Stannard A, Zachary I. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:2002–7. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 15.Yoo YG, Na TY, Yang WK, Kim HJ, Lee IK, Kong G, et al. 6-Mercaptopurine, an activator of Nur77, enhances transcriptional activity of HIF-1alpha resulting in new vessel formation. Oncogene. 2007;26:3823–34. doi: 10.1038/sj.onc.1210149. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Qin L, Bourbon PM, James L, Dvorak HF, Zeng H. Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12066–71. doi: 10.1073/pnas.1018438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circulation research. 2009;104:742–9. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 18.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circulation research. 2012;110:428–38. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–18. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Gong F, Dong X, Zhou W, Zeng Q. Regulation of vascular smooth muscle cell proliferation by nuclear orphan receptor Nur77. Molecular and cellular biochemistry. 2010;341:159–66. doi: 10.1007/s11010-010-0447-0. [DOI] [PubMed] [Google Scholar]
- 21.Pires NM, Pols TW, de Vries MR, van Tiel CM, Bonta PI, Vos M, et al. Activation of nuclear receptor Nur77 by 6-mercaptopurine protects against neointima formation. Circulation. 2007;115:493–500. doi: 10.1161/CIRCULATIONAHA.106.626838. [DOI] [PubMed] [Google Scholar]
- 22.Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, et al. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–5. doi: 10.1161/01.cir.0000028811.03056.bf. [DOI] [PubMed] [Google Scholar]
- 23.Wansa KD, Muscat GE. TRAP220 is modulated by the antineoplastic agent 6-Mercaptopurine, and mediates the activation of the NR4A subgroup of nuclear receptors. Journal of molecular endocrinology. 2005;34:835–48. doi: 10.1677/jme.1.01739. [DOI] [PubMed] [Google Scholar]
- 24.Hu YW, Zhang P, Yang JY, Huang JL, Ma X, Li SF, et al. Nur77 decreases atherosclerosis progression in apoE(-/-) mice fed a high-fat/high-cholesterol diet. PloS one. 2014;9:e87313. doi: 10.1371/journal.pone.0087313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiological reviews. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Liu Y, Yi B, Wang G, You X, Zhao X, et al. MicroRNA-638 Is Highly Expressed in Human Vascular Smooth Muscle Cells and Inhibits PDGF-BB Induced Cell Proliferation and Migration Through Targeting Orphan Nuclear Receptor NOR1. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Zhu N, Yi B, Wang N, Chen M, You X, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circulation research. 2013;113:1117–27. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Zhang J, Yi B, Chen M, Qi J, Yin Y, et al. Nur77 Suppresses Pulmonary Artery Smooth Muscle Cell Proliferation Through Inhibition of the STAT3/Pim-1/NFAT Pathway. American journal of respiratory cell and molecular biology. 2013 doi: 10.1165/rcmb.2013-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Tiel CM, de Vries CJ. NR4All in the vessel wall. J Steroid Biochem Mol Biol. 2012;130:186–93. doi: 10.1016/j.jsbmb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nature chemical biology. 2008;4:548–56. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 31.Liu JJ, Zeng HN, Zhang LR, Zhan YY, Chen Y, Wang Y, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer research. 2010;70:3628–37. doi: 10.1158/0008-5472.CAN-09-3160. [DOI] [PubMed] [Google Scholar]
- 32.Lam BY, Zhang W, Ng DC, Maruthappu M, Roderick HL, Chawla S. CREB-dependent Nur77 induction following depolarization in PC12 cells and neurons is modulated by MEF2 transcription factors. J Neurochem. 2010;112:1065–73. doi: 10.1111/j.1471-4159.2009.06521.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Dong X, Zhou W, Zeng Q, Mao Y. PDGF-induced proliferation of smooth muscular cells is related to the regulation of CREB phosphorylation and Nur77 expression. J Huazhong Univ Sci Technolog Med Sci. 2011;31:169–73. doi: 10.1007/s11596-011-0245-2. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Tai HH. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30:1606–13. doi: 10.1093/carcin/bgp161. [DOI] [PubMed] [Google Scholar]
- 35.Bonta PI, Matlung HL, Vos M, Peters SL, Pannekoek H, Bakker EN, et al. Nuclear receptor Nur77 inhibits vascular outward remodelling and reduces macrophage accumulation and matrix metalloproteinase levels. Cardiovasc Res. 2010;87:561–8. doi: 10.1093/cvr/cvq064. [DOI] [PubMed] [Google Scholar]
- 36.Haas JS, Stolz ED, Betti AH, Stein AC, Schripsema J, Poser GL, et al. The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta medica. 2011;77:334–9. doi: 10.1055/s-0030-1250386. [DOI] [PubMed] [Google Scholar]
- 37.Zi-ying Huang, J W, 1, Min-jie Huang, Jin-fu Wan. Determination of rutin,hyperoside,quercetin and kaempferol in Prunella vulgaris by HPLC. Chinese Traditional Patent Medicine. 2012;34:4. [Google Scholar]
- 38.Stojanovic G, Ethordevic A, Smelcerovic A. Do other Hypericum species have medical potential as St. John's wort (Hypericum perforatum)? Current medicinal chemistry. 2013;20:2273–95. doi: 10.2174/0929867311320180001. [DOI] [PubMed] [Google Scholar]
- 39.Choi JH, Kim DW, Yun N, Choi JS, Islam MN, Kim YS, et al. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. Journal of natural products. 2011;74:1055–60. doi: 10.1021/np200001x. [DOI] [PubMed] [Google Scholar]
- 40.Verma N, Amresh G, Sahu PK, Mishra N, Rao Ch V, Singh AP. Pharmacological evaluation of hyperin for antihyperglycemic activity and effect on lipid profile in diabetic rats. Indian journal of experimental biology. 2013;51:65–72. [PubMed] [Google Scholar]
- 41.Li W, Liu M, Xu YF, Feng Y, Che JP, Wang GC, et al. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncology reports. 2014;31:117–24. doi: 10.3892/or.2013.2811. [DOI] [PubMed] [Google Scholar]
- 42.Kim SJ, Um JY, Lee JY. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-kappaB activation in mouse peritoneal macrophages. The American journal of Chinese medicine. 2011;39:171–81. doi: 10.1142/S0192415X11008737. [DOI] [PubMed] [Google Scholar]
- 43.Ku SK, Kwak S, Kwon OJ, Bae JS. Hyperoside Inhibits High-Glucose-Induced Vascular Inflammation In Vitro and In Vivo. Inflammation. 2014 doi: 10.1007/s10753-014-9863-8. [DOI] [PubMed] [Google Scholar]
- 44.Li ZL, Liu JC, Hu J, Li XQ, Wang SW, Yi DH, et al. Protective effects of hyperoside against human umbilical vein endothelial cell damage induced by hydrogen peroxide. Journal of ethnopharmacology. 2012;139:388–94. doi: 10.1016/j.jep.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Li ZL, Hu J, Li YL, Xue F, Zhang L, Xie JQ, et al. The effect of hyperoside on the functional recovery of the ischemic/reperfused isolated rat heart: potential involvement of the extracellular signal-regulated kinase 1/2 signaling pathway. Free radical biology & medicine. 2013;57:132–40. doi: 10.1016/j.freeradbiomed.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1535–41. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2288–94. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Kim JY, Lee SJ, Oh CJ, Choi YK, Lee HJ, et al. alpha-Lipoic acid prevents neointimal hyperplasia via induction of p38 mitogen-activated protein kinase/Nur77-mediated apoptosis of vascular smooth muscle cells and accelerates postinjury reendothelialization. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2164–72. doi: 10.1161/ATVBAHA.110.212308. [DOI] [PubMed] [Google Scholar]
- 49.Liu WS, Lin PC, Chang LF, Harn HJ, Shiuan D, Chiou TW, et al. Inhibitory effect of n-butylidenephthalide on neointimal hyperplasia in balloon injured rat carotid artery. Phytotherapy research : PTR. 2011;25:1494–502. doi: 10.1002/ptr.3377. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–60. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 51.Paulsen RF, Granas K, Johnsen H, Rolseth V, Sterri S. Three related brain nuclear receptors, NGFI-B, Nurr1, and NOR-1, as transcriptional activators. Journal of molecular neuroscience : MN. 1995;6:249–55. doi: 10.1007/BF02736784. [DOI] [PubMed] [Google Scholar]
- 52.Shao Q, Shen LH, Hu LH, Pu J, Qi MY, Li WQ, et al. Nuclear receptor Nur77 suppresses inflammatory response dependent on COX-2 in macrophages induced by oxLDL. Journal of molecular and cellular cardiology. 2010;49:304–11. doi: 10.1016/j.yjmcc.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Liu J, Jia R, Song H. Nur77 inhibits androgen-induced bladder cancer growth. Cancer investigation. 2013;31:654–60. doi: 10.3109/07357907.2013.853077. [DOI] [PubMed] [Google Scholar]
- 54.Lee SO, Li X, Khan S, Safe S. Targeting NR4A1 (TR3) in cancer cells and tumors. Expert opinion on therapeutic targets. 2011;15:195–206. doi: 10.1517/14728222.2011.547481. [DOI] [PMC free article] [PubMed] [Google Scholar]