Abstract
Stevia rebaudiana (Bert.) is an emerging sugar alternative and anti-diabetic plant in Pakistan. That is why people did not know the exact time of propagation. The main objective of the present study was to establish feasible propagation methods for healthy biomass production. In the present study, seed germination, stem cuttings and micropropagation were investigated for higher productivity. Fresh seeds showed better germination (25.51–40%) but lost viability after a few days of storage. In order to improve the germination percentage, seeds were irradiated with 2.5, 5.0, 7.5 and 10 Gy gamma doses. But gamma irradiation did not show any significant change in seed germination. A great variation in survival of stem cutting was observed in each month of 2012. October and November were found the most suitable months for stem cutting survival (60%). In order to enhance survival, stem cuttings were also dipped in different plant growth regulators (PGRs) solution. Only indole butyric acid (IBA; 1000 ppm) treated cutting showed a higher survival (33%) than control (11.1%). Furthermore, simple and feasible indirect regeneration system was established from leaf explants. Best callus induction (84.6%) was observed on MS-medium augmented with 6-benzyladenine (BA) and 2,4-dichlorophenoxyacetic acid (2,4-D; 2.0 mg l−1). For the first time, we obtained the highest number of shoots (106) on a medium containing BA (1.5 mg l−1) and gibberellic acid (GA3; 0.5 mg l−1). Plantlets were successfully acclimatized in plastic pots. The current results preferred micropropagation (85%) over seed germination (25.51–40%) and stem cutting (60%).
Keywords: Stevia rebaudiana, Seed germination, Seed radiation, Stem cuttings, Micropropagation
1. Introduction
Stevia rebaudiana (S. rebaudiana) in the Asteraceae family is an emerging economical species throughout the world (Sreedhar et al., 2008). S. rebaudiana is considered as an important plant due to its active compound present in the leaves known as steviol glycosides. The purified form of steviol glycoside is known as Stevioside which is 300 times sweeter than commercially available sucrose (Hwang, 2006). Stevia species are specially used for the treatment of diabetic patients. The natural Steviosides cannot enter into the blood stream due to the absence of receptor for absorbance. Still today there are no reports that a single patient is completely recovered from diabetes after using different synthetic drugs (Thiyagarajan and Venkatachalam, 2012). It is expected that 57 million people would be affected by diabetes in the year of 2025. Stevia extracts have no reported side effects and can be used as an alternative to sugar and other synthetic sweeteners (Thiyagarajan and Venkatachalam, 2012).
This species originated from Paraguay and Brazil, and currently considered as an alternate substitute of cane and beet sugar (Ahmad et al., 2011a,b). According to the literature Brazilian and Paraguay tribes used this valuable species for the treatment of heartburn in medicinal teas and Yerba mate (Singh and Rao, 2005; Fazal et al., 2011). In vitro and in vivo experiments have been conducted on human beings and animals under the control of the WHO which showed that the intake of steviosides or rebaudiosides does not induce genotoxicity and the oxidative derivatives were not expressed in vivo (Ahmad et al., 2011a).
S. rebaudiana plants are conventionally propagated through cuttings, but this traditional method cannot produce a large number of plants. The seeds of this species are smaller in size and the germination % age is very low (Singh and Rao, 2005). Therefore modern techniques of propagation such as in vitro regeneration or tissue culture are needed to enhance the production of this important species. For these reasons tissue culture techniques are widely used to produce maximum mass from a single plant in a short span of time and also provide opportunities for germplasm conservation of important plants (Jagatheeswari and Ranganathan, 2012; Sivaram and Mukundan, 2003; Anbajhagan et al., 2010; Taware et al., 2010; Sabah and Rasha, 2013).
The main objective of the present study was to improve the production of Stevia rebaudiana using different propagation methods. This is an emerging species in Pakistan and most of the researchers are unaware regarding the exact time of seed sowing and cutting. In this study seed germination (irradiated) and stem cuttings were compared with micropropagation. So, we established a simple micropropagation system via indirect regeneration for healthy and consistent plantlets production from leaf explants. These results will provide an opportunity for selection of the best method of propagation for future studies.
2. Materials and methods
2.1. Seed collection and irradiation
Fresh seeds were collected from field grown plants of S. rebaudiana at the Nuclear Institute for Food and Agriculture (NIFA), Peshawar. Healthy and black coated seeds were selected for gamma irradiation. Two thousand seeds were irradiated with 2.5, 5.0, 7.5 and 10 Gy doses through 60Co gamma source in the presence of air and room temperature. The same number of untreated seeds was used as control.
2.2. Seed germination in petri plates, soil and on MS medium
Carefully 20 radiated seeds were placed in each petri plate along with the control (without radiation). Petri plates containing filter paper and 1 L bottle containing distilled water were autoclaved before seed inoculation. Seeds were surface sterilized with 0.2% mercuric chloride solution for 5 min and washed several times with sterile distilled water. Before inoculation the seeds were dried with the help of autoclaved filter paper. Irradiated seeds were placed in triplicate on a wet filter paper and the mean data was collected after 25 days. For seed germination in soil, 100 pots were filled with a mixture of sand, silt and clay. 20 pots were used for each dose (2.5, 5.0, 7.5 and 10 Gy) along with the control. Irradiated seeds were also placed on a solid MS-medium for germination without the addition of plant growth regulators (PGRs). A total of 180 seeds were inoculated on MS-medium along with the control. For each treatment 36 seeds were cultured in test tubes in three replicates. Untreated fresh seeds were also sown in the soil to test the best germination time for a period of 6 weeks.
2.3. Propagation through stem cuttings
Equal of Sixty stem cuttings were planted in each month of 2012 to obtain the best month for survival. In another set of experiment, stem cuttings of suitable length with 3–4 leaves were dipped for 1 min in NAA (500, 1000 ppm) and IBA (500, 1000 ppm) solutions respectively. Stem cuttings were then planted in pots containing combinations of soil, sand and manure in 2:1:1 ratio. Data on different parameters of shoot (length of cuttings, No. of leaves per cutting, No. of branches per cutting and internodes length) and root (length of longest root, No. of roots per cutting, fresh weight of roots and dry weight of roots) was collected after 90 days of culture.
2.4. Micropropagation
2.4.1. Collection and sterilization of leaf explants
Fresh leaf explants were taken from S. rebaudiana mother plant grown in the Nuclear Institute for Food and Agriculture (NIFA), Peshawar, Pakistan. The leaves were collected in a container with tap water to maintain viability. The leaves were then placed under running water for 15 min to remove dust particles. Ethanol (70%) for 1 min and mercuric chloride (0.2%) for 120 s were used for explant sterilization. The explants were then rinsed with double distilled water and dried with the help of filter paper (autoclaved).
2.4.2. Media and growth conditions
Murashige and Skoog, 1962 basal medium was selected for in vitro regeneration augmented with sucrose (3%) and then solidified with agar (8 g l−1, Merk). Plant growth regulators (PGRs) from stock solutions were added to the media and the pH (5.6–5.8) was adjusted through a pH meter (330i/SET, Germany). After pH adjustment the media were boiled in a microwave oven and then autoclaved (Hirayama, Japan) at 121 °C for 20 min. All prepared media were then placed in growth room (25 ± 1 °C) under a 16 h photoperiod.
2.4.3. Plant growth regulators for micropropagation
For callus induction, leaf explants were placed on MS medium incorporated with different concentrations (1.0–3.0 mg l−1) of 6-benzyladenine (BA) and 2,4-dichlorophenoxyacetic acid (2,4-D) alone (0.5–4.0 mg l−1) or in combination with BA (0.5–3.0 mg l−1). MS basal medium (MS0) was used as a control. About 2–5 explants were cultured in food jars containing 25–35 ml of media. Callus growth was visually observed after 4–5 weeks of explant inoculation. Fresh and green callus was selected for further organogenesis (shoots and roots). To study the effect of PGRs on shoot regeneration, the medium was supplemented with BA alone or in combination with GA3, or BA and GA3 in combination with naphthalene acetic acid (NAA) and indole butyric acid (IBA) or BA along with IBA or with GA3, BA with Zeatin or BA in combination with NAA or IBA alone or in combination with NAA. Data regarding different parameters of shoot organogenesis (Mean shoot length, number of shoots per explant and % shooting) was documented after 4 weeks of culturing. For rooting, MS-medium or B5 medium was incorporated with BA alone, or in combination with NAA and IBA, B5 medium with BA or half B5 medium with a combination of NAA and IBA or half MS-medium with similar composition of PGRs. Mature shoots were excised from the media and cultured on MS medium containing auxin and cytokinin for rooting. Mean root length, % rooting and number of roots per plantlet were recorded after 4 weeks. Regenerated plantlets were successfully transferred to pots containing combination of soil, sand, and manure in a ratio of 2:1:1.
2.5. Statistical analysis
For statistical analysis data was collected from triplicate experiments with 9 cultured flasks for each treatment during micropropagation. All other data were taken from triplicate independent experiments. Mean data with common letters and standard deviation were analyzed by using Statistix 8.1 software and all graphs were prepared by using OriginLab software (8.1).
3. Results and discussion
3.1. Effect of radiation doses on seed germination
The effect of different radiation doses on Stevia seed germination was investigated. The seeds were germinated on 3 different media for comparison including Petri plates, soil and MS-medium. Significant differences were observed in seed germination after growing on different substrates. Higher seed germination (23.31%) was observed in the control; however, 20% germination was recorded in petri plates after 10 Gy doses of gamma irradiation (Fig. 1). Similarly 13.44% seed germination in soil was observed when seeds were irradiated with 2.5 Gy doses (Fig. 1). Lower seed germination was observed in the MS-medium than Petri plates and a soil medium. The effect of radiation doses on seed germination in Stevia is not reported yet. However, the effect of gamma irradiation on other components of seed was recently reported by Ahmad et al., 2013a. Furthermore, many researchers observed that different doses of gamma irradiation frequently change the active components of many food and medicinal plants (Suk et al., 2005; Costa de Camargo et al., 2012; Pérez et al., 2007). In conclusion gamma irradiation did not show any significant change in germination because the untreated seeds showed better germination.
Figure 1.
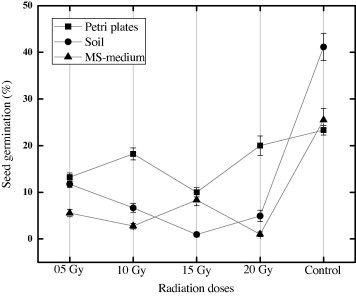
Effect of different gamma irradiations on seed germination in Stevia rebaudiana (Bert.). Treated seeds were germinated in Petri plates, soil and MS-medium. Data was collected from three independent experiments. Mean values (±SD) are significantly different when P < 0.05.
Fresh seeds were sown in soil to check the germination data periodically. Germination of seed started after day 9. Higher germination percentage (25.5%) was observed after 9 days of seed sowing (Fig. 2). But the germination percentage gradually decreases as the seeds were sown after 9 days and the least germination (6.12%) was observed after 37 days of sowing. It means that Stevia seeds lose viability when stored for longer period. These results suggest that fresh seeds should be used within first week of collection to obtain healthy stock.
Figure 2.
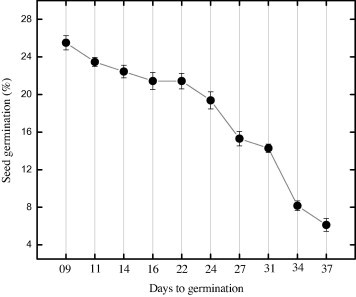
Days to seed germination of Stevia rebaudiana (Bert.). Mean values with standard deviation were taken from three replicates and significantly different when P < 0.05.
3.2. Conventional propagation through stem cuttings
Stem cutting was planted in each month of 2012. It has been observed that the best month for stem cutting was October and November. Generally growers tried to obtain higher biomass in the month of April. But we get 60% vigorous plants in these two months as compared to April (32%) as shown in Fig. 3. Therefore the current results suggest that stem cutting should be planted in October and November. Data regarding different parameters of shooting (length of cutting or shoot, internode length and, No. of leaves and branches per cutting) was taken after 90 days of sowing (Table 1). Data on % survival or sprouting of cuttings dipped in IBA and NAA solutions showed variation. Maximum survival rate (sprouting) of 33% was observed when cuttings were dipped in an IBA solution (1000 ppm) and NAA solution (1000 ppm; 22.2%). Smitha and Umesha (2012) more recently reported 76.8% sprouting with the application of IBA 500 and 1000 ppm solutions. However, 11.1% survival was observed in control and cuttings when dipped in IBA solution (500 ppm), but NAA (500 ppm) showed 2.66% survival. Furthermore, a maximum of 33.56 cm mean shoot length was recorded when cutting was dipped in an IBA solution (500 ppm) as shown in Table 1. Similarly, Ingle and Venugopal (2009) reported a shoot length of 37.83 cm when cuttings were exposed to IBA solution (500 ppm). The differences in data may be due to the dipping time and soil mixtures. But Smitha and Umesha (2012) mentioned that IBA 500 and 1000 ppm induced 19.98 and 19.18 cm sprout lengths in Stevia. Similarly 29.833 and 19.63 cm mean shoot lengths were recorded for IBA and NAA solutions (1000 ppm) as compared to control (20.83 cm). Maximum of 107.67 number of leaves per cutting was observed when cutting was dipped in the NAA solution (1000 ppm) as compared to control (91.3). The current data is in agreement with the reports of Chalapathi et al. (2001). But Ingle and Venugopal (2009) obtained the highest number of leaves (37.86) with the application of 500 ppm IBA solution. The highest number (10.3) of branches was observed when cuttings were dipped in NAA solution (1000 ppm) in comparison with control (6.67). Significantly similar internode length was recorded for cuttings dipped in IBA and NAA solutions (2.67, 2.83 and 2.29) as compared to control (1.65). Before data collection on rooting, roots were collected and carefully washed with distilled water to remove soil particles. Higher number of roots (24.0) per cutting was observed when dipped in IBA solution (500 ppm) as compared to control (12.35) (Table 1). Similarly, Ingle and Venugopal (2009) documented that IBA 500 ppm influenced a maximum number of roots in stem cutting of Stevia. Like the number of roots per cutting, IBA treated (500 ppm) roots showed a maximum length of 67.0 cm as compared to control (47.15 cm). All the roots collected from cuttings which were treated with IBA and NAA (1000, 500 and 1000 ppm) showed significantly similar fresh weight (1.65, 1.64 and 1.68 g) as compared to control (1.21). After fresh weight the roots were completely dried in an oven at 60 °C for 2 h. Maximum of 0.70 g of dry weight was observed for roots taken from IBA (1000 ppm) treated cuttings as compared to control (0.52 g). However, Ingle and Venugopal (2009) reported a maximum of 0.29 g of dry weight after treatment with IBA 500 ppm.
Figure 3.
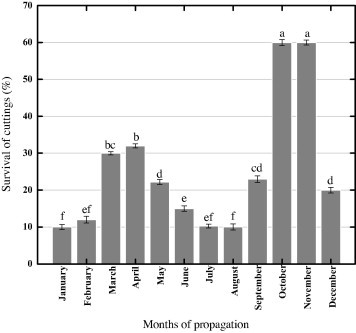
Survival percentage of stem cuttings planted in different months. Data (mean ± S.D + LSD) was collected from three replicates. Mean values with common letters on each bar are significantly different at P < 0.05.
Table 1.
Effect of IBA (500 and 1000 ppm) and NAA (500 and1000 ppm) solutions on shoot and root formations in stem cutting of Stevia rebaudiana (Bert.).
| PGRs (ppm) | % Survival | Length of cuttings (cm) | No. of leaves/cutting | No. of branches/cutting | Internodes length |
|---|---|---|---|---|---|
| Shoot emergence parameters | |||||
| IBA 1000 | 33.33a⁎ | 29.83ab | 90.0b | 06.0b | 02.67ab |
| NAA 1000 | 22.22b | 19.63b | 107.67a | 10.33a | 02.29b |
| IBA 500 | 11.11c | 33.57a | 102.33ab | 08.67ab | 02.83a |
| NAA 500 | 2.66 d | 10c | 21c | 2.33c | 0.67d |
| Control | 11.11c | 20.83b | 91.33b | 06.67b | 01.65 cd |
| Root formation parameters | |||||
| PGRs (ppm) | Mean length (cm) | No. of roots/cutting | Fresh weight of roots | Dry weight of roots | |
| IBA 1000 | 53.6b⁎ | 15.0b | 1.65ab | 0.7a | |
| NAA 1000 | 54.5b | 13.0bc | 1.68a | 0.56b | |
| IBA 500 | 67.0a | 24.0a | 1.64ab | 0.58b | |
| NAA 500 | 09d | 03c | 0.34c | 0.11c | |
| Control | 47.15c | 12.35bc | 1.21b | 0.51bc | |
The experiment was repeated twice and data was collected from replicated experiments. Mean values with least significant difference (LSD) are significantly different at P < 0.05.
3.3. Micropropagation
3.3.1. Effect of different PGRs on callus induction
Efficient regeneration system via callogenesis was established from leaf explants of S. rebaudiana (Fig. 4). Callus was induced on medium supplemented with various concentrations of BA alone (1.0, 2.0 and 3.0 mg l−1) or 2,4-D alone in various concentrations (0.5–4.0 mg l−1) or combination of BA and 2,4-D (0.5–3.0 mg l−1; Table 2). In the present study S. rebaudiana leaf explants responded to all plant growth regulators used. Best callus production (<84%) was observed on medium augmented with a combination of 2,4-D and BA (2.0 mg l−1; Table 2). Recently, Aman et al. (2013) reported that 2,4-D and BA along with different agar concentrations significantly enhanced callus formation in S. rebaudiana. These results are in agreement with those reported by Ahmad et al. (2011a) that the combination of BA and 2,4-D can induce callus in S. rebaudiana. Callus induction of >83% was recorded on a medium containing 1.0 mg l−1 of 2,4-D along with 1.0 mg l−1 of BA. Khalil et al. (2011) also observed that the combination of cytokinin and auxin can accelerate callus induction in Citrus sinensis. Significantly similar amount of callus (>83%) was also induced by 4.0 and 2.0 mg l−1 of 2,4-D. No callus was observed on MS0 medium. Similarly, the callus response of 70.06% was recorded on 2.0 mg l−1 of BA alone. A similar observation was also reported by Taware et al. (2010) also reported that the addition of 1.0 mg l−1 of 2,4-D to the medium enhance callus formation in S. rebaudiana. All the PGRs (Cytokinin and auxin) used alone or in combination induced higher amount of callus, however, BA (1.0 and 3.0 mg l−1) and 3.0 mg l−1 of 2,4-D were less effective in callus induction. The callus color, texture and growth were also recorded on various concentrations of BA and 2,4-D alone or in combination (Table 2). Various concentrations of 2,4-D induced yellowish green, granular and spongy callus. In comparison with 2,4-D, all the BA concentrations induced compact and green callus. However, combination of BA and 2,4-D produced granular and yellowish green callus.
Figure 4.
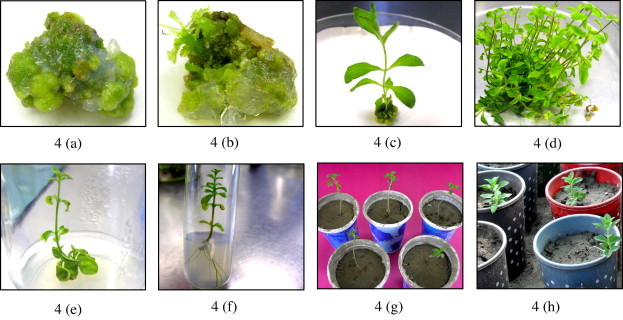
Simple and feasible indirect regeneration system from leaf explants in Stevia rebaudiana (Bert.), (4a) Callus induction (4bc) Shoot initiation from callus culture (4d) Shoot multiplication (4e) Shoot elongation (4f) Roots induction (4g and h) Successful acclimatization of in vitro regenerated plantlets.
Table 2.
Effect of different concentrations of auxin or cytokinin alone or in combination on percent callus formation, callus color, callus texture and callus growth in Stevia rebaudiana (Bert.).
| Plant growth regulators (mg l−1) | Callus formation (%) | Callus color | Callus texture | Callus growth |
|---|---|---|---|---|
| 2,4-D (0.5) | 66.48 ± 0.90cd⁎ | Yellowish green | Granular and spongy | +++ |
| 2,4-D (1.0) | 66.54 ± 1.62cd | Yellowish green | Granular and spongy | +++ |
| 2,4-D (1.5) | 66.94 ± 1.75c | Yellowish green | Granular and spongy | +++ |
| 2,4-D (2.0) | 83.23 ± 2.67a | Yellowish green | Granular | +++ |
| 2,4-D (3.0) | 50.37 ± 3.01e | Yellowish green | Granular | +++ |
| 2,4-D (4.0) | 83.25 ± 2.10a | Yellowish green | Granular | ++++ |
| BA (1.0) | 29.33±4.35g | Green | Compact | + |
| BA (2.0) | 70.06 ± 2.73b | Green | Compact | +++ |
| BA (3.0) | 40.38 ± 2.89f | Green | Compact | ++ |
| 2,4-D + BA (0.5) | 66.28 ± 1.25cd | Yellowish green | Granular | +++++ |
| 2,4-D+BA (1.0) | 83.22 ± 2.02a | Yellowish green | Granular | +++++ |
| 2,4-D + BA (2.0) | 84.60±1.27a | Yellowish green | Granular | +++++ |
| 2,4-D+BA (3.0) | 64.23 ± 3.21d | Yellowish green | Granular | +++++ |
| Control | 0 | NA | NA | NA |
+++++ Excellent, ++++ Very good, +++Good, ++Poor, + Very poor.
Indirect regeneration of S. rebaudiana via callus cultures from leaf explants. Data was taken from replicated experiments. Mean data in each column with common letters and standard deviation are not significantly different at P < 0.05.
3.3.2. Effect of different PGRs on shoot organogenesis
Data regarding different parameters on shoot organogenesis was documented after 4 weeks of callus inoculation. Presently BA (2.0 mg l−1) showed the greatest frequency of shoot regeneration (96%) after 4 weeks of callus culture (Table 3). Similar results were also reported by Ahmad et al. (2011a), that BA alone can influence shooting response in S. rebaudiana. BA (1.5 mg l−1) in combination with GA3 (0.5 mg l−1) also induce 90% shooting in S. rebaudiana. Furthermore, combinations of BA (1.0 mg l−1) with GA3 (0.3 mg l−1), NAA (0.3 mg l−1) and IBA (0.2 mg l−1) also produced more than 80% shoots from callus cultures. The current data is in agreement with the results of Aman et al. (2013). Least shoot organogenesis (20%) was recorded on BA combined with NAA and 2,4-D (Table 3). In the current experiment all the BA concentrations used were found effective in shoot induction. Presently, the highest (106) number of shoots per explant was documented when the medium was supplemented with BA (1.5 mg l−1) in combination with GA3 (0.5 mg l−1) as shown in Fig. 4d. Most recently, Aman et al. (2013) reported that BA alone in the medium induces 28 shoots per explant. But Thiyagarajan and Venkatachalam (2012) recently reported a total of 123 shoots per explant. The difference in data is due to explant types, we used indirect regeneration system in which multiple shoots were obtained from callus (leaf explant) but Thiyagarajan and Venkatachalam (2012) obtained direct shoots from nodal segments. Similarly, 96 number of shoots per explant was observed when the medium was supplemented with 2.0 mg l−1 BA (Table 3). Ahmad et al. (2011a) also reported that BA can produce the maximum number of shoots per explant in S. rebaudiana. Khalil et al. (2011) observed that BA in the medium enhances the number of shoots per explant. In the present investigation it was observed that, 2,4-D in combination with BA and NAA or IBA alone or in combination with NAA significantly inhibited the number of shoots per explant in S. rebaudiana (Table 3). When shoots were transferred to elongation medium, longest shoots (29.5 cm) were observed on MS-medium incorporated with 1.0 mg l−1 of BA in combination with 1.0 mg l−1 GA3 (Table 3). On the other hand 23.5 cm long shoots were recorded on 1.5 mg l−1 BA along with 0.5 mg l−1 GA3. Furthermore, the same result was also reported by Sabah and Rasha (2013).
Table 3.
Shoot and root organogenesis in Stevia rebaudiana (Bert.) on half and full MS or B5 medium supplemented with different concentrations of cytokinin (BA, Zeatin), gibberellic acid (GA3) and auxin alone (IBA, NAA, 2,4-D) or in combination (BA+ GA3; BA+IBA; BA+ GA3 + IBA + NAA; BA + zeatin; IBA + NAA).
| Plant growth regulators (mg l−1) | % Shooting mean ± SD | No. of shoots per explant | Mean shoot length (cm) | % Rooting mean ± SD | Mean root length (cm) | No. of roots per plantlet |
|---|---|---|---|---|---|---|
| BA 1.0 | 85.00 ± 0.67d⁎ | 20 ± 1.0e | 5.2 ± 2e | 53.13 ± 0.14e | 9.0 ± 0.5bc | 7.5 ± 0.37c |
| BA 2.0 | 96.00 ± 1.33a | 96 ± 4.8b | 6.3 ± 2e | 77.27 ± 0.24b | 4.5 ± 0.5 cd | 4 ± 0.2d |
| BA 3.0 | 70.00 ± 0.93 g | 106 ± 5.3a | 23.5 ± 0.5b | 46.14 ± 0.53f | 8.3 ± 0.6bc | 8.44 ± 0.5c |
| BA 4.0 | 60.00 ± 0.89jk | 37.5 ± 1.87d | 29.5 ± 0.59a | 46.43 ± 0.42f | 10.6 ± 1.5b | 13.5 ± 0.6b |
| BA 1.5 + GA3 0.5 | 90.00 ± 1.33b | 17 ± 0.85ef | 9.5 ± 0.5d | – | ||
| BA 1.0 + GA3 1.0 | 65.00 ± 0.67ij | 12 ± 0.6 fg | 11 ± 1.0 cd | – | ||
| BA 0.2 + GA3 0.2 | 66.60 ± 0.16hi | 45 ± 2.25c | 5.75 ± 0.7e | – | ||
| BA 1.0 + 0.1(GA3 + IBA + NAA) | 72.00 ± 1.33 fg | 44 ± 2.2c | 12 ± 1.0c | – | ||
| BA 1.0 + IBA 0.2 + 0.3(GA3 + NAA) | 86.66 ± 0.96 cd | 37.5 ± 1.8d | 5.1 ± 0.65e | – | ||
| BA + GA3 (0.2) + NAA + IBA (0.5) | 73.68 ± 0.75f | 12 ± 0.6 fg | 13.2 ± 1.0c | – | ||
| ½ MS + 0.2(BA + GA3) + 0.5(NAA + IBA) | 87.5 ± 0.96c | 23 ± 1.15e | 10 ± 1.0 cd | – | ||
| BA 0.5 + IBA 1.0 | 66.66 ± 0.16hi | 32.2 ± 1.6de | 11.5 ± 1.47 cd | – | ||
| BA 0.5 + ½ MS IBA | 80.00 ± 1.33e | 4.0 ± 0.2 g | 3.1 ± 1.0f | – | ||
| BA 1.0 + Zeatin 0.1 | 67.00 ± 0.67 h | 2.0 ± 0.1 g | 9.0 ± 0.5d | – | ||
| BA 1.0 + 0.5 (NAA + 2,4-D) | 20.00 ± 1.00 l | 2.5 ± 0.12 g | 3.0 ± 0.5f | – | ||
| IBA 1.0 | 64.28 ± 0.51j | 4.5 ± 0.25 g | 12 ± 0.5c | – | ||
| IBA 1.0 + NAA 0.5 | 50.00 ± 0.33 k | 2.5 ± 0.12 g | 5.5 ± 0.5e | – | ||
| IBA 0.5 + ½ MS NAA | 71.42 ± 0.41 g | 4.7 ± 0.17 g | 3.2 ± 0.19f | – | ||
| NAA 0.5 + IBA 0.5 | 71.42 ± 0.41 g | 2.3 ± 0.36 g | 3.7 ± 0.51f | 73.92 ± 0.41c | 7.4 ± 3.21c | 13.6 ± 0.8b |
| ½ MS + NAA 0.5 + IBA 0.5 | – | 85.72 ± 0.21a | 14.3 ± 1.0a | 19 ± 0.95a | ||
| BA 1.0 + B5 | – | 15.38 ± 0.38 g | 2.7 ± 2.0d | 4.5 ± 0.25d | ||
| BA 2.0 + B5 | – | 15.79 ± 0.21 g | 8.25 ± 0.5bc | 12 ± 0.6bc | ||
| ½ B5 + 0.5(IBA + NAA) | – | 70.00 ± 0.50d | 3.75 ± 3.0d | 7 ± 0.35c | ||
| Control | 8.00 ± 0.34 m | 1 ± 0.05 g | 5.5 ± 0.2e | 0 | 0 | 0 |
Data was taken from replicated experiments. Mean data in each column with common letters and standard deviation are not significantly different at P < 0.05.
3.3.3. Effect of different PGRs on root organogenesis
For root induction, elongated shoots were kept for more than 5 weeks on medium containing BA. Root initiation was started with very thin and weak roots. Maximum of 85% rooting was observed on medium with half strength basal salts containing NAA and IBA (0.5 mg l−1). However, Aman et al. (2013) reported that the addition of 2.0 mg l−1 IAA influence 100% rooting response when the agar concentration in the medium is reduced from 7.5 to 3.5 g l−1. Whereas, when the full MS-medium was incorporated with similar concentrations of auxin, more than 73% rooting was recorded (Table 3). Aman et al. (2013) also observed that the rooting response decreases with the addition of IAA, NAA and IBA to full MS-medium. However, BA (2.0 mg l−1) also produced similar % of rooting in regenerated shoots. Similar findings were also reported by Khalil et al. (2011) in C. sinensis. The current results are in line with the observation of Ahmad et al., 2013b. Furthermore, data regarding the number of roots/plantlets, maximum of 19 roots per plantlet was observed on MS medium with half strength basal salts and 0.5 mg l−1 NAA in combination with 0.5 mg l−1 IBA (Table 3). Similarly, Aman et al. (2013) reported the addition of higher concentrations of IBA and NAA with different agar concentrations significantly enhanced the number of roots (26.33) in S. rebaudiana. Maximum mean root length (14.33 cm) was recorded when half strength MS-medium was incorporated with similar concentrations of PGRs. These results suggest that all auxin are effective in root induction in S. rebaudiana.
3.3.4. Acclimatization of regenerated plantlets
Regenerated plantlets were successfully transferred to pots containing combinations of soil, sand and manure in a ratio of 2:1:1 (Fig. 4g and h). The pots containing plantlets were kept in growth chamber for 2 weeks and then transferred to greenhouse. Healthy plantlets from pots were then transferred to field conditions for survival. Cuttings were taken from these acclimated plants and sown in plastic bags for further studies.
The current results suggest that stem cutting and seed germination are not cost effective methods for higher biomass and healthy plantlets production, because the seed shortly loses viability after collection and further most of the seeds cannot germinate due to the presence of immature embryos. The seeds cannot be stored for longer period. It is better to collect fresh seeds and directly sown to get maximum plants. Similarly, stem cutting needs large input stock and labors. Stem cutting only showed maximum survival in November and October months. Therefore the best option for healthy biomass production is micropropagation. Here, we established a simple protocol from leaf explants for regeneration which is helpful for future studies on this species.
Conflict of interest
All the authors declare that they have no conflict of interests concerning this manuscript.
Acknowledgements
Authors are thankful to the Pakistan Science Foundation (PSF) for financial support to complete this research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad N., Fazal H., Zamir R., Khalil S.A., Abbasi B.H. Callogenesis and shoot organogenesis from flowers of Stevia rebaudiana (Bert.) Sugar Tech. 2011;13:174–177. [Google Scholar]
- Ahmad N., Fazal H., Abbasi B.H., Iqbal M. In vitro larvicidal potential against Anopheles stephensi and antioxidative enzyme activities of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous. Asian Pac. J. Trop. Med. 2011;4:169–175. doi: 10.1016/S1995-7645(11)60063-1. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Abbasi B.H., Fazal H. Evaluation of antioxidant activity and its association with plant development in Silybum marianum L. Ind. Crop. Prod. 2013;49:164–168. [Google Scholar]
- Ahmad N., Abbasi B.H., Fazal H., Rahman U.R. Piper nigrum L.: micropropagation, antioxidative enzyme activities and chromatographic fingerprint analysis for quality control. Appl. Biochem. Biotechnol. 2013;169:2004–2015. doi: 10.1007/s12010-013-0104-7. [DOI] [PubMed] [Google Scholar]
- Aman N., Hadi F., Khalil S.A., Zamir R., Ahmad N. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni) C. R. Biol. 2013;336:486–492. doi: 10.1016/j.crvi.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Anbajhagan M., Kalpana M., Rajendran R., Natarajan V., Dhanavel D. In vitro production of Stevia rebaudiana Bert. Emir. J. Food Agric. 2010;22:216–222. [Google Scholar]
- Chalapathi M.V., Thimmengowda S., Kumar N.D., Gangadhar G., Rao E., Mallikarjun K. Influence of length of cutting and growth regulators on vegetative propagation of Stevia (Stevia rebaudiana B.) Crop Res. 2001;21:53–56. [Google Scholar]
- Costa de Camargo A., de Souza Ferreira., Vieira T.M., Regitano-D Arce M.A.B., Calori-Domingues M.A., Canniatti-Brazac S.G. Gamma radiation effects on peanut skin antioxidants. Inter. J. Mol. Sci. 2012;13:3073–3084. doi: 10.3390/ijms13033073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal H., Ahmad N., Ullah I., Inayat H., Khan L., Abbasi B.H. Antibacterial potential in Parthenium hysterophorus L., Stevia rebaudiana and Ginkgo biloba. Pak. J. Bot. 2011;43:1307–1313. [Google Scholar]
- Hwang S.J. Rapid in vitro propagation and enhanced stevioside accumulation in Stevia rebaudiana Bert. J. Plant Biol. 2006;49:267–270. [Google Scholar]
- Ingle M.R., Venugopal C.K. Effect of different growth regulators on rooting of stevia (Stevia rebaudiana Bertoni) cuttings”, Karnataka. J. Agric. Sci. 2009;22:460–461. [Google Scholar]
- Jagatheeswari D., Ranganathan P. Studies on micropropagation of Stevia rebaudiana Bert. Inter. J. Pharm. Biol. Arch. 2012;3:315–320. [Google Scholar]
- Khalil S.A., Zamir R., Ahmad N., Sajid M., Fazal H., Khan M.A., Seema N., Alam R. In-vitro regeneration of plantlets from unpollinated ovary culture in sweet orange (Citrus sinensis L. Osbeck) African J. Biotechnol. 2011;10:15130–15134. [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Pérez M.B., Calderón N.L., Croci C.A. Radiation-induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.) Food Chem. 2007;104:585–592. [Google Scholar]
- Sabah A.H., Rasha M.A.K. Biotechnological studies for improving of stevia (Stevia rebaudiana Bertoni) in vitro plantlets. Middle-East J. Sci. Res. 2013;14:93–106. [Google Scholar]
- Singh S.D., Rao G.P. Stevia: the herbal sugar of 21st century. Sugar Tech. 2005;7:17–24. [Google Scholar]
- Sivaram L., Mukundan U. In vitro culture on Stevia rebaudiana Bert. In Vitro Cell. Dev. Biol. Plant. 2003;39:520–523. [Google Scholar]
- Smitha G.R., Umesha K. Vegetative propagation of stevia [Stevia rebaudiana (Bertoni) Hems.] through cuttings. J. Trop. Agric. 2012;50:72–75. [Google Scholar]
- Sreedhar R.V., Venkatachalam L., Thimmaraju R., Bhagyalakshmi N., Narayan M.S., Ravishankar G.A. Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol. Plant. 2008;52:355–360. [Google Scholar]
- Suk J.E., Hwa L.H., Sung K.J., Young L.S. Effects of low dose γ-ray irradiation on antioxidant activity of seeds and seedling growth in Raphanus sativus L. Korean J. Hort. Sci. Technol. 2005;23:245–249. [Google Scholar]
- Taware A.S., Mukadam D.S., Chavan A.M., Tawar S.D. Comparative studies of in vitro and in vivo grown plants and callus of stevia rebaudiana (Bertoni) Inter. J. Integr. Biol. 2010;9:10–15. [Google Scholar]
- Thiyagarajan M., Venkatachalam P. Large scale in vitro propagation of Stevia rebaudiana (Bert.) for commercial application: pharmaceutically important and antidiabetic medicinal herb. Ind. Crop. Prod. 2012;37:111–117. [Google Scholar]



