Abstract
Currently, 17–19% of all new primary malignancies occur in survivors of cancer, causing substantial morbidity and mortality. Research has shown that cancer treatments are important contributors to second malignant neoplasm (SMN) risk.
In this paper we summarise current knowledge with regard to treatment-related SMNs and provide recommendations for future research. We address the risks associated with radiotherapy and systemic treatments, modifying factors of treatment-related risks (genetic susceptibility, lifestyle) and the potential benefits of screening and interventions. Research priorities were identified during a workshop at the 2014 Cancer Survivorship Summit organised by the European Organisation for Research and Treatment of Cancer.
Recently, both systemic cancer treatments and radiotherapy approaches have evolved rapidly, with the carcinogenic potential of new treatments being unknown. Also, little knowledge is available about modifying factors of treatment-associated risk, such as genetic variants and lifestyle. Therefore, large prospective studies with biobanking, high quality treatment data (radiation dose–volume, cumulative drug doses), and data on other cancer risk factors are needed. International collaboration will be essential to have adequate statistical power for such investigations. While screening for SMNs is included in several follow-up guidelines for cancer survivors, its effectiveness in this special population has not been demonstrated. Research into the pathogenesis, tumour characteristics and survival of SMNs is essential, as well as the development of interventions to reduce SMN-related morbidity and mortality. Prediction models for SMN risk are needed to inform initial treatment decisions, balancing chances of cure and SMNs and to identify high-risk subgroups of survivors eligible for screening.
Keywords: Radiotherapy, Chemotherapy, Multiple primary malignancies or multiple primary neoplasms, Treatment-induced
1. Introduction
Currently, 17–19% of all new primary malignancies occur in individuals who have already survived a primary malignancy [1,2]. In the Netherlands, the proportion of second and subsequent malignancies (including second cancers in paired organs) increased from 10% in 1989 to 17% in 2013 [1]. In the U.S. National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) Programme, the proportion of cancers that are second and subsequent malignancies has more than doubled in the last three decades, from 9% in 1975–1979 to 19% in 2005–2009 [2].
Research conducted over the last three decades has clearly demonstrated that, paradoxically, some treatments used successfully to treat cancer have the potential to induce new (second) primary malignancies. Increased risks of second malignant neoplasms (SMNs) have been observed after radiotherapy, certain chemotherapy regimens and hormonal treatments. Of all late complications of treatment, SMNs are among the most serious because they cause not only substantial morbidity but also considerable mortality. For example, death due to SMNs is the largest contributor to long-term excess mortality among survivors of Hodgkin lymphoma (HL) [3,4].
Despite the importance of treatment-related SMNs, it must be recognised that SMNs may also be due to other causes. Aside from treatment the occurrence of two primary malignancies in the same individual may result from host susceptibility factors (e.g. genetic predisposition or immunodeficiency), carcinogenic influences in common, or indeed several of these factors may come into play [5–7]. Alternatively, the primary malignancies may be unrelated, and their occurrence in a single individual may arise by chance alone. In view of the high prevalence of cancer in the general population and the increasing incidence of most cancers with older age, background aetiological factors other than treatment are likely to be responsible for a substantial proportion of SMNs. Therefore, to properly evaluate the risk of SMN, comparison with cancer risk in the general population is important.
In this paper we summarise current knowledge regarding treatment-related SMNs, identify gaps in knowledge and provide recommendations for future research. In particular, we will address the risks associated with radiotherapy, systemic treatments including chemotherapy and hormonal agents and potential synergistic effects of different treatments. We will also describe the key factors that may modify the treatment-related SMN risks, including age, genetic susceptibility and other cancer risk factors such as cigarette smoking. Finally, we will discuss the potential benefits of screening and other interventions aimed at reducing SMN risk. The current perspective represents a summary of discussions during a workshop at the 1st Cancer Survivorship Summit organised by the European Organisation for Research and Treatment of Cancer (EORTC), which was held on January 30, 2014 in Brussels, Belgium. The EORTC Survivorship Summit focused on survivorship issues after malignancies occurring in adolescence and adulthood.
2. Radiotherapy
Extensive understanding of the cancer risks following ionising radiation exposure derives from studies of patients exposed to diagnostic or therapeutic irradiation, victims of nuclear accidents and survivors of the atomic bombings in Japan [8–11]. Particularly high risks following radiation exposure are evident for cancers of the brain, thyroid, female breast, skin (basal cell carcinoma), bone and soft tissue. Modestly increased risks also are reported for cancers of the lung, gastrointestinal tract and bladder, as well as myeloid leukaemias.
For most tissues, cancer risks increase linearly with increasing radiation dose, though the magnitude of the risk differs substantially. For example, the excess relative risk per Gy is 0.09 (95% confidence interval [CI] 0.04–0.21) for stomach cancer, 0.15 (0.06–0.39) for lung cancer and 0.15 (0.04–0.73) for breast cancer after HL [12–14], and 0.33 (0.01–1.71) for glioma, 1.06 (0.21–8.15) for meningioma and 1.32 (0.44–4.22) for sarcoma after childhood cancer [15,16]. In contrast, for thyroid cancer, risk increases linearly until approximately 20 Gy and declines thereafter, consistent with a model in which higher radiation doses kill rather than transform cells [17], and for leukaemia, some studies suggest a similar pattern, though with a decline at approximately 4 rather than 20 Gy [18]. However, the magnitude and shape of the radiation dose–response relation remain unknown for certain cancer types, such as colon and pancreatic cancers, particularly for the radiation doses experienced by patients undergoing radiotherapy.
In addition to the type of tissue exposed and the radiation dose, the time period over which the exposure occurs and the time since exposure are key determinants of radiation-related cancer risk. For example, patients undergoing radiotherapy, who generally receive fractionated exposures of 1–5 Gy per fraction and cumulative doses of 15–>50 Gy, have lower risks per unit dose than atomic bomb survivors, who received a single acute exposure primarily <2 Gy [19]. Finally, the time since exposure is an important determinant of subsequent cancer risk, with most radiation-related cancers not appearing for at least a decade following exposure, and increased risks persisting for decades thereafter [8–11,20,21].
Radiotherapy treatments have evolved substantially in recent decades, with certain patients treated with lower doses, smaller field sizes and novel techniques such as intensity-modulated radiation therapy (IMRT) and proton therapy [22]. Because radiation-related cancer risks often are not observed for at least a decade after exposure, the extent to which these changes will modify SMN risk is uncertain [23,24]. Although these contemporary treatments generally reduce the amount of normal tissue exposed to high radiation doses (>10 Gy), in some circumstances they can increase the amount exposed to lower doses (1–9 Gy). Thus, a better understanding of the dose-risk relationship across a range of normal tissue doses is needed to understand the SMN risks following contemporary radiotherapy. Currently, despite the ability of modern radiotherapy planning systems to quantify individuals’ normal tissue doses in detail, there is almost no capacity to utilise this information to either optimise treatment plans to minimise SMN risks without compromising initial cure, or to provide individual-level counselling about risks.
Additionally, future studies should account for the volume of tissue exposed at each dose level, particularly because larger radiation volumes have been shown to increase risk of SMNs [25]. Documentation of normal tissue doses for patients undergoing radiotherapy should also become a standard part of modern clinical practice. Finally, with the expanded use of proton therapy in the last decade, further research is needed to improve understanding of the biologic effects and SMN risks associated with proton (and neutron) exposures compared with photon exposures.
3. Systemic treatments
Some systemic anti-cancer therapies, including chemotherapeutic agents, hormone therapy and possibly immunomodulators, have been associated with increased risk of developing SMNs. The most well established association is for myeloid neoplasms, primarily therapy-related acute myeloid leukaemia (t-AML) and myelodysplastic syndrome (t-MDS) [26]. Relative risks (RRs) for t-AML/t-MDS tend to be very high (10–100-fold increased) but the absolute excess number of cases is rather low due to the low background risk [27,28]. Chemotherapies with well-known leukaemogenic potential include alkylating agents, topoisomerase II inhibitors and antimetabolites [7,26]. Dose-dependent risk of t-AML/t-MDS has been reported after almost all alkylating agents, such as mechlorethamine, cyclophosphamide, procarbazine, melphalan, busulfan and cisplatin [29–32], as well as topoisomerase II inhibitors [33–37]. However, the leukaemogenicity of different agents varies substantially. For example, melphalan is 10 times more leukaemogenic than cyclophosphamide [38], and RRs associated with mitoxantrone are five times higher than those for anthracyclines [35]. T-AML after alkylating agent exposure typically arises after a latency period of 5–8 years, is frequently preceded by myelodysplastic syndrome (MDS), and often has a complex karyotype with chromosome 5/7 abnormalities [39,40]. In contrast, t-AML after topoisomerase II inhibitors typically arises in <3 years, is rarely preceded by MDS, and is characterised by 11q23 or 15/17 aberrations [41]. Evidence increasingly suggests that chemotherapy also may play a role in the development of non-haematologic SMNs, which typically occur >10 years after exposure [7]. Alkylating agents have been reported to increase risks for lung, thyroid, gastrointestinal and bladder cancers as well as sarcoma. For example, lung cancer risk after HL is increased 2–>4-fold with increasing number of cycles of alkylating agent-containing chemotherapy, particularly mustine, vincristine, procarbazine, prednisolone (MOPP) [42–46]. Among childhood cancer survivors, receipt of any alkylating agent has been associated with 2.4-fold increased risk for thyroid cancer; receipt of procarbazine and platinum has been associated with 3.2- and 8.6-fold increased risk, respectively, of gastrointestinal cancer, and both alkylating agents and anthracyclines have been associated with sarcoma risk [16,47–49]. The causal link between cyclophosphamide and bladder cancer represents one of the few established relationships between a specific alkylating agent and carcinogenesis at a specific site, likely as a result of direct genotoxic exposure of bladder epithelium from cyclophosphamide metabolites [50,51]. Procarbazine-related risks for the gastrointestinal tract also may be related to direct exposure [12,16,52,53], whereas the mechanisms of carcinogenesis for agents administered intravenously and for other malignancies (e.g. lung) are unknown.
With increasing use of systemic therapy and rapid introduction of new drugs into the clinic, further research into potential risks of SMNs following systemic therapy is needed. Large sample sizes, long-term follow-up and a diverse patient population will be particularly important because the frequent use of combined modality therapy and multidrug regimens renders it difficult to disentangle the effects of specific agents. Examples are the introduction of new drugs such as taxanes, lenalidomide and monoclonal antibodies (e.g. rituximab); the use of more intensified regimens, growth factor support and targeted therapy (e.g. tyrosine kinase inhibitors); and increasing frequency of successful treatment for relapse, often resulting in larger cumulative doses. The importance of pursuing such research is emphasised by preliminary reports of SMN risks. Data on the role of taxanes in the development of t-AML are conflicting [54–58], partly due to their use in combination with various cytotoxic agents and in different intensity. In addition, taxane-containing regimens also frequently use granulocyte-colony stimulating factor (G-CSF), whose leukaemogenic potential continues to be debated [54,58,59]. Lenalidomide, an immunomodulator used in multiple myeloma treatment, may increase t-AML risk [60]. For rituximab-containing regimens (often used in non-Hodgkin lymphoma), there is also suggestive evidence for an association with acute myeloid leukaemia (AML) [61,62], but generally studies investigating the risk of SMNs with the wider use of monoclonal antibodies are lacking [63]. Finally, in terms of SMN risks and hormonal treatments, tamoxifen has been associated with two- to five fold duration-dependent increased risk of endometrial cancer [64–66]. However, long-term effects of aromatase inhibitors, which are increasingly used with or without tamoxifen, are not known.
4. Interactions between treatments
Evaluation of the carcinogenic effects of therapy is often complicated by the fact that therapeutic agents are frequently given in combination. SMN risk after combined modality treatment (radiotherapy and systemic therapy combined) may differ from the summed risks seen after either treatment type alone – with larger risks after combined modality treatment implying synergistic effects of different treatments, and smaller risks implying antagonistic effects.
Although of great interest, few high quality studies with sufficient sample size to evaluate potential treatment interactions have been conducted. A recently published international study on stomach cancer risk after HL treatment provided the first robust evidence for the possibility of supramultiplicative interaction between exposure to alkylating chemotherapy and irradiation with regard to solid cancer risk [12]. Radiation doses to the stomach of ⩾25 Gy combined with exposure to high-dose procarbazine (⩾5600 mg/m2) were associated with an odds ratio (OR) of 77.5 (95% CI, 14.7–1452), compared to ORs of 2.8 and 1.2 for exposure to ⩾25 Gy of radiation alone and exposure to high-dose procarbazine (⩾5600 mg/m2) alone, respectively (Fig. 2).
Fig. 2.
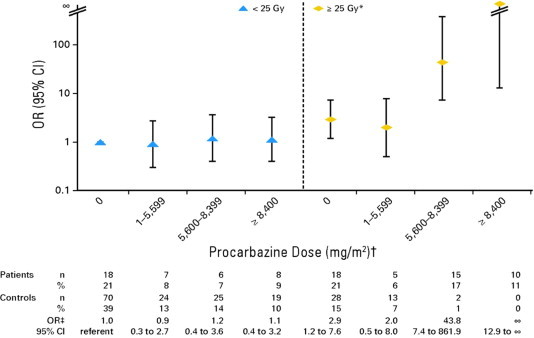
Risk of stomach cancer after Hodgkin lymphoma in relation to radiation dose to stomach and procarbazine dose. OR, odds ratio. (∗) Radiation dose was estimated to stomach tumour location (matched location for controls). (†) Assuming procarbazine dose of 1400 mg/m2 per cycle (14 days × 100 mg/m2 per day), categories correspond to zero, one to three, four to five, and ⩾six cycles of MOPP (mechlorethamine, vincristine, procarbazine and prednisone) or MOPP-like regimens. Other protocols (e.g. MOPP-ABV [MOPP–doxorubicin, bleomycin and vinblastine], BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone]) include procarbazine dose of 700 mg/m2 per cycle. (‡) ORs and 95% confidence intervals (CIs) were adjusted for receipt of any dacarbazine and unknown radiation dose.
From: Morton et al. [12].
In contrast with this report on stomach cancer risk, two studies have reported no apparent synergism between chemotherapy and radiotherapy on subsequent lung cancer risk [42,43]. Although the OR for lung cancer was 5.9 (95% CI 2.7–13.5) after >5 Gy to the lung without chemotherapy and 4.2 (95% CI, 2.1–8.8) after alkylating agents alone, the estimated RR of 8.0 (95% CI, 3.6–18.5) for patients who received both treatments did not deviate from the risk expected if the risks for the individual treatments were summed [42].
Results are inconsistent regarding the role of combined modality treatment and risk of subsequent leukaemia. However, in the largest case–control study to date, among HL patients with a given dose of alkylating chemotherapy, risk of leukaemia did not consistently increase with higher radiation dose while leukaemia risk clearly increased with increasing dose of alkylating agents within categories of radiation dose [67]. However even in the lowest dose category in this study (<10 Gy) many patients may have been exposed to myeloablative doses already and it may be that synergy between radiation and alkylating chemotherapy is only present in the lower dose range (<2–4 Gy).
The strongly reduced breast cancer risk among female childhood cancer and HL patients treated with chest irradiation and alkylating agent-containing chemotherapy compared to patients treated solely with chest irradiation may provide an example of antagonism between treatment modalities [13,25,68–71]. In a large British HL cohort breast cancer risk was 4.6-fold increased compared to the female general population among those treated with combined modalities and 14.4-fold after radiotherapy alone, while no breast cancers occurred among women treated solely with chemotherapy [68]. Breast cancer risk in patients treated with chest irradiation was also reduced in those patients who additionally had received pelvic radiation, suggesting that premature ovarian failure is a driving force behind this risk reduction [13,69,71]. Another example of a possible antagonistic relation between treatments is the risk of thyroid cancer after childhood cancer. In a long-term follow-up study of childhood cancer survivors, chemotherapy-related risks were only evident among children who did not receive radiation (OR = 4.6, 95% CI −.8–86.3) or who received <20 Gy to the thyroid (OR = 4.0, 95% CI 1.4–16.5), but not for children who received ⩾20 Gy to the thyroid (OR = 1.1, 95% CI 0.6–2.1) [72].
Because of the increasing use of combined modality therapy, additional large studies with high quality treatment data, including dosimetry data (dose–volume information) and cumulative doses of systemic therapy, are needed to obtain sufficient power to investigate potential interactions between radiation and chemotherapy as well as between various chemotherapy agents. Furthermore, additional research is needed to elucidate the mechanisms by which treatments may interact.
5. Modification of treatment-related second cancer risks by age
Numerous studies support the importance of age at initial treatment and age at SMN occurrence (i.e. attained age) as modifiers of treatment-related SMN risks. Generally, but not always, the relative risk (RR) of developing a SMN compared with the general population is higher at younger versus older ages, whereas the pattern of excess absolute risk (EAR) across different ages vary by type of both first and second cancer (Fig. 1) [20,21,25,27,73]. For example, a study of breast cancer after HL revealed that as the age at HL diagnosis increased from ⩽20 to 41–50 years, the RR declined from 17.9 to 1.4 and the EAR declined from 79 to 11 cases per 10,000 persons per year [25]. However, another study suggested that as the age at breast cancer occurrence (i.e. attained age) increased, the RR also declined but the EAR increased [20]. In contrast, the risk (RR and EAR) for thyroid cancer after HL did not vary by attained age [20]. Because most cancer rates in the general population increase substantially with increasing age, even steady or declining RRs with increasing age can be associated with strikingly increasing EARs, such as the pattern described above for breast cancer after HL [20].
Fig. 1.
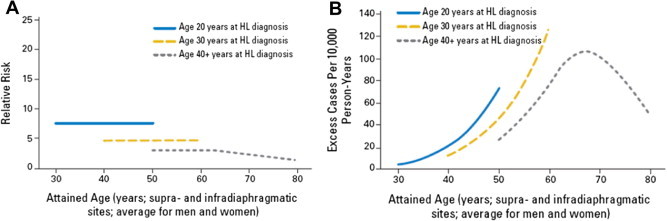
Relative risk (RR) and Absolute Excess Risk of supra- and infradiaphragmatic solid cancers according to age at Hodgkin lymphoma (HL) diagnosis and attained age. Panel A: RR of supra- and infradiaphragmatic solid cancers. Panel B: AER of supra- and infradiaphragmatic solid cancers.
From: Hodgson et al. [20].
The key factors that contribute to differences in SMN risks by age include the baseline cancer rate in the general population, potential differences in tissue susceptibility to radiation or chemotherapy exposure at different ages and the roles of other cancer risk factors. More research is needed to understand the independent and joint effects of age at exposure and attained age, particularly expanding beyond the research in survivors of HL to include survivors of other primary malignancies. Additionally, much of the research to date has focused on age-related effects of radiotherapy exposures. For example, childhood cancer survivors receiving cranial irradiation before age 5 years reportedly have significantly higher risk of subsequent glioma than children irradiated at age ⩾5 [15]. Similarly, thyroid cancer risk is higher among children irradiated before age 10 years [17]. These particularly elevated risks at younger ages have been attributed to radiosensitivity of developing tissues. For second primary breast cancer, the data on the role of age at exposure are conflicting. Although some studies have suggested that risks are the highest for girls receiving chest-directed radiotherapy during puberty, with lower risks both pre- and post-puberty, additional research with sufficient numbers of individuals exposed at a wide range of ages is needed [13,70,74,75]. Much less is known about potential age-related effects of chemotherapy exposures.
With the expansion of research into non-treatment risk factors for SMNs, the role of lifestyle and medical history factors in SMN aetiology is increasingly recognised. Because these exposures accumulate as ias individuals age, (Must be plural you can also say : As people age) age, they may play a greater role in SMNs occurring among older cancer survivors [76]. Further research will be needed to understand whether potential interactions between treatment and other cancer risk factors may depend on age.
6. Genetic susceptibility to treatment-related second cancer risks
Although genetic susceptibility to cancer in general is well established, little is known about genetic susceptibility to treatment-related SMNs. Individuals with Li–Fraumeni syndrome have long been thought to be highly radio-sensitive, yet no study has had the sample size and detailed treatment exposure and genetics data to quantify the risks, as exemplified by a recent review of the literature that yielded a total of 23 patients from 10 studies and case reports [77]. Outside of the context of cancer predisposition syndromes, most studies have investigated SMN risks in relation to specific genes, selected based on understanding of biologic pathways of drug metabolism and carcinogenesis. These studies have reported associations for variants in oxidative stress, DNA detoxification and DNA repair genes with treatment-related leukaemia [78–84], Ataxia Telangiectasia (AT) gene variants and mutations in DNA Damage Repair Pathway (DDRP) genes with contralateral breast cancer [85,86], and FGFR2 with breast cancer after supradiaphragmatic radiotherapy for HL [85].
More recently, genome-wide association studies (GWAS), which agnostically interrogate hundreds of thousands-to-millions of variants across the genome [87], have revealed genomic regions associated with treatment-related leukaemia [88] and with SMNs occurring among HL survivors initially treated with radiotherapy [89], supporting the idea of genetic susceptibility to treatment-related SMNs. A key limitation of previous studies has been a lack of detailed treatment data and/or sufficient sample size to quantify the effect of specific variants in individuals with differing treatment exposures (e.g. specific radiation dose). Integration of treatment exposure data to future genetic studies and replicating the findings across different patient populations will be critical for translating the findings into clinical practice. Because of the large sample sizes for such studies, international collaboration will be essential. Lending further support to the importance of this research area, several GWAS have identified genomic regions associated with toxicity after radiotherapy [90,91]. As such research expands, it will be important to investigate whether individuals who are susceptible to one treatment-related adverse effect may also be susceptible to others.
7. Modification of treatment-associated second cancer risk by lifestyle and environmental factors
Other cancer risk factors can play an important role in the development of SMNs. However, only a few studies have addressed whether these other factors may modify the risk of treatment-related SMNs, with most studies to date focusing on tobacco use and reproductive factors.
The influence of smoking on the risk of treatment-associated lung cancer has been examined in several studies [42,92–95]. In HL survivors, a large international case–control study examined lung cancer risk in relation to radiation dose, chemotherapy and smoking [14,42]. The increased RRs from smoking appeared to multiply the elevated risks from radiotherapy as well as chemotherapy, with the joint effects of smoking and treatment significantly stronger than the sum of the individual effects. Compared with non-smokers who received less than 5 Gy to the lung area and no alkylating agents, the largest risk (RR = 49.1) of lung cancer was observed among moderate-to-heavy smokers given both radiotherapy and alkylating agents, with a RR of 7.2 for non-smokers given radiotherapy and chemotherapy (Table 1). It was estimated that 9.6% of all lung cancers after HL were due to treatment, 24% were due to smoking, and 63% were due to treatment and smoking in combination [42]. The effect of smoking on radiation-associated lung cancer risk after breast cancer has also been examined. Several studies observed that excess lung cancer risk following post-mastectomy radiotherapy is restricted to smokers, pointing to strong interaction [93–95].
Table 1.
Risk of lung cancer in patients with Hodgkin lymphoma (HL) according to type of treatment and smoking category.a
| Treatment for Hodgkin’s disease |
RR (95% CI) by smoking category (No. of case patients; control patients)b |
||
|---|---|---|---|
| Radiation ⩾5 Gy | Alkylating agents | Non-smoker, light, otherc | Moderate-heavyd |
| No | No | 1.0e | 6.0 (1.9–20.4) |
| Yes | No | 7.2 (2.9–21.2) | 20.2 (6.8–68) |
| No | Yes | 4.3 (1.8–11.7) | 16.8 (6.2–53) |
| Yes | Yes | 7.2 (2.8–21.6) | 49.1 (15.1–187) |
Adapted from Travis et al. (2002) [42].
Represents estimated tobacco smoking habit 5 years before diagnosis date of lung cancer and corresponding date in control patients, with the use of information recorded up to 1 year before these dates. RR, relative risk; 95% CI, 95% confidence interval.
This group includes non-smokers, light current cigarette smokers (less than one pack per day), former cigarette smokers, smokers of cigar and pipes only and patients for whom tobacco smoking habit was not stated.
Moderate (one to two packs per day) and heavy (two or more packs per day) current cigarette smokers.
Reference group.
Several studies have examined the effect of reproductive factors on the risk of radiation-associated breast cancer. Menopausal age has been shown to modify the strongly increased risk of breast cancer in survivors of HL and childhood cancer treated with chest radiotherapy [13,25,69,75,96]. In a Dutch study [25], 30% of female HL survivors reached menopause before age 41 (related to intensive chemotherapy); such an early menopause was associated with a 60% (95% CI, 20–80%) reduced risk of RT-associated breast cancer. Women with less than 10 years of intact ovarian function after radiotherapy had a 70% (95% CI, 40–80%) decreased risk of breast cancer compared with women with 10–20 years of ovarian function after irradiation, while those with more than 20 years of intact ovarian function after radiotherapy had 5.3-fold (95% CI, 2.9–9.9) increased risk of breast cancer. These risk reductions were observed among women treated before age 31 but not among women treated between ages 31 and 40, possibly because these women were closer to natural menopause at time of treatment [25]. These results indicate that ovarian hormones are a crucial factor to promote tumorigenesis once RT has produced an initiating event. A recent British study observed stronger radiation-associated risk of breast cancer among women who were irradiated close to menarche, suggesting greater carcinogenicity of radiation when the breast is developing [75]. No modifying effects have been observed for other risk factors such as age at first birth, parity and weight, but none of the published studies included enough women to detect smaller interaction effects or risk modification by infrequent exposures. Studies had only limited information on hormone replacement therapy [25,75,96,97].
Knowledge regarding modifying effects of lifestyle and reproductive factors on treatment-associated SMN risk is only beginning to emerge. International collaborative studies are needed, including large numbers of survivors for whom not only treatment data are available, but also high-quality data on lifestyle, reproductive, environmental and occupational factors. The sequence of treatment exposures and other risk factors also deserves investigation, particularly for designing interventions to reduce treatment-related SMN risks where the treatments interact with modifiable risk factors. International pooling of data already available and data from new studies are essential to obtain sufficient power for interaction analyses allowing discrimination between additive, multiplicative and supra-multiplicative effects of treatment and other cancer risk factors.
8. Screening for second malignancy
Given the high relative and absolute risks of several SMNs identified in cancer survivors (see above), screening to detect cancers early or to detect pre-malignant lesions ought in principle to have considerable potential to reduce the incidence of and mortality from SMNs. This is dependent, however, on whether screening can detect cancers early, and whether early treatment improves prognosis. For many cancers with increased risks among certain cancer survivors, such as lung and stomach, no known screening method can affect prognosis. By contrast, for breast, cervix and colorectal cancers, there are well-established screening modalities for the general population, giving potential for screening programmes for cancer patients if they are at increased risk.
Research on screening of cancer survivors has largely focused on breast cancer after HL and childhood cancer. National recommendations on screening have been produced in the United Kingdom (UK) [98] and US [99–101], recommending that screening starts at a younger age (age 25–30 years, or 8 years after treatment), occurs more frequently (annually) and involves more modalities (Magnetic Resonance Imaging (MRI), ultrasound, mammography, alone or in combination) than in general population programmes. Research to investigate the value of such programmes, and to improve them, has been very limited, however, and take-up rates of breast screening have been found to be rather low [102,103]. Studies have shown 80–100% of tumours are detectable by mammography, and showed a recall rate greater than after general population screening [102,104–106]. In one study, mammography detected mainly Ductal Carcinoma in Situ of the breast (DCIS), while most invasive cancers were detected between screenings [106]. Breast cancers diagnosed after HL have been found more likely to be screen-detected, and more likely to be diagnosed at an earlier stage, than those in the general population [107], and there is some indication that the introduction of screening may have led to earlier stage diagnosis [108].
With respect to screening modality, it has been shown that for breast cancers occurring after HL, unlike those in BRCA mutation carriers, MRI does not have superior sensitivity to mammography [109,110]. However, mammography and MRI in combination give substantially greater sensitivity (>90%) than either alone (∼67%) [110].
Future research needs to address the age at which screening should begin, the frequency of screening and the modalities to be used. A specific issue is the benefit, if any, of screening before age 40, which has not been demonstrated in the general population or HL patients. Another issue is how long such patients need intensive screening to continue: in the UK, they revert at age 50 to the national 3-yearly screening programme, but their high continuing risk suggests more-intensive screening may be needed [71]. Most importantly, there needs to be investigation of whether screening can decrease mortality in these patients.
Research also needs to investigate whether colorectal cancer screening would benefit cancer patients at high risk [20,52] and the frequency, age at start of screening and modalities that would have benefit. Currently, in Ontario the take-up of routine colorectal cancer screening by HL patients has been found to be only 37% [103]. Research is also needed into whether the biology of SMNs differs from that of first primaries of the same site. Cancers induced by radiotherapy, chemotherapy or hormonal treatment may have a different pathogenesis, with potential different susceptibility to screening. For instance, endometrial cancers after tamoxifen treatment for breast cancer are skewed towards non-endometrioid tumours with poor survival [111,112], with potential consequences for the success of screening.
9. Intervention strategies to reduce second malignancy risk
Developing intervention strategies capable of reducing the incidence of SMNs is an attractive goal. These might be employed either during or after treatment for the first cancer although, of course, post-treatment interventions are the only options for patients already cured of a first cancer. Interventions may reduce the overall cancer causing potential of treatment by changing its dose or duration, or by focusing it more accurately on those patients who require it because of unfavourable disease characteristics. Alternatively, an additional therapeutic intervention may be identified that reduces the cancer causing potential of the treatment. Lifestyle interventions following treatment are another potentially important area of investigation.
For HL patients, radiotherapy has been identified as the main cause of solid SMNs [20]. Therefore, the wide field “mantle” [113] and “inverted Y” [114] techniques, the mainstay of HL treatment in the 1960s and 1970s, were gradually replaced by much smaller involved field approaches [115]. More recently “involved node”[116] and “involved site” [117] fields have been developed although clinical trials are required to evaluate the disease control characteristics and SMN risks associated with such techniques. “No radiotherapy” strategies have also been investigated. In a study in early stage HL, treatment with chemotherapy alone was compared with a radiotherapy based approach. When first reported this trial showed inferior disease control in the chemotherapy only arm [118] but in a subsequent analysis after longer follow-up, overall survival was superior in these patients due to an excess of SMNs and other deaths in the radiotherapy arm [119]. Although criticised for employing outmoded radiotherapy techniques, this trial raised the possibility of obtaining a better eventual outcome using chemotherapy alone. It also highlighted the importance of long follow-up if the full impact of treatment on survival in terms of both disease control and late toxicity are to be properly assessed.
More recently, response-adapted approaches whereby treatment is adjusted according to initial response have been investigated in HL [120]. A “negative” Positron Emission Tomography (PET) scan after initial chemotherapy in patients with early stage HL has identified a population with a very good prognosis without further treatment. If involved field radiotherapy is employed following chemotherapy, the 3-year progression-free survival is marginally better but this is bought at the expense of irradiating all patients, most of whom will have already been cured by chemotherapy alone.
This approach to individualisation of treatment will take a step further if prognostic gene signatures derived from tumour tissue [121] and capable of identifying low and high risk patients at diagnosis are integrated with the PET result. It can be envisaged that minimal therapy would be suitable for patients with low-risk signatures and a “negative” PET and more intensive treatment reserved for those with a high-risk signature and a “positive” PET. Clearly, in these circumstances, treatment associated with high SMN risk will be restricted to a section of the population rather than its entirety.
Additionally, therapeutic risk reduction interventions during or following treatment for the first cancer can be considered for some SMNs. In young women at high risk of breast cancer as a result of irradiation to breast tissue at a young age, anti-oestrogens, such as tamoxifen, or interventions to temporarily delayed onset of menarche may be protective and studies are currently underway to evaluate the effectiveness and acceptability of these approaches [122]. As the risk of breast cancer after chest radiotherapy at young ages is comparable to that of BRCA mutation carriers [71,123,124], bilateral prophylactic mastectomy may also be an appropriate consideration in some patients, especially when they also have a family history of breast cancer [125].
Lifestyle interventions after treatment may be effective in reducing the incidence of SMNs and should be evaluated in appropriately designed clinical trials. These interventions might include rigorous advice and help to quit smoking, reduce alcohol consumption, take regular exercise and lose weight [7,126]. Ideally, trials should incorporate predictive biomarkers so the value of the intervention can be assessed long before SMNs emerge, often many years later. Research should focus on the identification of such biomarkers.
10. Conclusions
Over the past decades much knowledge has been gained about treatment-related risk factors for SMNs. Recently, both systemic cancer treatments and radiotherapy approaches and techniques have evolved substantially. It is as yet unclear how the introduction of these new treatments will influence SMN risk. For changes in radiotherapy, such as the use of lower doses and smaller volumes, it may be possible to predict the associated SMN risk based on computational models for radiation dosimetry established on older regimens [127,128]. However, for completely new agents, drug combinations and radiation techniques, and interactions between these treatments, large studies with long-term follow-up are needed to assess SMN risk for at least several decades, in view of the long induction periods observed for older treatments. In such studies, high quality treatment data, including radiation dosimetry data (dose–volume information) and cumulative doses of systemic therapy, are essential. Complete follow-up of all patients is crucial to avoid selection bias, which can easily arise if information on SMNs is not derived from linkage with nationwide cancer registries, but from patient questionnaires or medical records. Several studies have shown that patients still under surveillance in the original treatment centre, or participants in questionnaire surveys, may represent either less healthy or healthier patients [129]. Routine collection and reporting of SMN data should become an integral part of randomised controlled trials in oncology.
Currently, very little knowledge is available about factors that may modify treatment-associated SMN risk, such as genetic variants and lifestyle or environmental factors. Although some candidate gene studies in t-AML have shown interesting results, genome-wide approaches with large sample sizes are needed to fully explore genetic susceptibility for treatment-related SMNs. For effective investigation of modifying factors large international collaborative studies with biobanking are needed to obtain sufficient power for interaction analyses. Such studies should prospectively collect not only extensive treatment and genetic information, but also high-quality data on lifestyle, reproductive and other risk factors. When addressing interaction, it is important to assess not only interaction at the multiplicative level, but also investigate whether the joint effects of treatment and a potential modifier are larger than the sum of the individual effects (interaction at the additive level), as such interaction may already impact substantially on the SMN burden of cancer survivors.
In many countries, recommendations on screening for SMN (especially breast cancer) have been issued for selected high-risk survivor groups. However, most guidelines are consensus- rather than evidence-based. Therefore, research is needed on the diagnostic value of screening tests in this specific population and the ultimate effect of screening on mortality. Effective screening is only possible with better understanding of the pathogenesis of treatment-related SMNs. Currently, such knowledge is lacking, so there is a strong need for studies investigating the mechanisms by which different treatments affect SMN pathogenesis, the clinicopathological characteristics of treatment-related SMNs and their prognosis.
Potentially, the burden of SMNs may also be reduced by lifestyle or drug interventions. Although smoking cessation can strongly reduce the risk of treatment-related lung cancer, no studies with regard to smoking or other interventions have been conducted. For future patients the most promising way to reduce SMN risk is through the introduction of new treatments with lesser carcinogenic potential and equivalent cure rates. Early biomarkers for SMN risk are currently not available, but would be helpful to use as intermediate end-points in such trials. Finally, prediction models for SMN risk are needed to enable more individualised treatment choices accounting for SMN risk.
While this paper focuses on SMN risk alone, it is important that late effects research incorporates also other adverse outcomes and assesses the total burden of adverse health outcomes in cancer survivors. Such studies could also investigate whether individuals who are susceptible to treatment-related SMNs, may also be susceptible to other adverse effects.
Conflict of interest statement
None declared.
References
- 1.Netherlands Cancer Registry. Personal communication Otto Visser, www.iknl.nl.; 2013.
- 2.Morton L., Onel K., Curtis R.E., Hungate E., Armstrong G.T. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol. 2014 doi: 10.14694/EdBook_AM.2014.34.e57. [in press] [DOI] [PubMed] [Google Scholar]
- 3.Aleman B.M.P., van den Belt-Dusebout A.W., Klokman W.J., van’t Veer M.B., Bartelink H., van Leeuwen F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21(18):3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 4.Ng A.K., Bernardo M.P., Weller E., Backstrand K.H., Silver B., Marcus K.C. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20(8):2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Boice J.D., Jr., Storm H.H., Curtis R.E., Jensen O.M., Kleinerman R.A., Jensen H.S. Introduction to the study of multiple primary cancers. Natl Cancer Inst Monogr. 1985;68:3–9. [PubMed] [Google Scholar]
- 6.Hodgson D., van Leeuwen F.E. Second malignancy risk after treatment of Hodgkin lymphoma. In: Engert A.H.S., editor. Hematologic malignancies: Hodgkin lymphoma a comprehensive update on diagnostics and clinics. Second ed. Springer Verlag; Berlin Heidelberg: 2011. pp. 305–331. [Google Scholar]
- 7.Travis L.B., Wahnefried W.D., Allan J.M., Wood M.E., Ng A.K. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 8.United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2006. Report: effect of ionizing radiation, Volume 1 – Report to the General Assembly, with Scientific Annexes A and B. Vienna; 2008.
- 9.IARC (International Agency for Research on Cancer). Monographs on the evaluation of carcinogenic risks to humans. Vol. 75. Ionizing radiation, part 1: X- and gamma-radiation, and neutrons. Lyon, France; 2000.
- 10.IARC (International Agency for Research on Cancer) Some antiviral and antineoplastic drugs, and other pharmaceutical agents. IARC Monogr Eval Carcinog Risk Chem Hum. 2000;76:177. [Google Scholar]
- 11.Committee to assess health risks from exposure to low levels of ionizing radiation: health risks from exposure to low levels of ionizing radiation, BEIR VII< Phase 2. Washington, DC; 2006. [PubMed]
- 12.Morton L.M., Dores G.M., Curtis R.E., Lynch C.F., Stovall M., Hall P. Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol. 2013;31(27):3369–3377. doi: 10.1200/JCO.2013.50.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis L.B., Hill D.A., Dores G.M., Gospodarowicz M., van Leeuwen F.E., Holowaty E. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin’s disease. JAMA. 2003;290(4):465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert E.S., Stovall M., Gospodarowicz M., van Leeuwen F.E., Andersson M., Glimelius B. Lung cancer after treatment for Hodgkin’s disease: focus on radiation effects. Radiat Res. 2003;159(2):161–173. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Neglia J.P., Robison L.L., Stovall M., Liu Y., Packer R.J., Hammond S. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 16.Henderson T.O., Rajaraman P., Stovall M., Constine L.S., Olive A., Smith S.A. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2012;84(1):224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatti P.V.L., Ronckers C.M., Sigurdson A.J., Stovall M., Smith S.A., Weathers R. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the Childhood Cancer Survivor Study. Radiat Res. 2010;174(6):741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boice J.D., Jr., Blettner M., Kleinerman R.A., Stovall M., Moloney W.C., Engholm G. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79:1295–1311. [PubMed] [Google Scholar]
- 19.Berrington de Gonzalez A., Gilbert E., Curtis R., Inskip P., Kleinerman R., Morton L. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose–response relationship. Int J Radiat Oncol Biol Phys. 2013;86(2):224–233. doi: 10.1016/j.ijrobp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson D.C., Gilbert E.S., Dores G.M., Schonfeld S.J., Lynch C.F., Storm H. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25(12):1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 21.Travis L.B., Fossa S.D., Schonfeld S.J., McMaster M.L., Lynch C.F., Storm H. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97(18):1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 22.Yoon M., Ahn S.H., Kim J., Shin D.H., Park S.Y., Lee S.B. Radiation-induced cancers from modern radiotherapy techniques: intensity-modulated radiotherapy versus proton therapy. Int J Radiat Oncol Biol Phys. 2010;77(5):1477–1485. doi: 10.1016/j.ijrobp.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Murray L.H.A., Hoskin P., Siebert F.A., Venselaar J. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol. 2014;110(2):213–228. doi: 10.1016/j.radonc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgson D.C. Late effects in the era of modern therapy for Hodgkin lymphoma. ASH Educ Prog Book. 2011;2011(1):323–329. doi: 10.1182/asheducation-2011.1.323. [DOI] [PubMed] [Google Scholar]
- 25.De Bruin M.L., Sparidans J., van’t Veer M.B., Noordijk E., Louwman M.W., Zijlstra J.M. Breast cancer risk in female survivors of Hodgkin’s lymphoma; lower risk after smaller radiation volumes. J Clin Oncol. 2009;27(26):4229–4231. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 26.Vardiman J.W. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010;184(1–2):16–20. doi: 10.1016/j.cbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Morton L.M., Dores G.M., Tucker M.A., Kim C.J., Onel K., Gilbert E.S. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone G., Fianchi L., Pagano L., Voso M.T. Incidence and susceptibility to therapy-related myeloid neoplasms. Chem Biol Interact. 2010;184(1–2):39–45. doi: 10.1016/j.cbi.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y., Wang H., Zhou S., Yu M., Wang X., Fu K. Risk of second malignant neoplasms after cyclophosphamide-based chemotherapy with or without radiotherapy for non-Hodgkin lymphoma. Leuk Lymphoma. 2013;54(7):1396–1404. doi: 10.3109/10428194.2012.743657. [DOI] [PubMed] [Google Scholar]
- 30.Diamandidou E., Buzdar A.U., Smith T.L., Frye D., Witjaksono M., Hortobagyi G.N. Treatment-related leukemia in breast cancer patients treated with fluorouracil-doxorubicin-cyclophosphamide combination adjuvant chemotherapy: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 1996;14(10):2722–2730. doi: 10.1200/JCO.1996.14.10.2722. [DOI] [PubMed] [Google Scholar]
- 31.Travis L.B., Holowaty E.J., Bergfeldt K., Lynch C.F., Kohler B.A., Wiklund T. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340(5):351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 32.Travis L.B., Andersson M., Gospodarowicz M., van Leeuwen F.E., Bergfeldt K., Lynch C.F. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92(14):1165–1171. doi: 10.1093/jnci/92.14.1165. [DOI] [PubMed] [Google Scholar]
- 33.RamaChandran S., Ariffin H. Secondary acute myeloid leukemia after etoposide therapy for haemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2009;53(3):488–490. doi: 10.1002/pbc.22063. [DOI] [PubMed] [Google Scholar]
- 34.Praga C., Bergh J., Bliss J., Bonneterre J., Cesana B., Coombes R.C. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(18):4179–4191. doi: 10.1200/JCO.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Le Deley M.-C., Suzan F., Cutuli B., Delaloge S., Shamsaldin A., Linassier C. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25(3):292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 36.Smith R.E., Bryant J., DeCillis A., Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21(7):1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 37.Boshoff C., Begent R.H., Oliver R.T., Rustin G.J., Newlands E.S., Andrews R. Secondary tumours following etoposide containing therapy for germ cell cancer. Ann Oncol. 1995;6:35–40. doi: 10.1093/oxfordjournals.annonc.a059037. [DOI] [PubMed] [Google Scholar]
- 38.Curtis R.E., Boice J.D., Jr., Stovall M., Bernstein L., Greenberg R.S., Flannery J.T. Risk of leukemia after chemotherapy and radiation treatment for breast cancer [see comments] N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 39.Döhner H., Estey E., Amadori S., Appelbaum F.R., Büchner T., Burnett A.K. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 40.Kayser S., Döhner K., Krauter J., Köhne C.H., Horst H.A., Held G. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 41.Pendleton M., Lindsey R.H., Felix C.A., Grimwade D., Osheroff N. Topoisomerase II and leukemia. Ann N Y Acad Sci. 2014;1310(1):98–110. doi: 10.1111/nyas.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travis L.B., Gospodarowicz M., Curtis R.E., Clarke E.A., Andersson M., Glimelius B. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94(3):182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow A.J., Schoemaker M.J., Allerton R., Horwich A., Barber J.A., Cunningham D. Lung cancer after Hodgkin’s disease: a nested case–control study of the relation to treatment. J Clin Oncol. 2001;19(6):1610–1618. doi: 10.1200/JCO.2001.19.6.1610. [DOI] [PubMed] [Google Scholar]
- 44.André M., Mounier N., Leleu X., Sonet A., Brice P., Henry-Amar M. Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood. 2004;103(4):1222–1228. doi: 10.1182/blood-2003-04-1124. [DOI] [PubMed] [Google Scholar]
- 45.Mudie N.Y., Swerdlow A.J., Higgins C.D., Smith P., Qiao Z., Hancock B.W. Risk of second malignancy after non-Hodgkin’s lymphoma: a British cohort study. J Clin Oncol. 2006;24(10):1568–1574. doi: 10.1200/JCO.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow A.J., Higgins C.D., Smith P., Cunningham D., Hancock B.W., Horwich A. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096–4104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
- 47.Henderson T.O.O.K., Whitton J., Leisenring W., Neglia J., Meadows A., Crotty C. Secondary gastrointestinal cancer in childhood cancer survivors: a cohort study. Ann Int Med. 2012;156(11):757–766. doi: 10.1059/0003-4819-156-11-201206050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucker M.A., D’Angio G.J., Boice J.D., Jr., Strong L.C., Li F.P., Stovall M. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 49.Veiga L.H.S., Bhatti P., Ronckers C.M., Sigurdson A.J., Stovall M., Smith S.A. Chemotherapy and thyroid cancer risk: a report from the childhood cancer survivor study. Cancer Epidemiol Biomark Prev. 2012;21(1):92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Travis L.B., Curtis R.E., Glimelius B., Holowaty E.J., van Leeuwen F.E., Lynch C.F. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1995;87:524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 51.Bermejo J.L., Sundquist J., Hemminki K. Bladder cancer in cancer patients: population-based estimates from a large Swedish study. Br J Cancer. 2009;101(7):1091–1099. doi: 10.1038/sj.bjc.6605325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nottage K., McFarlane J., Krasin M.J., Li C., Srivastava D., Robison L.L. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30(20):2552–2558. doi: 10.1200/JCO.2011.37.8760. [DOI] [PubMed] [Google Scholar]
- 53.van den Belt-Dusebout A.W., Aleman B.M., Besseling G., de Bruin M.L., Hauptmann M., van’t Veer L.J. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75(5):1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 54.Mackey J.R., Martin M., Pienkowski T., Rolski J., Guastalla J.-P., Sami A. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14(1):72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 55.Vay A., Kumar S., Seward S., Semaan A., Schiffer C.A., Munkarah A.R. Therapy-related myeloid leukemia after treatment for epithelial ovarian carcinoma: an epidemiological analysis. Gynecol Oncol. 2011;123(3):456–460. doi: 10.1016/j.ygyno.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 56.Jones S., Holmes F.A., O’Shaughnessy J., Blum J.L., Vukelja S.J., McIntyre K.J. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol. 2009;27(8):1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 57.Petrelli F., Borgonovo K., Cabiddu M., Lonati V., Barni S. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):335–346. doi: 10.1007/s10549-012-2121-6. [DOI] [PubMed] [Google Scholar]
- 58.Mamounas E.P., Bryant J., Lembersky B., Fehrenbacher L., Sedlacek S.M., Fisher B. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP b-28. J Clin Oncol. 2005;23(16):3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 59.Lyman G.H., Dale D.C. Long-term outcomes of myeloid growth factor treatment. J Natl Compr Canc Netw. 2011;9(8):945–952. doi: 10.6004/jnccn.2011.0077. [DOI] [PubMed] [Google Scholar]
- 60.Palumbo A., Bringhen S., Kumar S.K., Lupparelli G., Usmani S., Waage A. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15(3):333–342. doi: 10.1016/S1470-2045(13)70609-0. [DOI] [PubMed] [Google Scholar]
- 61.Zhao J., Xu Z., Liu D., Lu Q. Rituximab and new regimens for indolent lymphoma: a brief update from 2012 ASCO Annual Meeting. Cancer Cell Int. 2012;12(1):38. doi: 10.1186/1475-2867-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y., Tang G., Medeiros L.J., McDonnell T.J., Keating M.J., Wierda W.G. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25(2):237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 63.Baldo B. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. doi: 10.4161/onci.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swerdlow A.J., Jones M.E. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case–control study. J Natl Cancer Inst. 2005;97:375–384. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 65.Bergman L., Beelen M.L., Gallee M.P., Hollema H., Benraadt J., van Leeuwen F.E. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356(9233):881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 66.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) DC, Davies C., Godwin J., Gray R., Clarke M., Cutter D. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet Oncol. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaldor J.M., Day N.E., Clarke E.A., van Leeuwen F.E., Henry-Amar M., Fiorentino M.V. Leukemia following Hodgkin’s disease [see comments] N Engl J Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- 68.Swerdlow A.J., Barber J.A., Hudson G.V., Cunningham D., Gupta R.K., Hancock B.W. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18(3):498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 69.van Leeuwen F.E., Klokman W.J., Stovall M., Dahler E.C., van’t Veer M.B., Noordijk E.M. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95(13):971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 70.Inskip P.D., Robison L.L., Stovall M., Smith S.A., Hammond S., Mertens A.C. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27(24):3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swerdlow A.J., Cooke R., Bates A., Cunningham D., Falk S.J., Gilson D. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a national cohort study. J Clin Oncol. 2012;30(22):2745–2752. doi: 10.1200/JCO.2011.38.8835. [DOI] [PubMed] [Google Scholar]
- 72.Veiga L.H.L.J., Anderson H., de Vathaire F., Tucker M., Bhatti P., Schneider A. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res. 2012;178(4):365–376. doi: 10.1667/rr2889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng A., Kenney L., Gilbert E., Travis L. Secondary malignancies across the age spectrum. Sem Radiat Oncol. 2010;20(1):67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Little M.P., Weiss H.A., Boice J.D., Jr., Darby S.C., Day N.E., Muirhead C.R. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat Res. 1999;152(3):280–292. [PubMed] [Google Scholar]
- 75.Cooke R., Jones M.E., Cunningham D., Falk S.J., Gilson D., Hancock B.W. Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer. 2013;108(11):2399–2406. doi: 10.1038/bjc.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morton L.M., Chanock S.J. A step toward slaying the hydra of second cancers. Nat Med. 2011;17(8):924–925. doi: 10.1038/nm.2428. [DOI] [PubMed] [Google Scholar]
- 77.Suri J., Rednam S., Teh B., Butler E., Paulina A. Subsequent malignancies in patients with Li–Fraumeni syndrome treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(2, supplement):S71–S72. [Google Scholar]
- 78.Larson R.A., Wang Y., Banerjee M., Wiemels J., Hartford C., Beau M.M.L. Prevalence of the Inactivating 609C→T Polymorphism in the NAD(P)H:Quinone Oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia. Blood. 1999;94(2):803–807. [PubMed] [Google Scholar]
- 79.Allan J.M., Wild C.P., Rollinson S., Willett E.V., Moorman A.V., Dovey G.J. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc Natl Acad Sci. 2001;98(20):11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Worrillow L.J., Smith A.G., Scott K., Andersson M., Ashcroft A.J., Dores G.M. Polymorphic MLH1 and risk of cancer after methylating chemotherapy for Hodgkin lymphoma. J Med Genet. 2008;45(3):142–146. doi: 10.1136/jmg.2007.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Worrillow L.J., Travis L.B., Smith A.G., Rollinson S., Smith A.J., Wild C.P. An intron splice acceptor polymorphism in hMSH2 and risk of leukemia after treatment with chemotherapeutic alkylating agents. Clin Cancer Res. 2003;9(8):3012–3020. [PubMed] [Google Scholar]
- 82.Seedhouse C., Bainton R., Lewis M., Harding A., Russell N., Das-Gupta E. The genotype distribution of the XRCC1gene indicates a role for base excision repair in the development of therapy-related acute myeloblastic leukemia. Blood. 2002;100(10):3761–3766. doi: 10.1182/blood-2002-04-1152. [DOI] [PubMed] [Google Scholar]
- 83.Seedhouse C., Faulkner R., Ashraf N., Das-Gupta E., Russell N. Polymorphisms in genes involved in homologous recombination repair interact to increase the risk of developing acute myeloid leukemia. Clin Cancer Res. 2004;10(8):2675–2680. doi: 10.1158/1078-0432.ccr-03-0372. [DOI] [PubMed] [Google Scholar]
- 84.Bhatla D., Gerbing R.B., Alonzo T.A., Mehta P.A., Deal K., Elliott J. DNA repair polymorphisms and outcome of chemotherapy for acute myelogenous leukemia: a report from the Children’s Oncology Group. Leukemia. 2007;22(2):265–272. doi: 10.1038/sj.leu.2405000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brooks J.D., Teraoka S.N., Reiner A.S., Satagopan J.M., Bernstein L., Thomas D.C. Variants in activators and downstream targets of ATM, radiation exposure, and contralateral breast cancer risk in the WECARE study. Hum Mutat. 2012;33(1):158–164. doi: 10.1002/humu.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broeks A., Braaf L.M., Huseinovic A., Nooijen A., Urbanus J., Hogervorst F.B. Identification of women with an increased risk of developing radiation-induced breast cancer: a case only study. Breast Cancer Res. 2007;9(2):R26. doi: 10.1186/bcr1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung C., Chanock S. Current status of genome-wide association studies in cancer. Hum Genet. 2011;130(1):59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
- 88.Knight J.A., Skol A.D., Shinde A., Hastings D., Walgren R.A., Shao J. Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood. 2009;113(22):5575–5582. doi: 10.1182/blood-2008-10-183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Best T., Li D., Skol A.D., Kirchhoff T., Jackson S.A., Yasui Y. Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med. 2011;17(8):941–943. doi: 10.1038/nm.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerns S.L., Stock R.G., Stone N.N., Blacksburg S.R., Rath L., Vega A. Genome-wide association study identifies a region on chromosome 11q14.3 associated with late rectal bleeding following radiation therapy for prostate cancer. Radiother Oncol. 2013;107(3):372–376. doi: 10.1016/j.radonc.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kerns S.L., Stone N., Stock R.G., Rath L., Ostrer H., Rosenstein B.S. A 2-stage genome-wide association study to identify single nucleotide polymorphisms associated with development of urinary symptoms after radiotherapy for prostate cancer. J Urol. 2013;190(1):102–108. doi: 10.1016/j.juro.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 92.van Leeuwen FE, Klokman WJ, Stovall M, Hagenbeek A, van den Belt-Dusebout AW, Noyon R. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. J Natl Cancer Inst. 1995;87(20):1530–1537. doi: 10.1093/jnci/87.20.1530. [DOI] [PubMed] [Google Scholar]
- 93.Neugut A.I., Murray T., Santos J., Amols H., Hayes M.K., Flannery J.T. Increased risk of lung cancer after breast cancer radiation therapy in cigarette smokers [see comments] Cancer. 1994;73:1615–1620. doi: 10.1002/1097-0142(19940315)73:6<1615::aid-cncr2820730612>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 94.Kaufman E.L., Jacobson J.S., Hershman D.L., Desai M., Neugut A.I. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol. 2008;26(3):392–398. doi: 10.1200/JCO.2007.13.3033. [DOI] [PubMed] [Google Scholar]
- 95.Prochazka M., Hall P., Gagliardi G., Granath F., Nilsson B.N., Shields P.G. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23(30):7467–7474. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 96.Hill D.A., Gilbert E., Dores G.M., Gospodarowicz M., van Leeuwen F.E., Holowaty E. Breast cancer risk following radiotherapy for Hodgkin lymphoma: modification by other risk factors. Blood. 2005;106(10):3358–3365. doi: 10.1182/blood-2005-04-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kenney L.B., Yasui Y., Inskip P.D., Hammond S., Neglia J.P., Mertens A.C. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 98.Ralleigh G., Given-Wilson R. Breast cancer risk and possible screening strategies for young women following supradiaphragmatic irradiation for Hodgkin’s disease. Clin Radiol. 2004;59(8):647–650. doi: 10.1016/j.crad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 100.Mulder R.L., Kremer L.C.M., Hudson M.M., Bhatia S., Landier W., Levitt G. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14(13):e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henderson T., Amsterdam A., Bhatia S., Hudson M., Meadows A., Neglia J. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152(7):444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Howell S.J., Searle C., Goode V., Gardener T., Linton K., Cowan R.A. The UK national breast cancer screening programme for survivors of Hodgkin lymphoma detects breast cancer at an early stage. Br J Cancer. 2009;101(4):582–588. doi: 10.1038/sj.bjc.6605215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodgson D.C., Grunfeld E., Gunraj N., Del Giudice L. A population-based study of follow-up care for Hodgkin lymphoma survivors: opportunities to improve surveillance for relapse and late effects. Cancer. 2010;116(14):3417–3425. doi: 10.1002/cncr.25053. [DOI] [PubMed] [Google Scholar]
- 104.Yahalom J., Petrek J.A., Biddinger P.W., Kessler S., Dershaw D.D., McCormick B. Breast cancer in patients irradiated for Hodgkin’s disease: a clinical and pathologic analysis of 45 events in 37 patients [see comments] J Clin Oncol. 1992;10:1674–1681. doi: 10.1200/JCO.1992.10.11.1674. [DOI] [PubMed] [Google Scholar]
- 105.Diller L., Medeiros Nancarrow C., Shaffer K., Matulonis U., Mauch P., Neuberg D. Breast cancer screening in women previously treated for Hodgkin’s disease: a prospective cohort study. J Clin Oncol. 2002;20(8):2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 106.Lee L., Pintilie M., Hodgson D.C., Goss P.E., Crump M. Screening mammography for young women treated with supradiaphragmatic radiation for Hodgkin’s lymphoma. Ann Oncol. 2008;19(1):62–67. doi: 10.1093/annonc/mdm440. [DOI] [PubMed] [Google Scholar]
- 107.Elkin E.B., Klem M.L., Gonzales A.M., Ishill N.M., Hodgson D., Ng A.K. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin’s lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011;29(18):2466–2473. doi: 10.1200/JCO.2010.32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolden S.L., Hancock S.L., Carlson R.W., Goffinet D.R., Jeffrey S.S., Hoppe R.T. Management of breast cancer after Hodgkin’s disease. J Clin Oncol. 2000;18(4):765–772. doi: 10.1200/JCO.2000.18.4.765. [DOI] [PubMed] [Google Scholar]
- 109.Sung J.S., Malak S.F., Bajaj P., Alis R., Dershaw D.D., Morris E.A. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011;261(2):414–420. doi: 10.1148/radiol.11110091. [DOI] [PubMed] [Google Scholar]
- 110.Ng A.K., Garber J.E., Diller L.R., Birdwell R.L., Feng Y., Neuberg D.S. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of hodgkin lymphoma. J Clin Oncol. 2013;31(18):2282–2288. doi: 10.1200/JCO.2012.46.5732. [DOI] [PubMed] [Google Scholar]
- 111.Hoogendoorn W.E., Hollema H., van Boven H.H., Bergman E., Leeuw-Mantel G., Platteel I. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat. 2008;112(1):99–108. doi: 10.1007/s10549-007-9823-1. [DOI] [PubMed] [Google Scholar]
- 112.Jones M., van Leeuwen F., Hoogendoorn W., Mourits M., Hollema H., van Boven H. Endometrial cancer survival after breast cancer in relation to tamoxifen treatment: pooled results from three countries. Breast Cancer Res. 2012;14(3):R91. doi: 10.1186/bcr3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Page V., Gardner A., Karzmark C.J. Physical and dosimetric aspects of the radiotherapy of malignant lymphomas I the mantle field technique. Radiology. 1970;96(3):609–618. doi: 10.1148/96.3.609. [DOI] [PubMed] [Google Scholar]
- 114.Page V., Gardner A., Karzmark C.J. Physical and dosimetric aspects of the radiotherapy of malignant lymphomas II the inverted Y-technique. Radiology. 1970;96(3):619–626. doi: 10.1148/96.3.619. [DOI] [PubMed] [Google Scholar]
- 115.Engert A., Plütschow A., Eich H.T., Lohri A., Dörken B., Borchmann P. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 116.Girinsky T., van der Maazen R., Specht L., Aleman B., Poortmans P., Lievens Y. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol. 2006;79(3):270–277. doi: 10.1016/j.radonc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 117.Hoskin P.J., Díez P., Williams M., Lucraft H., Bayne M. Recommendations for the use of radiotherapy in nodal lymphoma. Clin Oncol. 2013;25(1):49–58. doi: 10.1016/j.clon.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 118.Meyer R.M., Gospodarowicz M.K., Connors J.M., Pearcey R.G., Bezjak A., Wells W.A. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(21):4634–4642. doi: 10.1200/JCO.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 119.Meyer R.M., Gospodarowicz M.K., Connors J.M., Pearcey R.G., Wells W.A., Winter J.N. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radford J., O’Doherty M., Barrington S., Qian W., Patrick P., Coltar S. Results of the 2nd planned interim analysis of the RAPID Trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1A and 2A Hodgkin lymphoma and a ‘negative’ FDG-PET scan after 3 cycles ABVD. ASH Annu Meet Abs. 2008;112(11):369. [Google Scholar]
- 121.Scott D.W., Chan F.C., Hong F., Rogic S., Tan K.L., Meissner B. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31(6):692–700. doi: 10.1200/JCO.2012.43.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palomares M. http://www.cancer.gov/clinicaltrials/search/view?cdrid=687084&version=HealthProfessional; 2013. [PubMed]
- 123.Taylor A.J., Winter D.L., Stiller C.A., Murphy M., Hawkins M.M. Risk of breast cancer in female survivors of childhood Hodgkin’s disease in Britain: a population-based study. Int J Cancer. 2007;120(2):384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 124.Moskowitz C., Chou J.F., Wolden S.L., Bernstein J.L., Malhotra J., Novetsky Friedman D. New insights into the risk of breast cancer in childhood cancer survivors treated with chest radiation: a report from the Childhood Cancer Survivor Study (CCSS) and the Women’s Environmental Cancer and Radiation Epidemiology (WECARE) Study. J Clin Oncol. 2012;30(18, Supplement) ASCO Annual Meeting Abstracts CRA9513. [Google Scholar]
- 125.Hartmann L.C., Schaid D.J., Woods J.E., Crotty T.P., Myers J.L., Arnold P.G. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340(2):77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 126.Ames B., Gold L.S., Willett W.C. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92(12):5258. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hodgson D.C., Koh E.-S., Tran T.H., Heydarian M., Tsang R., Pintilie M. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110(11):2576–2586. doi: 10.1002/cncr.23081. [DOI] [PubMed] [Google Scholar]
- 128.Ng A., Brock K.K., Sharpe M.B., Moseley J.L., Craig T., Hodgson D.C. Individualized 3D reconstruction of normal tissue dose for patients with long-term follow-up: a step toward understanding dose risk for late toxicity. Int J Radiat Oncol Biol Phys. 2012;84(4):e557–e563. doi: 10.1016/j.ijrobp.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 129.Oeffinger K.C., van Leeuwen F.E., Hodgson D.C. Methods to assess adverse health-related outcomes in cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2022–2034. doi: 10.1158/1055-9965.EPI-11-0674. [DOI] [PubMed] [Google Scholar]


