Highlights
-
•
Autophagy was augmented in RPE cells in the presence of a lipofuscin pigment, A2E.
-
•
RPE cell death was induced in the presence of A2E with an autophagy inhibitor.
-
•
Inhibition of autophagy resulted in the accumulation of abnormal mitochondria.
-
•
Autophagy in RPE cells prevents the accumulation of damaged cellular molecules.
Abbreviations: 3MA, 3-methyladenine; AMD, age-related macular degeneration; mROS, mitochondrial reactive oxygen species; RPE, retinal pigment epithelial cells; SEM, scanning electron microscopy; STGD, Stargardt disease; TEM, transmission electron microscopy
Keywords: Autophagy, Retinal pigment epithelium, Lipofuscin, Mitochondria, Reactive oxygen species
Abstract
In this study, we show augmented autophagy in the retinal pigment epithelial cell line ARPE-19 when cultured in the presence of the lipofuscin pigment A2E. A2E alone does not induce RPE cell death, but cell death was induced in the presence of A2E with the autophagy inhibitor 3-methyladenine (3MA), with a concomitant increase in the generation of mitochondrial reactive oxygen species. On the other hand, the ATP production capacity of mitochondria was decreased in the presence of A2E, and pharmacological inhibition of autophagy had no additional effects. The altered mRNA expression level of mitochondrial function markers was confirmed by real-time polymerase chain reaction, which showed that the antioxidant enzymes SOD1 and SOD2 were not reduced in the presence of A2E alone, but significantly suppressed with the addition of 3MA. Furthermore, transmission electron micrography revealed autophagic vacuole formation in the presence of A2E, and inhibition of autophagy resulted in the accumulation of abnormal mitochondria with loss of cristae. Spheroid culture of human RPE cells demonstrated debris accumulation in the presence of A2E, and this accumulation was accelerated in the presence of 3MA. These results indicate that autophagy in RPE cells is a vital cytoprotective process that prevents the accumulation of damaged cellular molecules.
1. Introduction
Retinal pigment epithelial (RPE) cells, a monolayer of postmitotic cells located between photoreceptors of the retina and choriocapillaris, play multiple vital roles in photoreceptor function and survival, such as photoreceptor outer segment phagocytosis, maintenance of the visual cycle, and supply of nutritional factors, and are possibly associated with the pathogenesis of diverse diseases [1,2]. Intense light exposure, together with an ample oxygen supply from the choroid, exposes RPE to constant oxidative stress [1], leading to lipid peroxidation of the disk membranes in the photoreceptor outer segment, which may be counteracted by the phagocytosis of oxidized tips of the outer segments by RPE cells [3]. However, phagocytosis of barely digestible materials results in the accumulation of a complex of highly oxidized and degraded molecules with autofluorescence called lipofuscin in RPE cells [2]. Lipofuscin is amassed with age in normal healthy RPE cells, as well as diseased RPE cells. Several clinical observations suggest that excessive lipofuscin accumulation is related to RPE cell damage. It is generally accepted that RPE cell dysfunction due to abnormal lipofuscin accumulation is involved in all key pathological pathways of retinal diseases including Stargardt disease (STGD) and age-related macular degeneration (AMD) [4,5]. Lipofuscin accumulation was significantly increased in RPE cells of STGD patients compared with age-matched controls [6,7]. Moreover, a relationship was noted between lipofuscin accumulation (i.e., increased autofluorescence by fundus autofluorescence) and soft drusen, especially in cases with large soft drusen areas [8,9]. Thus, elucidating the molecular mechanisms underlying impaired RPE cell function appears crucial both for improving our understanding of the pathogenesis of retinal disease and for identifying novel targets for prophylaxis and therapeutic intervention. Among more than 25 components of lipofuscin, A2E is the most major fluorophore identified in aged human eyes and is characterized the most intensively [10,11]. Previous laboratory investigations suggested that A2E exerts toxic effects on RPE cells through diverse mechanisms. It was suggested that A2E oxidation products may trigger the complement system, which predisposes the macula to disease and contributes to chronic inflammation [12]. Additionally, A2E interferes with cholesterol efflux from RPE late endosomes/lysosomes, which in turn inhibits acid lipase and results in a feed-forward cycle of cholesterol accumulation in RPE cells [13]. A study suggested that A2E impairs lysosomal acidification [14], but a later study demonstrated that at the physiological level, A2E does not inhibit the activity of several lysosomal enzymes [13]. A2E also targets specific molecules; for example, it inhibits isomerohydrolase in the visual cycle, thereby disrupting the 11-cis retinal supply to the retina, resulting in photoreceptor dysfunction [15] and activating the retinoic acid receptor, thereby upregulating pro-angiogenic molecules [16]. Additionally, A2E has been shown to destabilize cellular membranes, inhibit respiration in mitochondria, and impair degradation of phospholipids from phagocytosed outer segments [11].
Possible protecting functions of RPE cells against A2E loading as a senescent change may involve autophagy, which is the major mechanism for renewing all cytoplasmic parts of post-mitotic cells. In mammals, three types of autophagy have been described, i.e., microautophagy, chaperone-mediated autophagy, and macroautophagy [17]. Among others, macroautophagy is the most prevalent form of autophagy. The macroautophagy process can be divided into induction, initiation/nucleation, elongation and closure, i.e., engulfment of cytoplasmic proteins, lipids, and damaged organelles, maturation and fusion with primary lysosomes, and finally, degradation, in which contents are degraded by lysosomal enzymes. Previous studies demonstrated that the factors related to autophagy are expressed in RPE cells. Moreover, diverse cellular stress, such as oxidative stress, lipid oxidation products, and intense light exposure, can increase autophagic activity in RPE cells [18–21], suggesting that autophagy plays important roles in the maintenance of RPE cells. Additionally, a recent study demonstrated involvement of noncanonical autophagy in the visual cycle [20]. Given that A2E interferes with various RPE cell functions and is related to the production of lipid peroxidation products such as 4-hydroxynoneal and malondialdehyde, A2E may alter autophagic activity in RPE cells.
Here we investigated the effects of A2E on RPE cells with a focus on its relationship with autophagy. Our results indicate that autophagy functions against A2E-mediated cytotoxic effects and plays a pivotal role in the pathogenesis of lipofuscin-related retinal diseases.
2. Results
2.1. Autophagy is induced in A2E-laden RPE cells
First, we examined the amount of the lipidated form of microtubule-associated protein light chain, which correlates closely with autophagosome number. Consequently, when ARPE-19 cells were incubated with A2E, we observed autophagy induction as demonstrated by the conversion of autophagy protein LC3 (form I) into the lipidated form (form II) (Fig. 1A). Lipidation of LC3 resulted in cytosolic punctate localization of LC3. Consistent with the Western blot analysis, LC3 punctae were confirmed in green fluorescent protein (GFP)–LC3 transfected cells, which showed perinuclear localization after A2E treatment (Fig. 1B). An ubiquitin ligase, Parkin, is recruited from the cytosol to mitochondria and promotes mitochondria-specific autophagy known as mitophagy [22]. Interestingly, Parkin punctae were also found to be localized in the cytosol in GFP–Parkin transfected cells after A2E treatment (Fig. 2B), suggesting the involvement of mitophagy.
Fig. 1.
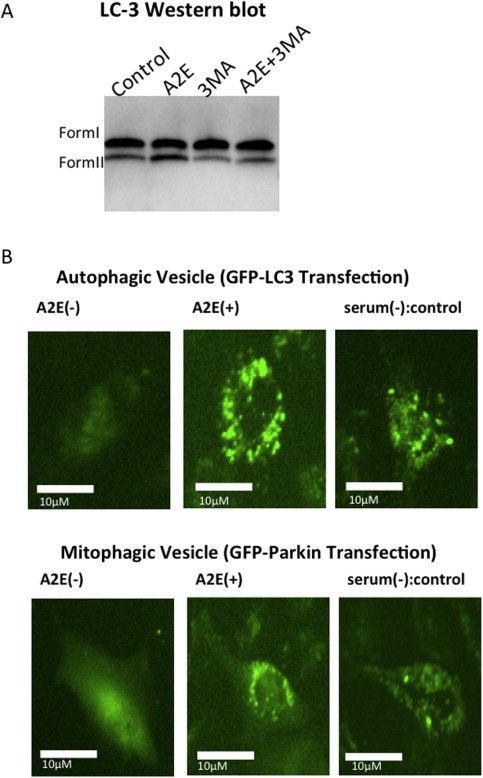
Treatment with increased autophagic flux. (A) Western blot analysis was performed for LC-3 in ARPE-19 cells treated for 12 h. Levels of LC-3-II, the active form, were higher with the addition of A2E (10 μM). With 3MA (10 mM) treatment, the relative levels of LC-3 II decreased, suggesting 3MA inhibited autophagic vacuole formation. (B) (Top) Autophagic vesicle formation visualized by transfection of green fluorescent protein (GFP)–LC3. ARPE-19 cells were transfected with a plasmid that expresses GFP–LC3 fusion protein. After 24 h, the cells were incubated for 12 h in 10 μM A2E in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) or without serum. The cells were immediately visualized by fluorescence microscopy. (Bottom) Mitophagic vesicle formation visualized by transfection of GFP–Parkin. ARPE-19 cells were transfected with a plasmid that expresses GFP–Parkin fusion protein. After 24 h, cells were incubated for 12 h in 10 μM A2E in DMEM containing 10% FBS or without serum. The cells were immediately visualized by fluorescence microscopy.
Fig. 2.
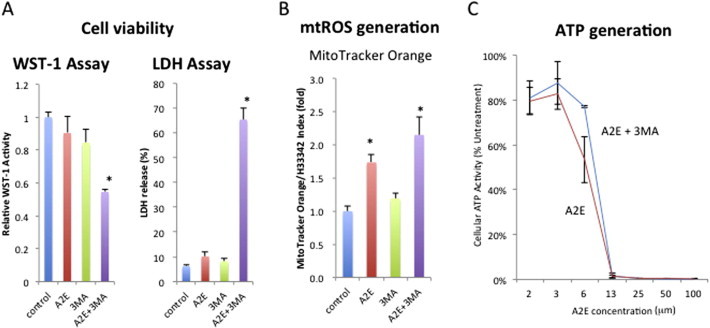
Inhibition of autophagy in A2E-laden RPE cells reduced cell viability and increased mitochondrial ROS generation. (A) Combined treatment of A2E (10 μM) and 3MA (10 mM) reduced the viability of ARPE-19 cells. Cells were treated with the indicated compounds for 24 h and subjected to the WST1 assay and LDH assay, respectively. (B) Increased mitochondrial reactive oxygen species with A2E and 3MA stimulation. ARPE-19 cells were seeded in 96-well plates and stimulated with A2E (10 μM) and/or 3MA (10 mM). After 12 h incubation, the cells were labeled with MitoTracker CMTMRos, which is a mitochondria-specific fluorescence dye that indicates mitochondrial ROS activity. Fluorescence intensity was measured in a Microplate Reader (ARVO, Perkin Elmer, CA, USA). The ratios of MitoTracker/Hoechist 33342 fluorescence were calculated in each well. (C) Reduction of ATP by A2E treatment. ARPE-19 cells were seeded in 96-well plates and stimulated with A2E at the indicated concentrations and/or 3MA (10 mM). After 12 h incubation, the cells were labeled with ToxGlo, an ATP detection reagent. Fluorescence intensity was measured in a Microplate Reader (ARVO).
2.2. Inhibition of autophagy in A2E-laden RPE cells reduces cell viability and increases mitochondrial reactive oxygen species generation
To determine the role of autophagy on RPE cell function, we applied pharmacological inhibition of autophagy using 3-methyladenine (3MA), as widely reported in the literature. Western blot analysis demonstrated that 3MA treatment decreased the relative levels of LC-3 II, corroborating that 3MA inhibited autophagic vacuole formation (Fig. 1A). Similar to previous studies, A2E alone did not affect the viability of ARPE-19 cells under the present conditions, as measured by WST-1 and LDH release assays (Fig. 2A). However, pharmacologic inhibition with 3MA remarkably increased RPE cell death. Similar results were obtained with cultured human RPE cells, suggesting autophagy is required for RPE cell survival (Fig. 2A).
Given that A2E affects mitochondrial function, we first characterized mitochondrial mass by staining with MitoTracker Orange, a dye that binds to mitochondrial reactive oxygen species (mROS). Concomitantly, we measured the mitochondrial ATP generation. Interestingly, in the presence of A2E, mROS generation was increased (Fig. 2B). As expected, in the presence of A2E, ATP generation was decreased in a dose dependent manner. Thus, the increased production of mROS may partly contribute to the reduced synthesis of the mitochondrial ATP. Addition of 3MA alone had no effect on the production of mROS or ATP. mROS generation was further increased in the presence of A2E plus 3MA (Fig. 2B). However, with regard to ATP production, addition of 3MA to A2E had no synergistic effect on the reduction of ATP synthesis (Fig. 2C), suggesting that mROS generation is not due to mitochondrial ATP, but rather due to cellular antioxidant(s) or antioxidant enzyme(s) quenching mROS.
2.3. Acceleration of decreased SOD gene expression in A2E-laden RPE cells with pharmacological inhibition of autophagy
First, reverse transcription polymerase chain reaction (RT-PCR) was performed to examine the mRNA expression levels of the genes involved in mitochondrial biogenesis and mROS quenching. Among the genes examined, PPAR g coactivator (PGC1a), PPAR g coactivator (PCG1b), and PGC-1-related coactivator (PRC) were upregulated in the presence of A2E, whereas mitochondrial transcription factor (TFAM) and nuclear respiratory factor (NRF1) mRNAs were questionably but significantly upregulated in ARPE-19 cells in the presence of A2E (Fig. 3). However, inhibition of autophagy by 3MA did not change the mRNA levels of these factors, and the mRNA expression levels of these genes, upregulated by A2E, were unchanged even in the presence of 3MA. Thus, the genes involved in mitochondrial biogenesis were induced by A2E treatment independent of autophagy. In sharp contrast, the genes for the antioxidant enzymes superoxide dismutase 1 (SOD1) and superoxide dismutase 2 (SOD2) were unchanged in the presence of A2E alone; however, the expressions of these genes was downregulated with the addition of 3MA (Fig. 3).
Fig. 3.

Expression of mitochondrial genes in response to A2E and 3MA treatment. ARPE-19 cells were treated with A2E (10 μM) and/or 3MA (10 mM) for 24 h. RNA was extracted and analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR). The mRNA levels of mitochondrial genes were normalized to those of GAPDH.
2.4. Autophagic vacuole formation in A2E-laden RPE cells and abnormal mitochondrial accumulation with the inhibition of autophagy
We further corroborated the morphological findings by transmission electron microscopy (TEM). Preliminary analysis revealed that mitochondria were more easily observed in cultured human RPE cells compared with ARPE-19 cells. Thus, cultured human RPE cells were used for analysis. In cultured human RPE cells, autophagic vacuoles were not frequently found, as expected. Induction of autophagosomes was evident in the RPE cells after A2E treatment. Cytoplasmic vacuole formation and electron lucent cytoplasmic vacuoles, which often accompanies with increased autophagic influx [23], were also observed. In the presence of 3MA alone, no morphological changes were evident. However, A2E plus 3MA treatment resulted in a number of mitochondria with a disorganized structure of disrupted cristae and a swollen appearance (Fig. 4).
Fig. 4.
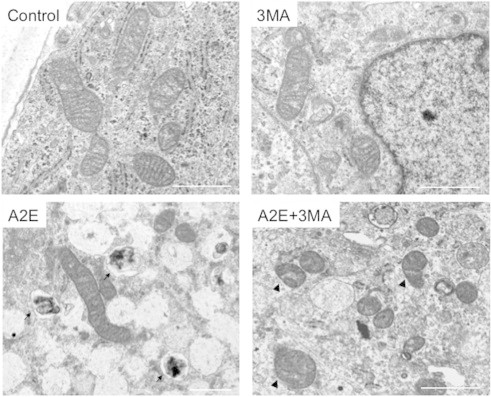
Transmission electron microscopy (TEM) analysis of human RPE cells. Human primary RPE cells were cultured in the presence of A2E (10 μM) and/or 3MA (10 mM) and subjected to TEM analysis. Note that in the presence of A2E, an autophagic vacuole is visible (arrows). In the presence of A2E and 3MA, some mitochondria exhibited a disorganized structure with disrupted cristae and a swollen appearance (arrowheads). Bars = 1 μm.
2.5. Inhibition of autophagy enhances the accumulation of debris in A2E-laden RPE spheroid culture
Both SOD1 and SOD2 play important roles in RPE homeostasis, including Bruch’s membrane integrity and basal laminar deposition. Thus, three-dimensional spheroidal culture of human RPE cells, which provides a three-dimensional view of the structure on the basal side of RPE, was used to investigate the morphology of the basal side of RPE and Bruch’s membrane. Moreover, it was previously documented that spheroid culture system of RPE cells is different from monolayer culture in that the RPE cells in the spheroid phagocytose inner material derived from apoptotic cells, and RPE cells produce more evident lipid nanoparticles in the spheroid culture than those observed in monolayer culture, suggesting increased lipogenesis [24]. As reported previously [24], a Bruch’s membrane-like structure, which possessed an elastin layer as the main component of Bruch’s membrane, was generated on the surface of spheroids of human RPE cells (Fig. 5A), and scanning electron microscopy (SEM) revealed a smooth surface on the spheroids (Fig. 6). In contrast, A2E treatment resulted in morphological alterations involving budding or segmentation of RPE cells and basal laminar deposits and membranous debris-like materials (Figs. 5A and 6). Elastin immunostaining revealed granular staining covering the spheroid, suggesting partial disruption of elastin layers. 3MA treatment alone had no apparent effect; however, in the presence of A2E plus 3MA, these morphological alterations were more remarkable, presumably resulting from growth defects of the lamellipodia of RPE or increased accumulation of debris due to oxidative cellular stress. Elastin immunostaining revealed pronounced disruption of the elastin layer covering the surface of the spheroids. RT-PCR demonstrated that the elastin gene was upregulated in the presence of A2E, which was further increased with an addition of 3MA (Fig. 5B). TEM demonstrated that 3MA treatment alone resulted in infrequent vacuole formation, while the cellular structure of RPE was markedly disorganized with numerous vacuole-like structures, cellular materials, and increased lipid deposition with the treatment with A2E plus 3MA (Fig. 6).
Fig. 5.
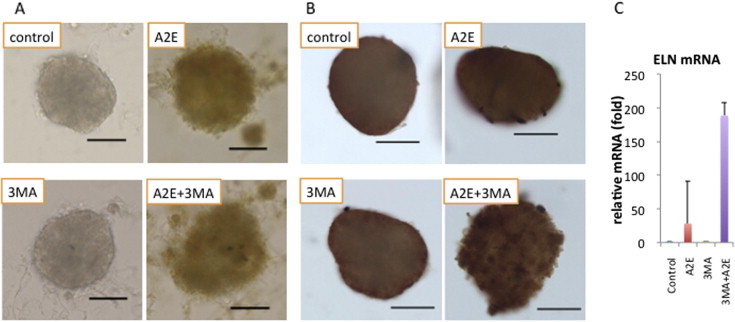
Morphological analysis of human RPE spheroids. (A) Light microscopy and (B) immunocytochemistry of human RPE spheroids. Human primary RPE cells were cultured in RPE spheroids for 7 days and subjected to A2E (10 μm) and/or 3MA (10 mM) treatment. A Bruch’s membrane-like structure with an elastin layer was generated on the surface of the human RPE spheroids. Elastin staining was diffuse on control spheroids such as those seen in the layer of Bruch’s membrane, while A2E treatment showed irregular patchy staining, suggesting remodeling of Bruch’s membrane. Bars = 100 μm. (C) RT-PCR demonstrated that this morphological alteration of the elastin layer was accompanied by overexpression of elastin at the mRNA level.
Fig. 6.
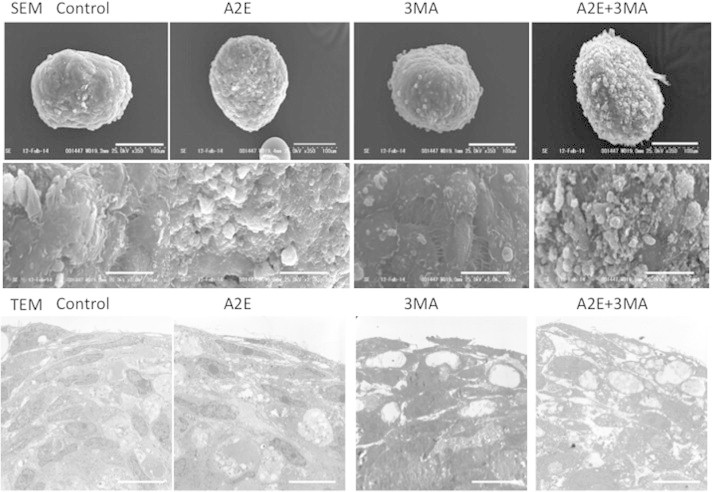
Ultrastructural analysis of human RPE spheroids. Top to bottom. SEM images (and magnified insets), and TEM images of human RPE spheroids. Human primary RPE cells were cultured in spheroids for 7 days and subjected to A2E (10 μm) and/or 3MA (10 mM) treatment. SEM revealed a smooth surface on the spheroids. With A2E treatment, budding or segmentation of RPE cells, accompanied by basal laminar deposits and membranous debris-like materials, was observed and was further pronounced in the presence of A2E and 3MA. Bars = 100 (top) and 20 (bottom) μm, respectively. TEM revealed that 3MA treatment alone resulted in infrequent vacuole formation, a markedly disorganized cellular structure of RPE with numerous vacuole-like structures, cellular materials, and increased lipid deposition in response to treatment with A2E plus 3MA. Bars = 20 μm.
3. Discussion
To assess the role of the lipofuscin pigment A2E in RPE cell function, we investigated whether lipofuscin accumulation affects autophagy. Incubation of human RPE cells with A2E resulted in pronounced influx of autophagy. Since normal and pathological aging are often associated with reduced autophagic potential [25–27], we tested the effect of an early stage inhibitor of autophagy, 3MA, which acts on an upstream regulator of autophagy. We found that inhibition of autophagy resulted in cell death and increased the accumulation of debris in the RPE cells in the presence of A2E. 3MA alone neither affected cell viability nor resulted in the accumulation of debris. Concomitantly, accumulation of damaged mitochondria was remarkably increased in the presence of A2E, especially when autophagy was inhibited. Therefore, autophagy in RPE cells is considered a vital cytoprotective process for preventing the accumulation of damaged cellular molecules.
The present study implicated ROS generation, due to mitochondrial damage at least in part, in RPE damage. Clinical findings from STGD and AMD patients have revealed that excessive lipofuscin accumulation precedes atrophy of the outer retinal layers and the subsequent loss of visual function. Cumulative evidence supports an increase in mitochondrial stress and dysfunction in the RPE cells of AMD patients [28,29]. The increase in the structural alterations of mitochondria coincides with the pathology of AMD [28]. Oxidative stress leads to mitochondrial DNA damage, increases ROS generation, and reduces metabolic capacity [29]. Furthermore, oxidative stress and lipid peroxidation products have been shown to upregulate autophagy to prevent the accumulation of damage. Damaged mitochondria activate inflammasomes by increasing oxidative stress and/or through direct activation by oxidized mitochondrial DNA [30,31]. The present study extends these previous findings by showing that A2E accelerates mitochondrial damage and suggests that the removal of damaged mitochondria by autophagy is essential for RPE cell survival. Together with previous studies demonstrating the involvement of autophagy in RPE homeostasis [18–21], the present study suggests that autophagy is vital for cellular homeostasis in RPE cells.
Among the factors investigated, the mRNA expression levels of SOD1 and SOD2 were downregulated. In the presence of A2E alone, these genes were not significantly downregulated, but in the presence of A2E and 3MA, significant downregulation of these genes was evident. If antioxidant enzyme systems fail to prevent oxidative damage, autophagy takes effect as a second-line protection response by oxidatively degrading damaged cellular structures. Both SOD1 and SOD2 play important roles in RPE homeostasis and Bruch’s membrane integrity [32]. Sod1−/− mice have accelerated age-related pathologic changes in the retina reminiscent of AMD, including drusen, thickening of Bruch’s membrane, and choroidal neovascularization [33]. Sod2 knockdown mice had RPE and Bruch’s membrane changes and accumulated A2E and lipofuscin granules in the RPE [34]. These results emphasize the role of antioxidant enzymes in RPE pathogenesis, as the phenotype includes RPE hypopigmentation and increased lipofuscin, Bruch’s membrane thickening, basal laminar deposits, and photoreceptor disorganization. Additionally, a recent study identified major AMD risk alleles; the ARMS2/HTRA1 risk alleles decrease the SOD2-mediated anti-oxidative pathway [35]. The present study demonstrated the downregulation of SOD1 and SOD2 in the presence of A2E plus 3MA. Using a three-dimensional culture system, it was demonstrated that the exposure to A2E alone moderately disrupted Bruch’s membrane formation and that the inhibited autophagy in the presence of A2E resulted in the accumulation of basal-laminar deposit-like materials. There were marked morphological difference between the RPE cells treated with A2E alone and A2E + 3MA in the spheroid culture system. Moreover, the difference was more evident when RPE cells were cultured in the spheroid than the monolayer system. In the spheroid culture, RPE cells are supposed to be phagocytically active and there is a metabolic challenge to the lysosomal system, which is continuously degrading phagocytosed materials [24]. Considering that the endolysosomal system is also important for autophagy, the marked difference between the RPE cells in the spheroid culture and monolayer culture is attributable to their difference in phagocytotic activity. TEM revealed that debris accumulation was rare in the presence of A2E, but pronounced in the presence of A2E plus 3MA. Thus, the present study clearly demonstrated that A2E and the loss of autophagy lead to RPE damage and debris accumulation. This was observed at A2E levels that occur in vivo in the RPE of aged individuals. Taken together with the aforementioned studies [32–34], the present results suggest that reduced autophagy activity in the presence of A2E results in the reduced expression of antioxidant enzymes, accelerating cellular degeneration.
The present study demonstrated that in our in vitro system, autophagy is upregulated in the presence of A2E after short-term exposure; however, it should be noted that age-related lipofuscin accumulation may eventually lead to impairment of autophagy in RPE cells in vivo for the following reasons. Firstly, a previous study described a 30% decrease in the intracellular ATP levels in aged RPE [36]. Reduced capacity of energy supply in aged cells is due mainly to decreased electron transfer in the mitochondrial respiratory chain [36]. Autophagy is a highly energy-consuming process which may be affected by ATP deficit. Indeed, moderately reduced ATP levels may affect autophagy in RPE cells [37]. Secondly, although still controversial, A2E or lipofuscin may impair lysosomal function [14,38]. In phagocytic cells, including in the RPE, acidic compartments of the endolysosomal system are important regulators of both phagocytosis and autophagy. Thus, decreased lysosomal capacity might also result in decreased autophagy in RPE cells, not affecting autophagosome biogenesis or fusion with lysosomes, but rather impairing the terminal stage of the degradation process. Thus, A2E accumulating in aged RPE cells, in which autophagy is already impaired, may exert RPE cell damage. It is also possible that A2E inhibited lysosomal function resulting in accumulation of autophagosomes rather than increased autophagic flux through this pathway. Also, since only one inhibitor of autophagy is used in this study, it is important to note any potential off-target effects of the drug. Therefore, the clarification of specific molecules of autophagic pathway that are crucial to maintain RPE homeostasis are an important issue for future studies.
In summary, the present study demonstrated that A2E and impaired autophagy mediate RPE cell damage. Future studies should address the link between reduced autophagy in aged RPE and lipofuscin-related degenerative retinal disorders, such as STGD and AMD. Identification of molecules involved in RPE homeostasis will unravel the potential target molecule(s).
4. Materials and methods
4.1. Cell culture
The ARPE-19 human RPE cell line was cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Primary human RPE cells (Takara, Tokyo, Japan) were cultured in F-12 medium containing 10% FBS and diagnostic medium (Glutamax II, Invitrogen, CA, USA). For mitochondrial ATP assays, ARPE-19 cells were cultured in DMEM containing galactose instead of glucose.
A2E was prepared as previously described [16]. Typically, a mixture of all-trans-retinal (50 mg) and ethanolamine (4.6 mg) in ethanol (1.5 ml) was stirred in the presence of acetic acid (4.7 μl) at room temperature under dark conditions for 2 days. The reaction mixture was evaporated and then purified by silica gel column chromatography. After elution with MeOH:CH2Cl2 (5:95) to remove less polar byproducts, further elution with MeOH:CH2Cl2 (5:95) including 0.1% trifluoroacetic acid gave A2E.
For uptake into cultured ARPE-19 cells, A2E was delivered at 10 μM (except for mitochondrial activity assays in which the indicated concentrations of A2E were used) into the culture media, and confluent cell cultures were incubated with A2E or ethanol (control) for the indicated period. 3MA, a specific inhibitor of autophagy, was added at a concentration of 10 mM. Mitochondrial ROS measurements, visualization of autophagic vacuoles, and TEM were performed 12 h after stimulation before the beginning of apparent cell death (data not shown), and cell viability assays were performed 24 h after stimulation.
4.2. Cell viability and mitochondrial activity assays
Cell viability assays (staining with WST1, Roche, Basel, Switzerland; LDH, Takara; and Hoechst 33342, Life Technologies, CA, USA) were performed according to the manufacturers’ instructions. Cellular ATP levels were quantified in ARPE-19 cells. Mitochondrial activity assays (Mitochondrial ToxGlo Assay, Promega, CA, USA; and Mito Tracker, Life Technologies) were performed according to the manufacturers’ instructions.
4.3. Vesicle formation visualization and Western blot analysis
Autophagic and mitophagic vesicle formation was visualized under an epifluorescent microscope in cells transfected with a plasmid expressing GFP–LC3 and GFP–Parkin fusion proteins, respectively. Western blot analysis was performed in a standard manner. ARPE-19 cells were collected in lysis buffer, and cell lysates were separated by SDS–PAGE. Anti-LC3 antibody was used at a concentration of 1:1000. Binding was detected with the appropriate HRP-conjugated secondary antibody at a concentration of 1:1000 and the ECL-Plus Western Blotting Detection System (GE Healthcare, PA, USA).
4.4. Quantitative RT-PCR
For quantitative real-time RT-PCR of the mitochondrial genes, 2 μg total RNA extracted from ARPE-19 cells with Trizol (Life Technologies) was reverse transcribed using the Superscript VILO cDNA Synthesis Kit (Life Technologies). After reverse transcription, 4 μl cDNA was used as a template for quantitative real-time PCR performed on a Thermal Cycler Dice Real Time System II (Takara) using SyBR Premic Ex Taq II (Tli RNaseH Plus, Takara). PCR was carried out in a 20-μl volume using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) for 30 s at 95 °C for denaturing, followed by 55 cycles at 95 °C for 5 s, 55 °C for 5 s, and 72 °C for 10 s in a Roche LightCycler. The values of each gene were normalized to the expression levels of GAPDH. RT-PCR primers sequences are shown in a supplementary file (Supplementary file).
4.5. Spheroid culture
The primary human RPE cells were cultured essentially as described previously [24]. After conventional culture, 4500 human RPE cells were resuspended in 150 μl culture medium with 20% stock methylcellulose solution (v/v) in nonadherent 96-well culture plates with a rounded bottom (Nunc, Langenselbold, Germany). After 7 days of culture, the spheroids were exposed to A2E and/or 3MA for 24 h, harvested, and subjected to immunohistochemistry with a primary antibody against elastin and electron microscopy. For immunohistochemistry, anti-elastin antibody was used at a concentration of 1:1000 and visualized with the appropriate HRP-conjugated secondary antibody at a concentration of 1:1000 visualized by diaminobenzidine staining.
4.6. Ultrastructural analysis
SEM and TEM were performed with reference to a previous report [39]. A monolayer of cultured human RPE cells or spheroids were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M PBS at pH 7.4. After dehydration and embedding, cells or semi-thin sections of spheroids were analyzed by electron microscopy (JEOL JEM-200EX, Akishima, Japan).
4.7. Statistical analysis
Results are presented as the mean (±SE). Values of P < 0.05 were considered significant. Statistical analysis was performed in SPSS 17.0 for Windows (SPSS Inc., NY, USA).
5. Authors contribution
Y.Y. planned the experiments; K.A.S., Y.M., X.T., and Y.N. performed the experiments; K.A.S., Y.M., T.Y., Y.I., and Y.Y. analyzed the data; W.D.J. and E.O. contributed reagents and other essential materials; and Y.Y. wrote the paper.
Acknowledgement
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (Grant No. 9037642).
Appendix A. Supplementary data
This Supplementary data contains a table.
References
- 1.Rattner A., Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 2.Kopitz J., Holz F.G., Kaemmerer E., Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J., Cai B., Jang Y.P., Pachydaki S., Schmidt A.M., Sparrow J.R. Mechanisms for the induction of HNE-, MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 2005;80:567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Weng J., Mata N.L., Azarian S.M., Tzekov R.T., Birch D.G., Travis G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow J.R., Zhou J., Ben-Shabat S., Vollmer H., Itagaki Y., Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest. Ophthalmol. Vis. Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 6.Eagle R.C., Jr., Lucier A.C., Bernardino V.B., Jr., Yanoff M. Retinal pigment epithelial abnormalities in fundus flavimaculatus: a light and electron microscopic study. Ophthalmology. 1980;87:1189–1200. doi: 10.1016/s0161-6420(80)35106-3. [DOI] [PubMed] [Google Scholar]
- 7.Birnbach C.D., Jarvelainen M., Possin D.E., Milam A.H. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101:1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- 8.Smith R.T., Chan J.K., Busuoic M., Sivagnanavel V., Bird A.C., Chong N.V. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47:5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimura S., Ueta T., Takahashi H., Obata R., Smith R.T., Yanagi Y. Characteristics of fundus autofluorescence and drusen in the fellow eyes of Japanese patients with exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. = Albrecht von Graefes Arch. Klin. Exp. Ophthalmol. 2013;251:1–9. doi: 10.1007/s00417-013-2363-y. [DOI] [PubMed] [Google Scholar]
- 10.Eldred G.E., Miller G.V., Stark W.S., Feeney-Burns L. Lipofuscin: resolution of discrepant fluorescence data. Science. 1982;216:757–759. doi: 10.1126/science.7079738. [DOI] [PubMed] [Google Scholar]
- 11.Sparrow J.R., Gregory-Roberts E., Yamamoto K., Blonska A., Ghosh S.K., Ueda K., Zhou J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012;31:121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J., Jang Y.P., Kim S.R., Sparrow J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakkaraju A., Finnemann S.C., Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holz F.G., Schutt F., Kopitz J., Eldred G.E., Kruse F.E., Volcker H.E., Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest. Ophthalmol. Vis. Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 15.Moiseyev G., Nikolaeva O., Chen Y., Farjo K., Takahashi Y., Ma J.X. Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17551–17556. doi: 10.1073/pnas.1008769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iriyama A., Fujiki R., Inoue Y., Takahashi H., Tamaki Y., Takezawa S., Takeyama K., Jang W.D., Kato S., Yanagi Y. A2E, a pigment of the lipofuscin of retinal pigment epithelial cells, is an endogenous ligand for retinoic acid receptor. J. Biol. Chem. 2008;283:11947–11953. doi: 10.1074/jbc.M708989200. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Krohne T.U., Stratmann N.K., Kopitz J., Holz F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Kaarniranta K., Sinha D., Blasiak J., Kauppinen A., Vereb Z., Salminen A., Boulton M.E., Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.Y., Zhao H., Martinez J., Doggett T.A., Kolesnikov A.V., Tang P.H., Ablonczy Z., Chan C.C., Zhou Z., Green D.R., Ferguson T.A. Noncanonical autophagy promotes the visual cycle. Cell. 2013;154:365–376. doi: 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klettner A., Kauppinen A., Blasiak J., Roider J., Salminen A., Kaarniranta K. Cellular and molecular mechanisms of age-related macular degeneration: from impaired autophagy to neovascularization. Int. J. Biochem. Cell Biol. 2013;45:1457–1467. doi: 10.1016/j.biocel.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L., Alva A., Su H., Dutt P., Freundt E., Welsh S., Baehrecke E.H., Lenardo M.J. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 24.Sato R., Yasukawa T., Kacza J., Eichler W., Nishiwaki A., Iandiev I., Ohbayashi M., Kato A., Yafai Y., Bringmann A., Takase A., Ogura Y., Seeger J., Wiedemann P. Three-dimensional spheroidal culture visualization of membranogenesis of Bruch’s membrane and basolateral functions of the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2013;54:1740–1749. doi: 10.1167/iovs.12-10068. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuervo A.M., Bergamini E., Brunk U.T., Droge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 27.Del Roso A., Vittorini S., Cavallini G., Donati A., Gori Z., Masini M., Pollera M., Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp. Gerontol. 2003;38:519–527. doi: 10.1016/s0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 28.Ambati J., Fowler B.J. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 30.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 31.Oka T., Hikoso S., Yamaguchi O., Taneike M., Takeda T., Tamai T., Oyabu J., Murakawa T., Nakayama H., Nishida K., Akira S., Yamamoto A., Komuro I., Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramkumar H.L., Zhang J., Chan C.C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog. Retin. Eye Res. 2010;29:169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamura Y., Noda S., Hashizume K., Shinoda K., Yamaguchi M., Uchiyama S., Shimizu T., Mizushima Y., Shirasawa T., Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice. a model of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Justilien V., Pang J.J., Renganathan K., Zhan X., Crabb J.W., Kim S.R., Sparrow J.R., Hauswirth W.W., Lewin A.S. SOD2 knockdown mouse model of early AMD. Invest. Ophthalmol. Vis. Sci. 2007;48:4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Li Y., Chan L., Tsai Y.T., Wu W.H., Nguyen H.V., Hsu C.W., Li X., Brown L.M., Egli D., Sparrow J.R., Tsang S.H. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum. Mol. Genet. 2014;23:3445–3455. doi: 10.1093/hmg/ddu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Y., Tombran-Tink J. Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv. Exp. Med. Biol. 2010;664:165–183. doi: 10.1007/978-1-4419-1399-9_20. [DOI] [PubMed] [Google Scholar]
- 37.Schutt F., Aretz S., Auffarth G.U., Kopitz J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2012;53:5354–5361. doi: 10.1167/iovs.12-9845. [DOI] [PubMed] [Google Scholar]
- 38.Shamsi F.A., Boulton M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest. Ophthalmol. Vis. Sci. 2001;42:3041–3046. [PubMed] [Google Scholar]
- 39.Yuda K., Takahashi H., Inoue T., Ueta T., Iriyama A., Kadonosono K., Tamaki Y., Aburatani H., Nagai R., Yanagi Y. Adrenomedullin inhibits choroidal neovascularization via CCL2 in the retinal pigment epithelium. Am. J. Pathol. 2012;181:1464–1472. doi: 10.1016/j.ajpath.2012.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This Supplementary data contains a table.


