Abstract
The SynCardia total artificial heart (TAH) currently provides the most definitive option for patients with biventricular failure who are not candidates for isolated left ventricular (LV) assist device placement. The techniques for implantation are adaptable to almost all patients with advanced heart failure, including those with severe biventricular cardiomyopathy, complex congenital heart disease, failed LV assist devices, failed transplantations, and acquired structural heart defects that have failed or are not amenable to conventional surgical treatment. Over the years, the implantation technique has evolved in order to minimize the surgical invasiveness of the procedure, in anticipation of additional future surgery. Meticulous hemostasis with double layer sutures, use of Gore-Tex sheets around the TAH and the pericardial cavity, and use of tissue expanders to avoid contraction of pericardial cavity around the device are discussed in detail in the following report. Additionally, we will provide our experience with implantation of TAH in various challenging scenarios, such as patients with a small chest cavity, congenital heart defects, and simultaneous use of extracorporeal membrane oxygenation (ECMO).
Keywords: Total artificial heart (TAH), SynCardia, cardiowest, implantation technque
Introduction
Initial efforts into creating an artificial complete cardiac replacement organ began in the mid-1960s (1). Several devices have been developed over the past 50 years; however, the SynCardia total artificial heart (TAH) is the only one currently commercially available and used in clinical practice.
Implanted in patients with biventricular failure, the SynCardia TAH has asserted itself as a reliable bridge to transplantation (2-4) and was approved by the United States Food and Drug Administration (FDA) in 2004 and the Centers for Medicare & Medicaid Services in 2008 (5).
Clinical indications for the SynCardia TAH include biventricular failure, left ventricular (LV) failure with prior mechanical heart valves, LV failure with severe anatomical damage (ventricular septal defect, atrioventricular disruption), intractable malignant arrhythmias, massive ventricular thrombus, cardiac allograft failure, hypertrophic or restrictive cardiomyopathy and complex congenital heart disease. Contraindications are generally attributed to size mismatch between the device and patients [body surface area (BSA) <1.7 m2 or anteroposterior diameter of the chest at level of 10th vertebral body <10 cm].
In this article, we present the surgical technique for implantation of the SynCardia TAH.
Operative technique
Device preparation
The arterial outflow connectors are prepared and preclotted or sprayed with CoSeal (Baxter, Deerfield, Illinois, USA) on the back table. We prefer to use CoSeal sprayed with a special applicator onto the stretched graft, and letting the grafts dry while stretched.
The quick connections of the atrial cuffs are trimmed and cut in a circular fashion, leaving about three to five mm of sewing cuff.
All four connectors are stretched with a clamp to facilitate later connection to the device ventricle (Figure 1).
Figure 1.
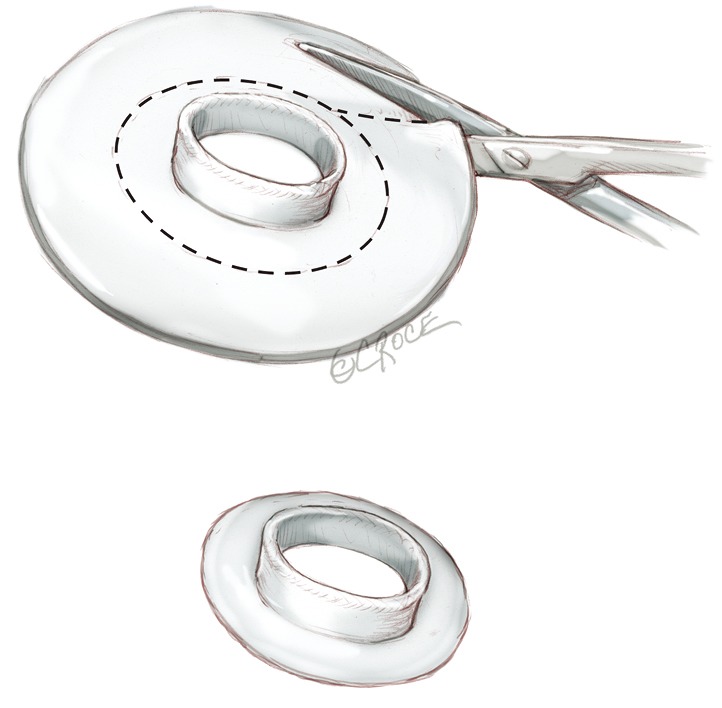
Device preparation: the quick connects of the atrial cuffs are trimmed and cut in a completely circular fashion leaving about three to five mm of sewing cuff. All four connectors are stretched with a clamp to facilitate later connection to the device ventricle.
Surgery preparation
The patient is prepared in a supine position. Under general anesthesia, a central venous access and transesophageal echocardiogram (TEE) probe are inserted. No Swan-Ganz catheter is required; if the patient has a pulmonary artery catheter, this should be removed and exchanged for a short central venous catheter. Before prepping the patient, we mark any previous surgical scars as well as the future exit sites of the drivelines in the epigastrium: a first mark is pointed 7-10 cm below the costal margin along the left midclavicular line. A second mark for the exit of the right ventricle driveline is pointed five cm medially from the first one.
The chest and abdomen are prepared and draped in a routine fashion for cardiac surgery. Preoperatively, access to peripheral vessels for planned or emergent cannulation is obtained as necessary. If required, a high-risk sternotomy may be done under peripheral cardiopulmonary bypass.
Sternotomy and surgical access
The procedure is accomplished through a median sternotomy. Exposure and dissection of the heart and great vessels is similar to that for heart transplantation. Heparin is administered, and the ascending aorta is cannulated distally. We prefer to cannulate both cavae through the right atrium [as opposed to direct cannulation of the superior vena cava (SVC) and inferior vena cava (IVC)] to preserve those sites for use during subsequent transplantation. Dissection around the great vessels is limited in anticipation of additional future surgery. Snares may be placed around both cavae to facilitate total cardiopulmonary bypass. However, we do not find it necessary to snare the venae cavae with vacuum-assisted cardiopulmonary bypass. Cardiopulmonary bypass is instituted (Figure 2).
Figure 2.
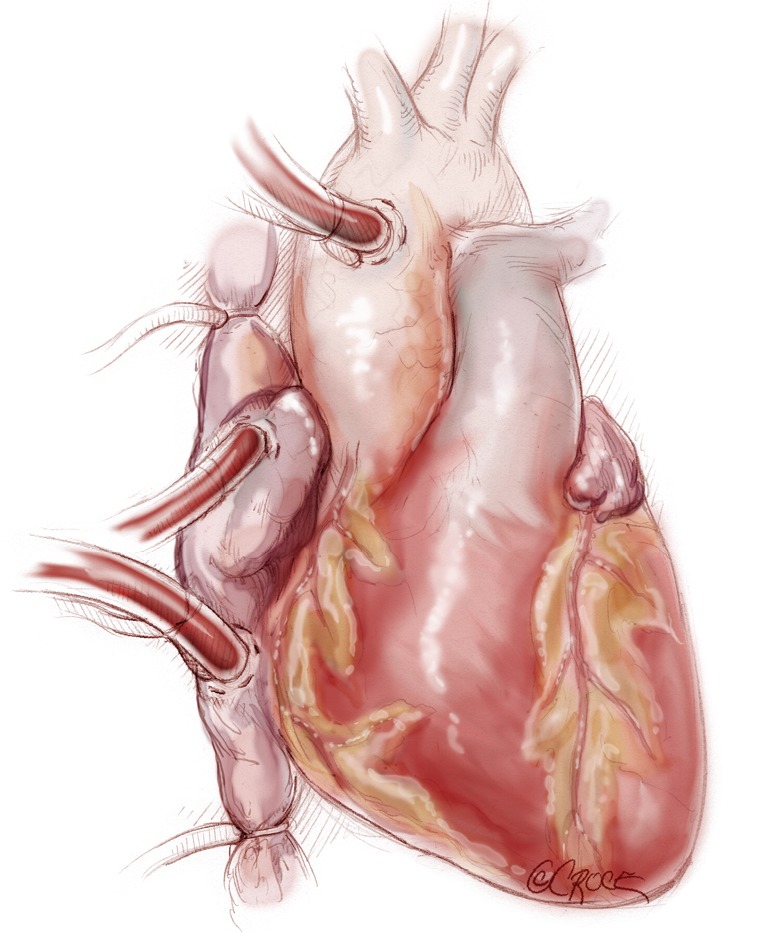
Cardiopulmonary bypass institution: the ascending aorta is cannulated distally. We prefer to cannulate both cavae through the right atrium [vs. direct cannulation of the superior vena cava (SVC) and inferior vena cava (IVC)] to preserve those sites for the heart transplantation surgery.
Cardiac excision
The aorta is clamped. In a large heart or where there are dense adhesions from prior surgery, cardiac dissection and excision may be facilitated through induced cardiac arrest either by administration of cardioplegia or by electrical fibrillation. Adhesions are divided to free the heart down to the pulmonary veins posteriorly. Excision of the heart begins (Figure 3). The purpose of the explantation of the heart is to leave both atria in place, as well as one to two cm of ventricle muscle around each atrioventricular valve plane, preserving both annuli of the mitral and tricuspid valves. The excision is started with a large blade (#10) scalpel by incising the right ventricle about one cm from and parallel to the right atrioventricular groove, extending to the acute margin of the right ventricle. This line is extended anteriorly across the right ventricular outflow tract. The pulmonary trunk is transected, preserving as much length of the pulmonary artery as possible. The original line on the acute margin of the right ventricle is then extended posteriorly toward the interventricular septum. Any pacing wires are divided. The left ventricle is entered again, preserving at least one to two cm of ventricular muscle attached to the mitral valve. This incision is continued circumferentially around the left ventricle. The ascending aorta is transected at the sinotubular junction. The aortic root is dissected caudally off the left atrial roof. The aortic valve is partly excised, preserving the aspect of the valve and aortic root attached to the mitral valve (mitral- aortic curtain), as that will be part of the left atrial cuff. The excised ventricles are now removed from the surgical field.
Figure 3.
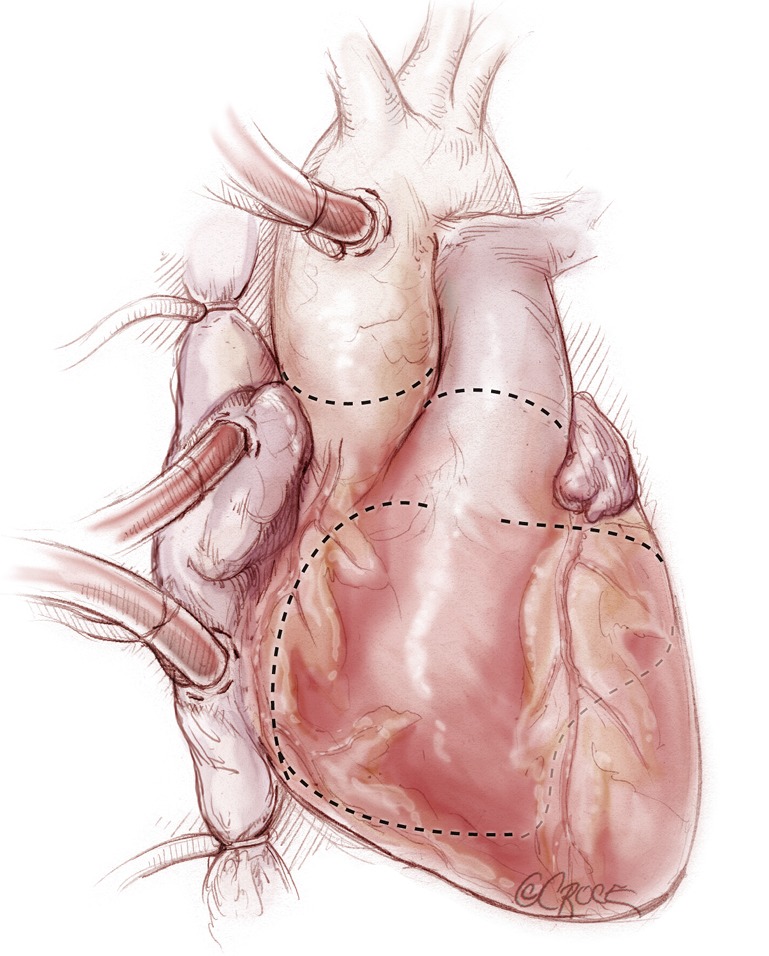
The excision of the heart proceeds along the lines depicted in the figure. The purpose of the explantation of the heart is to leave in place both atria and 1-2 cm of ventricle muscle around each atrioventricular valve plane preserving both annuli of the mitral and tricuspid valves.
Preparation for anastomosis
Excess muscle is trimmed away to leave about 1 cm of ventricular muscle attached to the atrioventricular valves. Any pacing leads are traced through the tricuspid valve into the SVC and dissected free from the endocardium, then divided at the level of the SVC. The external components of the pacemaker and remaining intravascular leads will be removed at a later stage after reversal of heparin and before chest closure. The right atrium is inspected for in-dwelling central venous catheters, and these are divided or retrieved if the tips lie close to the tricuspid valve. The cavae are snared at this stage if desired, taking care to maintain adequate positioning of the venous cannulae. The mitral and tricuspid valves are then excised, including all leaflet tissue, chordae and sub valvular apparatus The coronary sinus is oversewn from within the atrium, as is the left atrial appendage. Alternatively, the appendage could be ligated externally, but this may promote adhesion formation, potentially complicating later transplantation. The atrial septum is inspected and any septal defects are closed (Figure 4).
Figure 4.
Native heart is removed. Preparation for anastomosis: the excess muscle is trimmed away around the atrioventricular junction. Coronary sinus and left atrial appendage are oversewn.
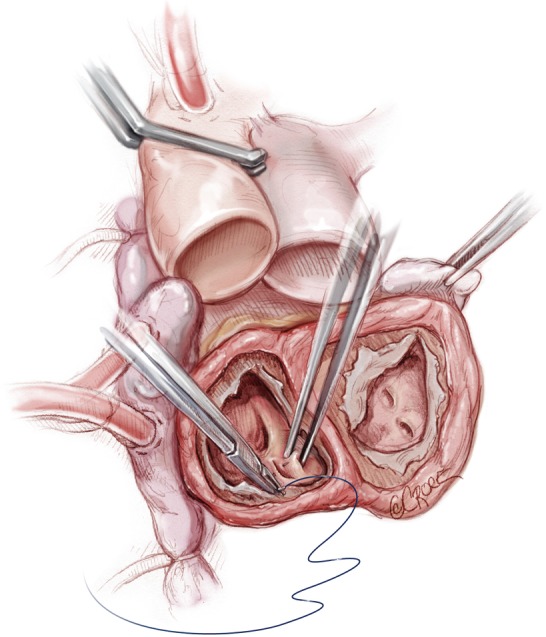
Anastomosis
The left atrial connector is placed by the left atrial cuff. Using a continuous 3-0 prolene suture the quick atrial connector is anastomosed to the left atrial cuff. In the area of the aortic-mitral curtain, the sutures will pass through the aortic root and the fibrous valve trigone. A second running 3-0 prolene suture is then applied to ensure total hemostasis. After completing the left side, a similar procedure is done with the right quick connector, also with a double layer of 3-0 prolene suture (Figure 5). In suturing, the surgeon must be aware that there will be intense scarring around this suture line and therefore if bites are taken deep into the atrium, this will compromise the amount of healthy tissue available for anastomosis at transplantation. This is especially important in the region of the left pulmonary veins. With the double layer suture technique, it is rare to experience bleeding or require any extra sutures on the atrial suture line. There is no need to test the anastomosis with pressurized saline injection. An alternative method of anastomosis involves using felt strips to reinforce the atrial cuffs, but this can result in intense inflammation and dense fibrous scarring, which can complicate cardiac excision at future transplantation. Therefore, we prefer the double suture technique.
Figure 5.
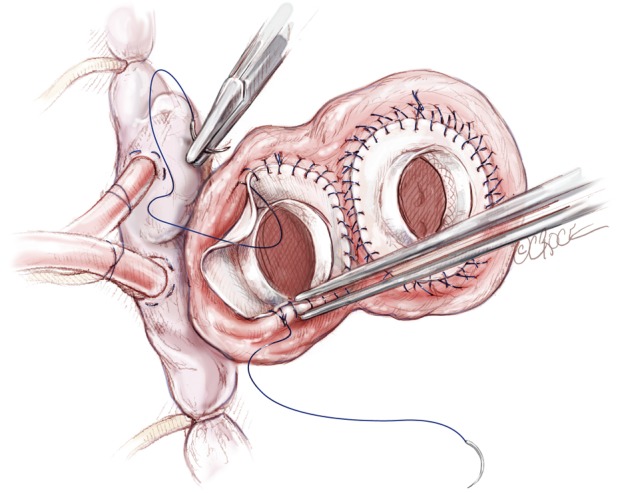
Atrial connectors are placed in the right and left cuffs with a double layer of prolene 3-0. We do not use any Felt Strip reinforcement around the atrial cuffs.
Next, the anastomosis of the great arteries is performed (Figure 6). The outflow conduits are trimmed to size; generally the length of the pulmonary conduit is about five to six cm while the aortic one is shorter around three to four cm. The distal sutures of each conduit are made with 4-0 prolene in a continuous end-to-end fashion suture. We use double layers of suturing for the atrial cuffs, to ensure adequate haemostasis.
Figure 6.
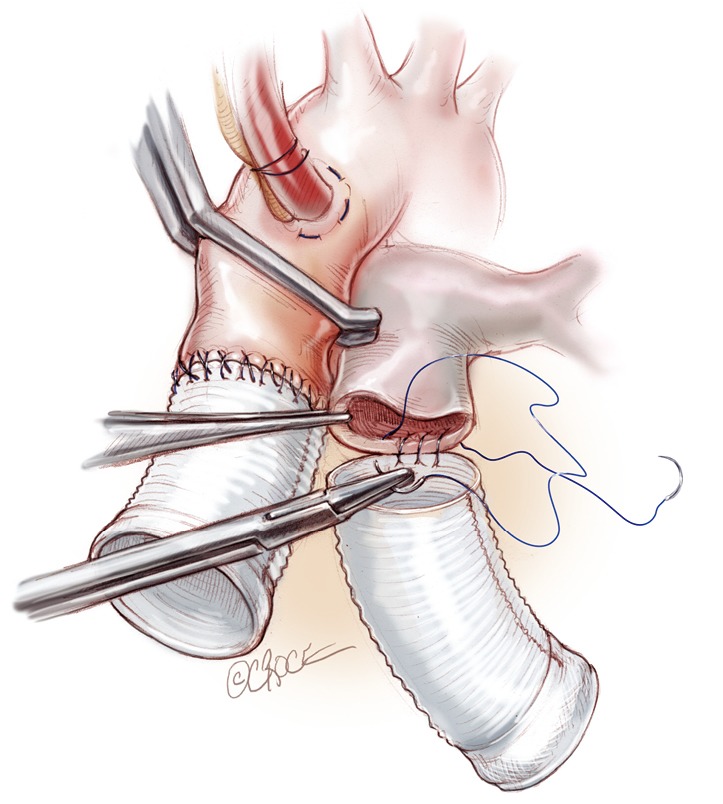
Anastomosis of the great arteries is performed. The outflow conduits are trimmed to size (3-4 cm for the aortic graft; 6-7 cm for the pulmonary one). The end-to-end anastomosis is done with a double layer 4-0 prolene.
Implantation of ventricles
Before placement of the ventricles, we suture two large sheets of Gore-Tex to the atrioventricular groove such that the entire ventricles and in particular, the atrial suture lines, will not be in direct communication with the posterior or lateral pericardium (Figure 7). This facilitates device explantation at transplantation. The drivelines for the ventricles are passed through the skin. First a one cm long curvilinear skin incision is made in the left side of the epigastrium along the midclavicular line, seven to 10 cm below the costal margin. This will represent the exit site for the left ventricle drive line. Another skin incision, for the right one, is made five cm medial to the previous one. This distance is important to avoid any skin necrosis between the two exit sites. The pathway is enlarged with a clamp and a size 40 F chest tube is grasped from the pericardium and pulled out through the skin. The driveline tubing of the left ventricle is placed in the chest tube and pulled out through the skin. A similar process is done for the right ventricle (Figure 8).
Figure 7.
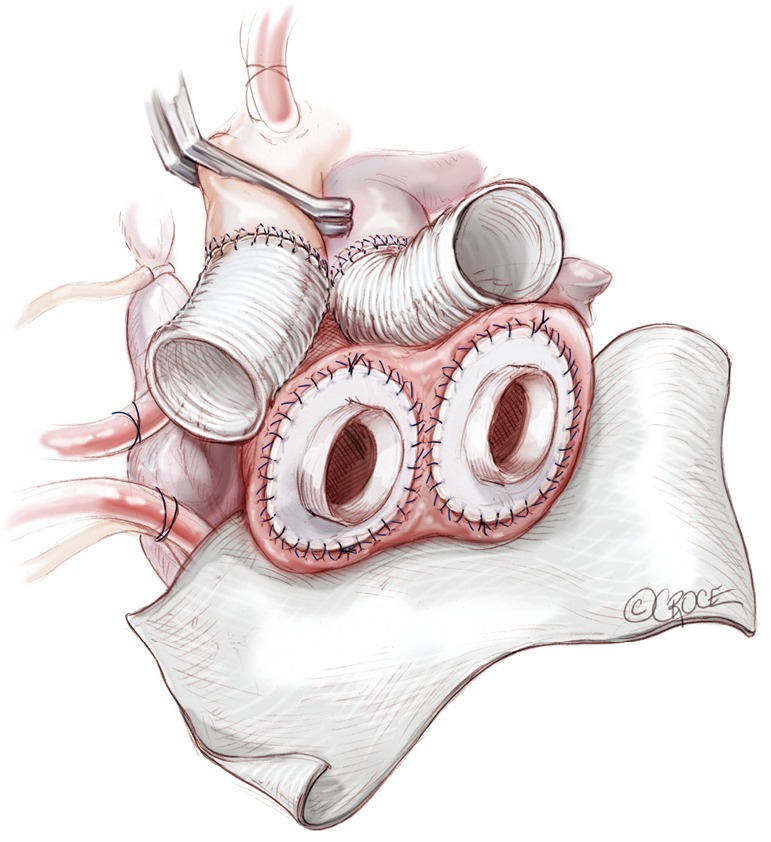
Before placement of the ventricles, we suture two large sheets of Gore-Tex to the atrioventricular groove in order to facilitate the reentry at the time of transplantation.
Figure 8.
The two drivelines are tunnelled under the skin and the artificial ventricle brought into the surgical field. The exit of the left driveline is seven to 10 cm below the left costal margin along the mid clavicular line. The exit of the right driveline is generally five cm medially from the left one.
Connection and deairing
The left ventricle is connected first. Careful assessment of the exact position of the ventricle and its orientation is crucial at this stage. The left atrial quick connector is grasped with two Mayo clamps and while pulling on them, the ventricle is pushed into the quick connector in its final position (Figure 9). The ventricle is rotated slightly to adjust position as necessary to allow a good course for the outflow graft. Ventilation is resumed to allow filling of the ventricle. The aortic cross clamp may be removed under low bypass flow for a few seconds to fill the aortic conduit. At this time, the aortic quick connection to the ventricle is accomplished, avoiding any twisting of the graft. The graft is examined. If there is felt to be a kink, the outflow conduit is disconnected by placing a spatula or flat instrument between the quick-connect and the ventricular connection to disarticulate the connection. Once the connection is deemed satisfactory, the patient is placed in a steep Trendelenburg position and a needle vent is placed in the aortic graft. Ventilation is restarted, as well as gentle suction through the vent. The right artificial ventricle is then connected in a similar fashion, starting with the atrial side first and then the pulmonary side. Before connecting the pulmonary graft, the right side is deaired by partially occluding the venous line (Figure 10).
Figure 9.
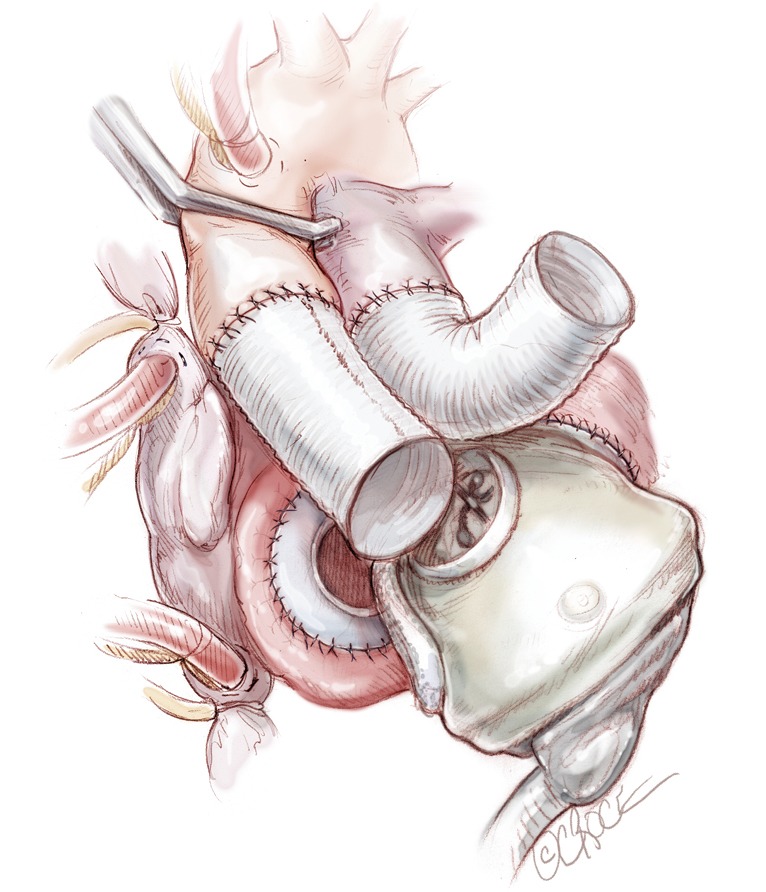
The left ventricle is connected first. Careful assessment of the exact position of the ventricle and its orientation is crucial at this stage. The left atrial quick connector is grasped with two Mayo clamps and while pulling on them, the ventricle is pushed into the quick connector in its final position.
Figure 10.
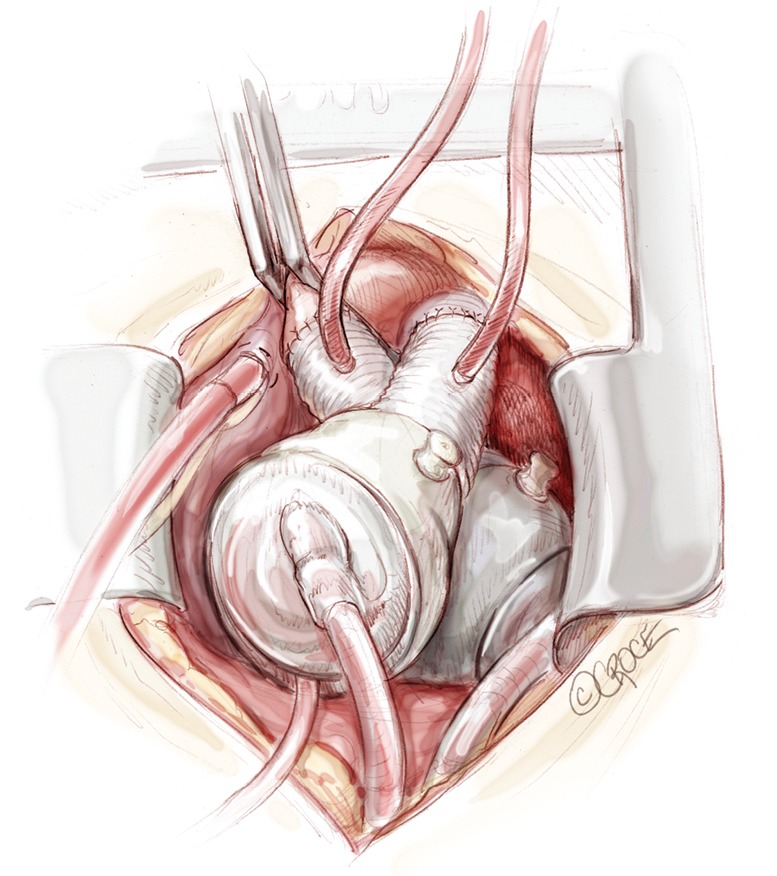
Total artificial heart in final position inside the chest. A vent is placed in the pulmonary graft and in the aortic graft before releasing the aortic cross clamp.
A needle vent is placed in the aorta and another in the pulmonary outflow conduit. The cross clamp is removed. With the patient in Trendelenburg and lungs being ventilated, pumping is begun at a very slow rate with deairing through the aortic vent. Further de-airing, guided by TEE, is accomplished by gentle agitation of the ventricles and atria. This process takes generally 10 to 15 minutes. Meanwhile, the TAH is pumping, increasing the heart rate, and patient is weaned from the cardiopulmonary bypass.
Closure
Protamine is administered and cardiopulmonary bypass cannulae are removed. Careful hemostasis is accomplished—in the very coagulopathic patient, this process might be protracted, but it is imperative to aim to not to leave the operating room until the field is dry. Before chest closure, a Gore-Tex membrane is wrapped around the anterior surface of both artificial ventricles as well as the tunnelled drivelines. Contraction of the pericardial cavity around the device has been observed in numerous TAH patients and can limit the space available for subsequent transplantation. A saline filled breast implant or tissue expander may be used to maintain the apical pericardial space for subsequent transplant (Figure 11). The chest is then closed in standard fashion. Because sternal dehiscence and mediastinitis is catastrophic in the setting of the TAH, closure must be done in a meticulous fashion. For large patients, use of closure devices (other than simple wires) may be considered.
Figure 11.
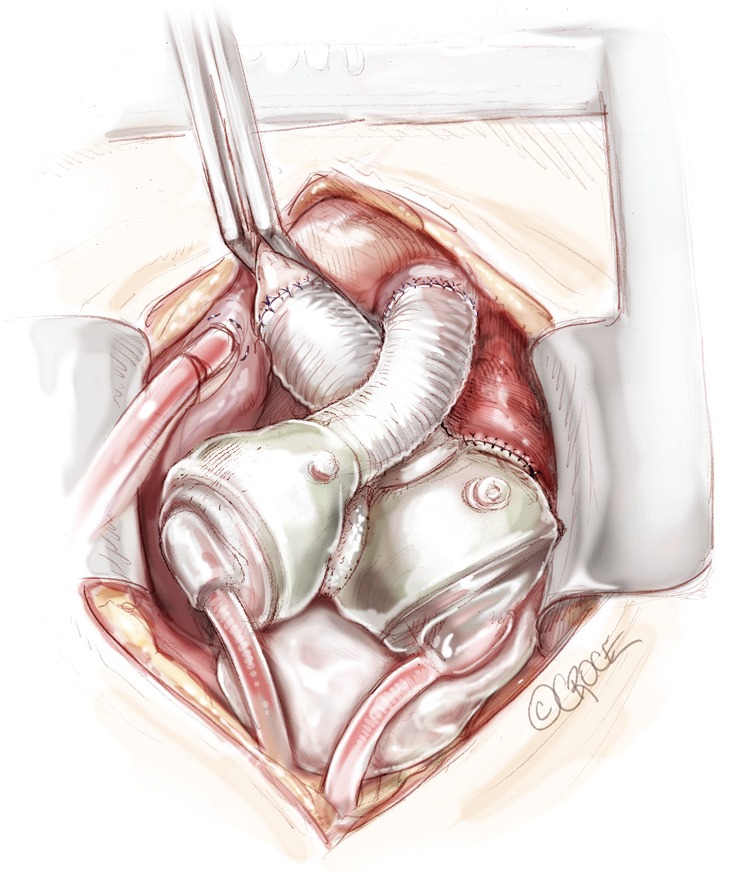
A breast implant is generally left in the pericardium to avoid contraction of the mediastinum around the total artificial heart (TAH) and leave space inside the chest cavity for the donor heart at the time of transplantation.
Challenging scenario
Small chest
Traditionally, a BSA less than 1.7 m2 or a distance between the sternum and the anterior vertebral body of less than 10 cm were considered absolute contraindications to implant the SynCardia TAH. In recent years, some groups have reported successful implantation of SynCardia TAH in small patients with a BSA less than 1.7 m2 (6). Specialized manoeuvres are generally required in this setting. It is critical to mobilize the diaphragmatic attachment of the pericardium to allow the device to sit more leftward and posteriorly in the chest. This requires opening of the left pleura and allows the TAH to slightly migrate into the left pleural space. The left ventricle should be wrapped with a Gore-Tex membrane preventing lung adherence to the device. The IVC is particularly susceptible to compression in patients with small chest cavities. The left sided pulmonary veins are also susceptible to compression by a tightly fitting device. Anchoring both ventricles to a rib on the left chest wall may be helpful in order to laterally displace the TAH away from the IVC and left pulmonary veins. The ventricles then lie mostly to the left of the sternum, with the left ventricle in the left pleural cavity, as opposed to the usual placement where the right ventricle is immediately posterior to the sternum.
Umbilical tape or a heavy silk or polyester suture is generally wrapped two or three times around the main body of the left artificial ventricle; the two ends of the tape or suture are then tunnelled outside the chest through an intercostal space and tied around one of the ribs in order to displace the left artificial ventricle (Figure 12). The ideal position of the ventricle is determined by a combination of echocardiographic and hemodynamic evaluation combined with observation of the TAH fill-volumes. Once the ideal position is identified, the suture or tape is tied. Further adjustments may still be required when the sternum is closed. It is critical for the echocardiographer to confirm laminar flow into all left-sided veins and the IVC prior to chest closure. Ventricular filling should also be maintained after closure. In some cases, all attempts to close the chest will result in venous obstruction despite corrective manoeuvres—in this case, the chest is left open and the patient returned to intensive care. Chest closure can be re-attempted at a later date. In rare cases, if chest closure cannot be accomplished, then musculocutaneous flap closure may be required.
Figure 12.
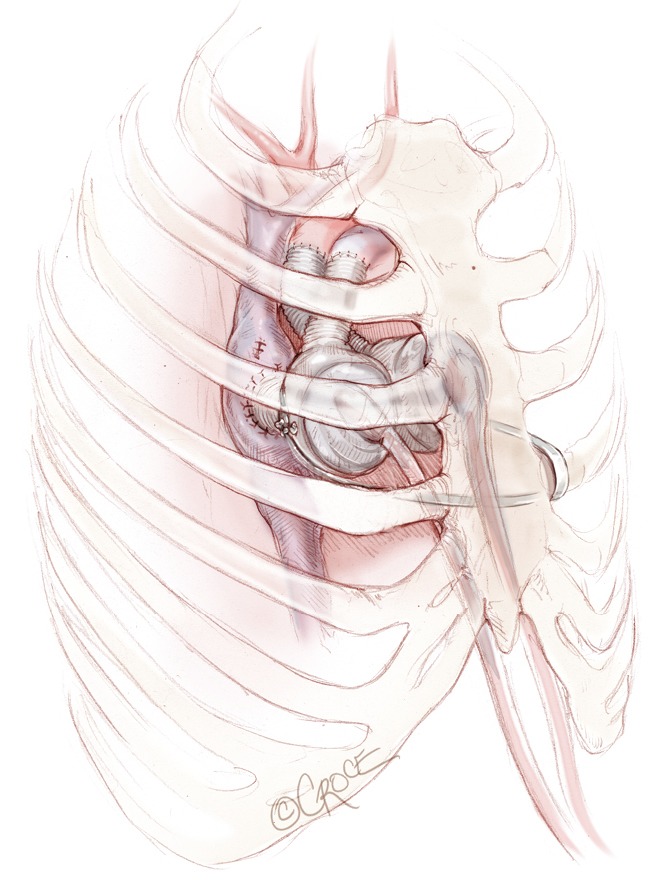
Umbilical tape or a heavy silk or polyester suture is generally wrapped two or three times around the main body of the left artificial ventricle; the two ends of the tape or suture are then tunnelled outside the chest through an intercostal space and tied around one of the rib in order to displace the left artificial ventricle.
Congenital heart disease
Patients with complex congenital heart disease and end-stage cardiomyopathy are ideal for SynCardia TAH placement, as most intracardiac anomalies will be excluded by excision of the heart. Following cardiac excision, a TAH can usually be implanted in a similar way to orthotopic heart transplantation. This surgery is ofetn easier than attempts at repeat technical correction. There are, however, some situations where congenital cardiac disease poses unique surgical challenges for TAH implantation. These are typically situations where a patient has a univentricular physiology or where there is transposition of the great vessels.
In case of failed ‘Fontan’ operations, the TAH has been implanted after constructing a ‘neo right atrium’ (neo RA) in order to fit the right atrial quick connector (7). The extracardiac conduit and IVC are both separated from the right pulmonary artery, which is reconstructed with a bovine pericardial patch or autologous tissue explanted from the failing heart. Next, a 24 or 26 mm Gore-Tex graft, is inserted as an interposition graft between the SVC and IVC to serve as ‘neo RA’. The left atrial cuff is prepared in a standard fashion and any interatrial communication is carefully addressed at this stage. The ‘neo RA’ tubing graft is opened in a generous elliptic shape in order to accommodate the right atrial quick connector. The outflow grafts are then sutured in an end-to-end fashion on the aorta and end to side fashion at the pulmonary artery bifurcation. The length of the pulmonary graft is generally longer than the usual six to seven cm and should be addressed carefully before proceeding with the distal anastomosis (7) (Figure 13).
Figure 13.
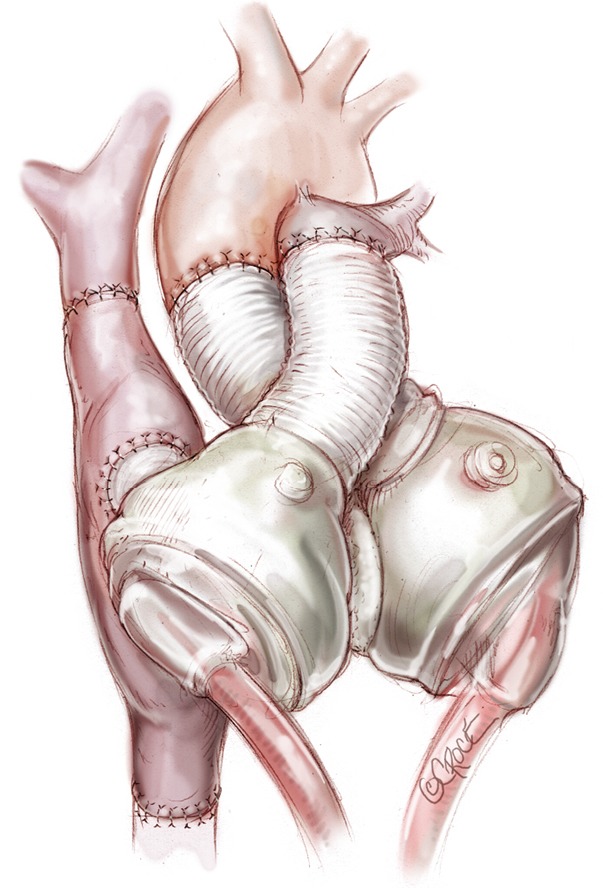
Total artificial heart (TAH) in a Fontan physiology patient.
The SynCardia TAH has also been used in cases of congenital corrected transposition of the great arteries (cc-TGA). In this congenital heart disease, the challenge of TAH implantation is represented by the position of the great arteries (aorta and pulmonary artery), with the aorta anterior and leftward to the pulmonary artery, which require the right and left pumps to be implanted in a parallel rather than normal criss-cross arrangement (8).
Extracorporeal membrane oxygenation (ECMO) at the time of TAH
Pulmonary edema, is often present in cardiogenic shock patients requiring biventricular support and may necessitate ECMO to maintain adequate oxygenation.
Veno-arterial ECMO may be undertaken but will result in two parallel circuits with reduced flow through the TAH. Flow is balanced to allow the lowest ECMO flow that will ensure an acceptable level of oxygenation. However, such set-up could predispose to thrombosis of the mechanical valves due to lower TAH flow and therefore not ideal if a prolonged period of ECMO is anticipated. Veno-venous ECMO is therefore preferable as it allows the ECMO and TAH circuits to run in series with maximal flows permissible on both. Veno-venous ECMO can be instituted in different ways while a TAH is in placed. A single dual-lumen catheter (Avalon Elite®, Maquet Cardiovascular, Germany) may be placed via the internal jugular vein. Care must be taken when introducing the cannula with the Seldinger technique, so as not to entrap the wire or the cannula into the mechanical right atrioventricular valve of the TAH. This technique is particularly suited to the patient who develops respiratory failure in the intensive care unit hours or days after TAH implant. For patients who require ECMO for weaning from cardiopulmonary bypass, other options are required, as the right internal jugular vein is generally not accessible. Our preferred approach is to place a femoral venous cannula and advance it such that the tip lies in the intrahepatic portion of the inferior IVC—this will serve as the inflow for the ECMO (9). For the ECMO outflow, a separate venous cannula via the other femoral or a subclavian vein, is placed and advanced a few centimeters into the right atrium under sonographic guidance. If ECMO is anticipated preoperatively, then cannulae are placed before instituting cardiopulmonary bypass, with final adjustment of outflow cannula position made under direct vision after excision of the native heart to ensure adequate distance of the cannula tip from the right-sided atrioventricular valve. The ECMO circuit therefore draws blood from the infrahepatic IVC and returns it to the right atrium. Blood returning via the SVC is not oxygenated. This setting allows the explantation of veno-venous ECMO at the bedside of the patient. Adequate distance between the tips of both cannulae should be ensured to avoid short-circuit phenomenon.
An alternative approach which has been reported (10) involves placing a cannula in the main pulmonary artery for ECMO inflow and another in the left atrium for outflow. This allows a parallel circuit and has the theoretical advantage of excluding the lungs from a substantial part of cardiac output, thereby promoting recovery of the edematous lung. However, this approach is cumbersome and may risk systemic embolization, necessitating chest re-exploration for decannulation.
Comments
Methods for implanting the TAH are now well established, making this a versatile tool that can be applied to a diversity of patients with advanced biventricular cardiac failure. Operative results have improved considerably over the last two decades, and despite the severity of illness of recipients, several centers reported successful bridge to transplant rates of up to 70%. The SynCardia TAH therefore currently provides the most definitive option for most patients who are not candidates for isolated LV assist device placement. The techniques for implantation are adaptable to almost all patients with advanced heart failure, including those with severe biventricular cardiomyopathy; complex congenital heart disease; failed LV assist devices; failed transplantations; and acquired structural heart defects that have failed or are not amenable to conventional surgical treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.DeVries WC, Anderson JL, Joyce LD, et al. Clinical use of the total artificial heart. N Engl J Med 1984;310:273-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Banayosy A, Arusoglu L, Morshuis M, et al. CardioWest total artificial heart: Bad Oeynhausen experience. Ann Thorac Surg 2005;80:548-52. [DOI] [PubMed] [Google Scholar]
- 3.Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [DOI] [PubMed] [Google Scholar]
- 4.Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg 2012;143:727-34. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch ME, Nguyen A, Mastroianni C, et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at la pitié hospital. Ann Thorac Surg 2013;95:1640-6. [DOI] [PubMed] [Google Scholar]
- 6.Leprince P, Bonnet N, Varnous S, et al. Patients with a body surface area less than 1.7 m2 have a good outcome with the CardioWest Total Artificial Heart. J Heart Lung Transplant 2005;24:1501-5. [DOI] [PubMed] [Google Scholar]
- 7.Rossano JW, Goldberg DJ, Fuller S, et al. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg 2014;97:1438-40. [DOI] [PubMed] [Google Scholar]
- 8.Adachi I, Morales DS. Implantation of total artificial heart in congenital heart disease. J Vis Exp 2014;(89). [DOI] [PMC free article] [PubMed]
- 9.Hosseinian L, Levin MA, Fischer G, et al. Hemodynamic deterioration during ECMO weaning in a patient with a total artificial heart. Critical Care Medicine 2014. (In press) [DOI] [PubMed] [Google Scholar]
- 10.Anderson E, Jaroszewski D, Pierce C, et al. Parallel application of extracorporeal membrane oxygenation and the CardioWest total artificial heart as a bridge to transplant. Ann Thorac Surg 2009;88:1676-8. [DOI] [PubMed] [Google Scholar]


