Summary
Opioid prescription at baseline was significantly associated with greater self-reported disability in back pain patients at 6 months after adjusting for confounders.
Keywords: Disability, Low back pain, Opioids, Propensity score, Prospective cohort
Abstract
Opioid prescribing for chronic noncancer pain is increasing, but there is limited knowledge about longer-term outcomes of people receiving opioids for conditions such as back pain. This study aimed to explore the relationship between prescribed opioids and disability among patients consulting in primary care with back pain. A total of 715 participants from a prospective cohort study, who gave consent for review of medical and prescribing records and completed baseline and 6 month follow-up questionnaires, were included. Opioid prescription data were obtained from electronic prescribing records, and morphine equivalent doses were calculated. The primary outcome was disability (Roland-Morris Disability Questionnaire [RMDQ]) at 6 months. Multivariable linear regression was used to examine the association between opioid prescription at baseline and RMDQ score at 6 months. Analyses were adjusted for potential confounders using propensity scores reflecting the probability of opioid prescription given baseline characteristics. In the baseline period, 234 participants (32.7%) were prescribed opioids. In the final multivariable analysis, opioid prescription at baseline was significantly associated with higher disability at 6-month follow-up (P < .022), but the magnitude of this effect was small, with a mean RMDQ score of 1.18 (95% confidence interval: 0.17 to 2.19) points higher among those prescribed opioids compared to those who were not. Our findings indicate that even after adjusting for a substantial number of potential confounders, opioids were associated with slightly worse functioning in back pain patients at 6-month follow-up. Further research may help us to understand the mechanisms underlying these findings and inform clinical decisions regarding the usefulness of opioids for back pain.
1. Introduction
Opioid prescribing for chronic noncancer pain has increased in recent years [6], and chronic low back pain (LBP) is among the most common nonmalignant disorders associated with prescribed opioid use in primary care [1], [26]. Although opioids are an accepted treatment for LBP, there is limited evidence of their efficacy [7], [9], [18], [19]. The evidence is largely derived from randomized controlled trials with short-term (⩽16 weeks) follow-up, in highly selected populations, and functional outcomes are considered only in a minority of studies. In addition to the uncertainty around efficacy, the epidemiological literature raises concern about potential harms. A Danish study found that opioid use for chronic pain was significantly associated with reporting of severe pain, poor self-rated health, unemployment, higher health care use, and lower self-rated quality of life [11]. In the United States, Kidner et al. [17] reported that patients with chronic musculoskeletal pain who were taking opioids reported higher pain severity, greater disability, and higher levels of depression compared with those who were not. Saunders et al. [29] reported a twofold increase in the fracture risk for older patients prescribed ⩾50 mg daily morphine equivalent dose (MED), and Dunn et al. [10] reported an increasing incidence of accidental overdose with increasing strength of prescribed opioids.
Two previous studies [14], [36] explored the relationship between prescribed opioids and disability in acute LBP and reported an association between early prescription of opioids for LBP and higher long-term disability in U.S. workers’ compensation claimants. A Canadian study [15] looked at the relationship between opioid prescribing and continued disability in a broader range of painful musculoskeletal conditions and also reported that, after adjusting for injury severity, those receiving an early opioid prescription were less likely to return to work. However, this study identified a similar association with receipt of an early prescription for nonsteroidal anti-inflammatory drugs (NSAIDs) and muscle relaxants, raising the possibility of confounding by indication. Given the considerable overlap in the factors influencing LBP-related disability and opioid use, there are a number of potential confounders, many of which could not be adjusted for in these studies. Both long-term disability and opioid use have previously been reported to be associated with baseline disability [30], [32], radiculopathy [30], [32], and a number of psychosocial factors [4], [8], [31]. There are conflicting reports about the influence of pain intensity on both disability [5], [8] and opioid use [12], [31]. Furthermore, previous studies in this field are confined to populations of workers in North America, using continued receipt of wage replacement benefits as a surrogate measure of disability, and the generalizability of the findings outside this population is uncertain.
This study sought to assess the relationship between opioid prescribing at baseline and self-reported disability, as measured by the Roland and Morris Disability Questionnaire (RMDQ), at 6-month follow-up in a UK population of primary care consulters with LBP. We used a propensity score approach to adjust for a substantial number of important potential confounders in the relationship between opioid use and disability.
2. Methods
This study is a secondary analysis nested in a prospective cohort study of patients consulting in UK primary care with LBP (the BeBack study). Ethical approval for the BeBack study was obtained from the North Staffordshire and Central Cheshire Research Ethics Committees.
2.1. Population and study design
The original study recruited 1591 patients ages 18 to 60 years consulting in primary care with LBP between September 2004 and April 2006. Full details of this study are available elsewhere [13]. Study participants received postal questionnaires at baseline (soon after their index consultation) and 6 months later. For nonresponders, a reminder postcard was sent after 2 weeks with a reminder questionnaire after 4 weeks. Participants’ consent to review of their medical records was also requested. Electronic prescribing records for consenting study participants were obtained as part of the medical record information from the primary care practices participating in the BeBack study, for the period between recruitment (from September 2004 to April 2006) and 6-month follow-up. As part of the Keele GP Research Partnership, these practices participate in regular training and audit to ensure the quality of data recording [25].
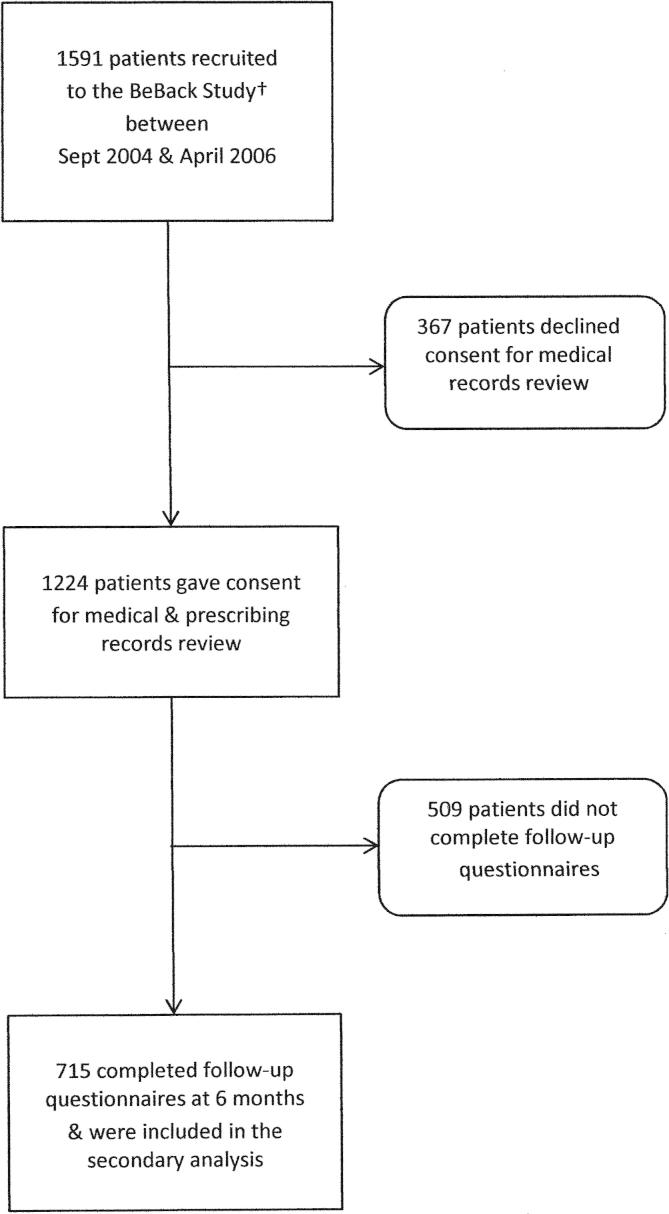
A total of 1224 (77%) patients in the original cohort gave consent for review of medical and prescribing records. Of these, 715 (58%) completed a 6-month follow-up questionnaire and were included in this nested study, as shown in Fig. 1. The potential for nonresponse bias due to loss of follow-up was addressed in the original study through comparisons of the baseline characteristics of responders and nonresponders, and the baseline data for employment status, symptom duration, and RMDQ score were almost identical [13].
Fig. 1.
Flow chart. †Foster et al., 2008 [13].
2.2. Measures
2.2.1. Opioid prescription
Data regarding prescribed opioids were obtained from electronic prescribing records. Back pain consulters were identified from participating practices on a weekly basis, and baseline questionnaires were sent in the week after consultation. To ensure inclusion of prescribing data from the time of the index consultation, the baseline period for this study was defined as the 28-day period starting 14 days before the date the baseline questionnaire was sent. Baseline opioid use was defined as receipt of 1 or more prescriptions for an opioid analgesic in the baseline period. The duration of opioid therapy prior to the baseline period was unknown. The total opioid medication prescribed in the baseline period was obtained by multiplying the total number of tablets or patches prescribed in the 28-day period by the dose of each tablet or patch. This was then converted to an MED using previously published equianalgesic conversion factors [35]. Average daily MED was then calculated by dividing the total MED prescribed in the baseline period by 28 and classified into 4 dose groups (none = 0 mg/d, low = 1 to 19 mg/d, medium = 20 to 49 mg/d, and high = ⩾50 mg/d) based on previously published work [29].
2.2.2. Outcomes
The RMDQ [27] was used to measure self-reported disability. It includes 24 items and is scored from 0 (no disability) to 24 (highest disability). The primary outcome measure of the study was self-reported disability based on RMDQ from the 6-month follow-up questionnaire.
2.2.3. Baseline covariates (potential confounders)
Baseline variables with the potential to affect disability (RMDQ) at follow-up based on theoretical considerations and the published literature were included as potential confounders. Sociodemographic information including age, gender, socioeconomic status (based on job title) [21], [22], education, employment status, and work loss due to back pain was obtained at baseline.
In addition to RMDQ, usual pain intensity on a 0 (no pain) to 10 (worst pain) numerical rating scale, duration of symptoms, and presence of leg pain below the knee were also reported on the questionnaire.
From the prescribing records, information was obtained regarding baseline NSAID use, defined as receipt of 1 or more prescriptions for an NSAID in the baseline period (defined as for opioid medication), and a count of all prescribed nonopioid medications in the same period was used as a surrogate measure for comorbidity [24].
A range of psychological measures were included in the questionnaires: The Hospital Anxiety and Depression Scale (HADS) [38] provides subscale scores from 0 to 21 for anxiety and depression. Given that anxiety and depression are frequently highly correlated, we merged these 2 variables to facilitate more efficient adjustment for confounding. We used the established cut-off of 8 on each subscale to indicate likely depression or anxiety [3] to categorize patients into 3 groups for distress dependent on whether they had a HADS score of ⩾8 for both depression and anxiety, a score of ⩾8 for either depression or anxiety, or a score of <8 on both anxiety and depression. Fear of movement was assessed using the Tampa Scale of Kinesiophobia [34]. This comprises 17 items, each scored on a 4-point Likert scale (range of scores: 17 to 68); higher scores indicate greater levels of fear avoidance. Coping style was assessed using the 4 subscales of the Coping Strategies Questionnaire [16], with higher scores on each subscale indicating higher frequency of the specific coping style: catastrophizing (6 items; subscale score: 0 to 36), diversion (6 items; 0 to 36), reinterpretation (6 items; 0 to 36), and cognitive coping (5 items; 0 to 30). We used the Pain Self-Efficacy Questionnaire [20] to assess patients’ beliefs about their ability to accomplish activities despite pain. It has 10 items, each with a 6-point Likert scale (scale score: 0 to 60), and higher scores indicate stronger self-efficacy beliefs.
2.3. Statistical analyses
Initial analyses were carried out to explore the unadjusted relationship between receipt of an opioid prescription in the baseline period and disability (RMDQ score) at 6 months. We then adjusted solely for baseline RMDQ score because baseline disability is likely to be the strongest determinant of disability at follow-up [8], [32], [33]. For comparison, we also explored the relationship between receipt of an NSAID prescription in the baseline period and disability at 6 months in order to ascertain whether any association observed between opioids and future disability was also observed with other prescribed analgesics and therefore likely to be due to a general association with analgesic medication. The main analysis was confined to investigating the effect of receiving an opioid prescription (yes or no). We had also intended to investigate the effect of opioid dose on disability, but were unable to do so due to the small numbers in the higher-dose opioid groups.
Propensity scores are a statistically efficient method of adjusting for multiple confounding factors. Using this method permits the amalgamation of potential confounders into a single variable (the propensity score), which reflects the probability of an individual receiving an opioid prescription given their baseline characteristics. Linear regressions were performed for each baseline covariate separately with RMDQ at follow-up as the dependent variable to identify the extent of the unadjusted association of these potential confounders with RMDQ at 6 months.
The propensity score was then estimated using a logistic regression model with opioid prescription status (yes or no) as the dependent variable and all baseline covariates as the explanatory variables. The propensity to receive an opioid at baseline was calculated for all participants, and participants were divided into 5 groups based on the quintile propensity scores. Checks were performed to ensure balance within the 5 propensity groups between those who did receive an opioid and those who did not; this was performed by checking that within each group, the distribution of baseline variables was comparable for participants who were and were not prescribed an opioid during the study baseline period. In the event of an imbalance for particular variables, an interaction term was introduced for these variables in the propensity model in an attempt to obtain improved balance within groups. In the event of imbalance once interaction terms had been investigated, the remaining variables were included as an independent predictor of the outcome, along with the propensity score and opioid status. Multivariable linear regression was then used to examine the association between prescription of an opioid in the baseline period and disability as measured by RMDQ at 6-month follow-up, adjusting for propensity score group.
3. Results
In the baseline period, 234 of the 715 participants (32.7%) were prescribed opioids. For those prescribed opioids, the mean daily MED was 22.74 mg (SD 31.57) and the median daily opioid dose was 16.07 mg (interquartile range 5.36 to 26.70). When classified according to average daily MED, the majority, 152 (21.3% of the study population), were prescribed low dose (1 to 19 mg MED/day), 57 (8.0%) medium dose (20 to 49 mg MED/day), and 25 (3.5%) high dose (⩾50 mg MED/day) opioids.
The baseline characteristics of the study participants, according to their opioid prescription status, are shown in Table 1. Patients prescribed any dose of opioid in the baseline period had higher mean usual pain intensity (P < .001), had higher disability on RMDQ (P < .001), and were more likely to report higher distress (anxiety and depression scores ⩾8 on HADS scale) (P ⩽ .001), lower self-efficacy (P < .001), greater fear of movement (Tampa Scale of Kinesiophobia P < .001), and a greater tendency to catastrophize (Coping Strategies Questionnaire-catastrophizing P < .001) at baseline compared with those who were not prescribed opioids. Among those prescribed opioids, the mean reported levels of pain, disability, and distress increased from the low-dose to the high-dose groups, as did the score for catastrophizing. Self-efficacy scores were lower in the higher dose groups. Those prescribed opioids were also significantly more likely to receive an NSAID prescription and had significantly greater comorbidity scores, with the highest comorbidity scores in the high-dose group. There was no significant difference in terms of mean age, but the proportion of female patients was significantly greater in those receiving an opioid prescription, particularly in the higher dose groups.
Table 1.
Baseline characteristics by opioid prescription status (n = 715).
| No opioid prescription | Opioid prescription group (morphine equivalent dose) |
t test/χ2 P value⁎ |
|||
|---|---|---|---|---|---|
| None | Low | Medium | High | ||
| Baseline characteristic | n = 481(67.3%) | n = 152(21.3%) | n = 57(8.0%) | n = 25(3.5%) | |
| Age (years), mean (SD) | 45.77 (9.87) | 46.27 (9.36) | 44.07 (10.69) | 47.64 (9.39) | .891 |
| Gender, female | 56.1% | 67.1% | 73.7% | 76.0% | <.001⁎ |
| Socioeconomic status Professional/manager |
33.7% | 26.3% | 26.4% | 24.0% | .022⁎ |
| Employment status Employed and working as usual |
63.4% | 40.8% | 26.3% | 24.0% | <.001⁎ |
| Duration of current episode of lower back pain >3 months |
34.3% | 34.9% | 43.9% | 36.0% | .450 |
| Leg pain below the knee | 32.0% | 39.5% | 49.1% | 68.0% | .001⁎ |
| Usual pain intensity Numerical rating scale (0–10), mean (SD) |
4.28 (2.49) | 5.43 (2.66) | 6.21 (2.25) | 7.08 (2.31) | <.001⁎ |
| Disability Roland-Morris Disability Questionnaire (0–24), mean (SD) |
7.37 (5.18) | 10.74 (5.99) | 13.05 (5.99) | 15.56 (5.21) | <.001⁎ |
| Distress Hospital Anxiety and Depression Questionnaire score ⩾8 for anxiety and depression |
26.2% | 36.8% | 50.9% | 56.0% | <.001⁎ |
| Fear of movement Tampa Scale of Kinesiophobia (17–68), mean (SD) |
38.70 (6.83) | 40.32 (6.78) | 41.01 (6.72) | 42.97 (7.12) | <.001⁎ |
| Self efficacy Pain Self-Efficacy Questionnaire (0–60), mean (SD) |
41.60 (12.79) | 34.05 (14.96) | 27.97 (15.26) | 26.84 (13.23) | <.001⁎ |
| Coping style | |||||
| CSQ—diversion (0–36), mean (SD) | 14.98 (8.15) | 16.53 (6.50) | 16.31 (6.59) | 18.92 (6.65) | .008⁎ |
| CSQ—catastrophizing (0–36), mean (SD) | 8.64 (6.93) | 11.05 (8.35) | 13.31 (9.23) | 16.5 (8.98) | <.001⁎ |
| CSQ—reinterpretation (0–36), mean (SD) | 7.63 (6.98) | 7.60 (6.49) | 6.94 (6.50) | 7.50 (5.20) | .714 |
| CSQ—cognitive coping (0–30), mean (SD) | 17.56 (6.12) | 15.84 (6.50) | 15.70 (6.59) | 15.48 (5.87) | <.001⁎ |
| Nonsteroidal anti-inflammatory drug Prescribed at baseline |
32.0% | 49.3% | 43.9% | 48.0% | <.001⁎ |
| Comorbidity Number of prescribed nonanalgesic medications, mean (SD) |
0.88 (1.64) | 2.05 (3.12) | 2.35 (3.75) | 3.28 (3.54) | <.001⁎ |
CSQ = Coping Strategies Questionnaire.
Comparison of any opioid prescription at baseline versus no opioid prescription at baseline.
Across the study population, self-reported disability, pain, self-efficacy, anxiety, and depression showed some improvement during the 6-month follow-up period; the degree of improvement was similar for those who were prescribed opioids in the baseline period and those who were not (Table 2).
Table 2.
Changes between baseline and follow-up by opioid prescription status.
| No opioid prescription in baseline period |
Opioid prescription in baseline period |
|||
|---|---|---|---|---|
| Baseline score | Follow-up (6 months) | Baseline score | Follow-up (6 months) | |
| Disability Roland-Morris Disability Questionnaire⁎ (0–24), mean (SD) |
7.37 (5.19) | 4.92 (5.18) | 11.74 (6.11) | 9.02 (7.24) |
| Usual pain intensity Numerical Rating Scale⁎ (0–10), mean (SD) |
4.27 (2.50) | 2.77 (2.54) | 5.79 (3.56) | 4.21 (3.10) |
| Depression Hospital Anxiety and Depression Questionnaire⁎ (0–21), mean (SD) |
5.69 (3.77) | 4.45 (3.79) | 7.95 (4.60) | 6.50 (4.89) |
| Anxiety Hospital Anxiety and Depression Questionnaire⁎ (0–21), mean (SD) |
7.83 (4.27) | 6.09 (4.23) | 9.16 (4.73) | 7.72 (5.01) |
| Self-efficacy Pain Self-Efficacy Questionnaire† (0–60), mean (SD) |
41.60 (12.79) | 43.97 (12.07) | 31.79 (15.11) | 34.46 (16.28) |
Lower score indicates improvement.
Higher score indicates improvement.
Results of unadjusted regression analyses demonstrating the relationship between all of the baseline characteristics and RMDQ score at 6 months are given in Table 3. Receipt of an opioid prescription at baseline predicted a higher RMDQ score at 6 months in the unadjusted regression analysis (coefficient = 4.21, 95% confidence interval [CI] 3.28 to 5.14). This remained significant after adjusting for baseline RMDQ score (Table 4), although the coefficient was reduced (coefficient = 1.23, 95% CI 0.45 to 2.00). Receipt of an NSAID prescription in the baseline period was not significantly associated with RMDQ score at follow-up in the unadjusted analysis (coefficient = 0.33 95% CI −0.62 to 1.28, P = .50).
Table 3.
Unadjusted associations between baseline characteristics and disability at 6 months.
| Baseline variable | Regression coefficient⁎ | 95% confidence interval | Standardized regression coefficient (β) | P value |
|---|---|---|---|---|
| Opioid prescription at baseline Opioid prescription vs no opioid at baseline |
4.21 | 3.28 to 5.09 | 0.32 | <.001† |
| Age Per1-year increase |
<0.01 | −0.04 to 0.05 | <0.01 | .884 |
| Gender Male vs female |
−0.80 | −1.73 to 0.14 | −0.06 | .096 |
| Social class Professional/managerial vs lower occupation classification |
−2.35 | −3.33 to −1.37 | −0.17 | <.001† |
| Education Educated up to 16 years vs beyond 16 years |
2.60 | 1.67 to 3.53 | 0.20 | <.001† |
| Work Employed and working as usual vs unemployed/not working or working reduced hours |
−4.93 | −5.77 to −4.08 | −0.39 | <.001† |
| Usual pain intensity Per 1-unit increase (Numerical Rating Scale, range 0–10) |
1.09 | 0.94 to 1.25 | 0.46 | <.001† |
| Disability Per 1-unit increase (Roland-Morris Disability Questionnaire, range 0–24) |
0.706 | 0.65 to 0.76 | 0.67 | <.001† |
| Duration of pain Duration >3 months vs ⩽3 months) |
4.75 | 3.85 to 5.64 | 0.63 | <.001† |
| Leg pain Pain below the knee vs no leg pain below knee |
3.75 | 2.84 to 4.67 | 0.29 | <.001† |
| Distress Hospital Anxiety and Depression Questionnaire score ⩾8 vs Hospital Anxiety and Depression Questionnaire score <8 for anxiety and depression |
5.09 | 4.18 to 6.01 | 0.38 | <.001† |
| Fear of movement Per 1-unit increase (Tampa Scale of Kinesiophobia, range 17–68) |
0.38 | 0.32 to 0.44 | 0.42 | <.001† |
| Self-efficacy Per 1-unit increase (Pain Self-Efficacy Questionnaire, range 0–60) |
−0.26 | −0.28 to −0.23 | −0.59 | <.001† |
| Coping style CSQ—diversion Per 1-unit increase (range 0–36) |
0.13 | 0.08 to 0.19 | 0.17 | <.001† |
|
CSQ—catastrophizing Per 1-unit increase (range 0–36) |
0.41 | 0.36 to 0.46 | 0.51 | <.001† |
| CSQ—reinterpretation Per 1-unit increase (range 0–36) |
0.04 | −0.03 to 0.11 | 0.05 | .221 |
| CSQ—cognitive coping Per 1-unit increase (range 0–30) |
−0.20 | −0.27 to −0.13 | −0.20 | <.001† |
| NSAID at baseline NSAID prescription vs no NSAID at baseline |
0.33 | −0.62 to 1.28 | 0.03 | .498 |
| Comorbidity Per additional medication prescribed (nonopioid prescribed medication count) |
0.71 | 0.52 to 0.89 | 0.27 | <.001† |
Coefficients from separate linear regressions.
CSQ = Coping Strategies Questionnaire; NSAID = nonsteroidal anti-inflammatory drug.
Mean increase in follow-up Roland-Morris Disability Questionnaire score per unit change in independent variable.
Statistically significant P ⩽ .05.
Table 4.
Unadjusted and adjusted associations between baseline opioid prescription and disability (RMDQ score) at 6 months.
| Unadjusted analysis |
Adjusted analysis† |
Multivariable analysis‡ |
P value | ||||
|---|---|---|---|---|---|---|---|
| Coefficient⁎ | 95% CI | Coefficient⁎ | 95% CI | Coefficient⁎ | 95% CI | ||
| Opioid prescription in baseline period | 4.21 | 3.28–5.14 | 1.23 | 0.45–2.00 | 1.18 | 0.17–2.19 | .022 |
CI = confidence interval; RMDQ = Roland-Morris Disability Questionnaire.
Regression coefficient denotes the mean difference in 6-month RMDQ score compared to a subject without an opioid prescription at baseline.
Analysis adjusted for baseline RMDQ score.
Multivariable model adjusted for propensity score.
In the propensity score model, it was necessary to introduce interaction terms to balance some of the variables. These interactions were (1) age and pain, (2) CSQ reinterpretation and CSQ diversion, (3) RMDQ at baseline and comorbidity (count of prescribed nonanalgesic medications), and (4) NSAID prescription status and distress. Once these 4 variables were included in the propensity score model, balance was found across all variables, and therefore the final model included opioid prescription status and propensity score group.
In the multivariable analysis, opioid prescription at baseline predicted higher disability (RMDQ score) at 6 months (P = .022) after adjusting for propensity score. The mean 6-month RMDQ score was 1.18 (95% CI 0.17 to 2.19) units higher for those prescribed opioids at baseline compared with those who were not prescribed any opioids (Table 4).
4. Discussion
We observed a significant association between receipt of an opioid prescription in the baseline period and self-reported disability at follow-up, even after adjusting for a large number of potential confounders. However, the increase in mean RMDQ score associated with receipt of an opioid prescription was small and therefore unlikely to be clinically important [23]. Our findings imply, however, that receipt of an opioid prescription at baseline is not associated with improved outcome.
Almost one third (32.7%) of patients with LBP in this study received an opioid prescription within the baseline period, although the majority of patients who received opioids were prescribed only a low dose. The duration of opioid therapy either before or after the baseline period is unknown. Our findings indicate that patients who received an opioid prescription in the baseline period differed significantly in their baseline characteristics from those who were not prescribed opioids and that these differences were more marked for those in the higher dose groups. Those prescribed opioids reported higher pain intensity, including leg pain, and greater disability, and were also more likely to receive medication for comorbid conditions and to report higher distress, greater fear of movement, a greater tendency to catastrophize, and lower self-efficacy.
The proportion of subjects receiving an early opioid prescription for LBP is higher in this study than the 21.2% reported by Webster et al. [36] in the United States and substantially higher than the 7.1% reported by Gross et al. [15] in Canada, although it is similar to that found by Franklin et al. [14] in the United States. It is likely that the variation in reported levels of opioid prescribing reflect differences in study setting, methods, and population.
Our findings are consistent with those of previous studies [14], [15], [36], which reported higher disability, as measured by continued receipt of wage replacement benefits, in those who received an early opioid prescription for LBP. However, these studies, although they attempted to adjust for some measure of injury severity and basic demographics, were not able to adjust for important potential confounders, including psychosocial variables. Gross et al. [15] found a similar association between prescribed nonopioid analgesics and future disability, and concluded that their findings were likely to be explained by pain severity or other unmeasured confounders. It is perhaps surprising that in our study the association between an opioid prescription at baseline and disability at follow-up remained significant after adjusting for pain intensity and a large number of other potential confounders, whereas no significant association was demonstrated between an NSAID prescription and disability at follow-up, even in the unadjusted analysis. It is difficult to offer a plausible explanation, on purely pharmacological grounds, for why opioids should be associated with greater disability even at very low doses, and although we controlled for a substantial number of known and observed potential confounders, we were not able to adjust for past history of opioid use, smoking history, or the presence of all other painful conditions, and the possibility of residual confounding by unknown factors cannot be excluded.
The major strength of this study lies in combining the use of electronic prescribing records with self-reported measures, allowing us to accurately calculate the morphine equivalent dose and to adjust for a large number of potential confounders, including pain severity, nonopioid analgesic (NSAID) prescription, and psychosocial variables. Other strengths of the study include its prospective design, sample size, and unique primary care setting, which, in the UK health service, is where most new episodes of LBP are managed.
The use of a propensity score approach to adjust for multiple confounders is more efficient than a multivariable regression that includes each variable separately. This leads to less bias in the estimates and more generalizable results [28]. There was loss to follow-up in the original cohort. The 715 participants included in the sample had a slightly higher mean (SD) age than the original study cohort and were slightly more likely to be female, but there were no significant differences in terms of key sociodemographic characteristics, employment status, duration of back pain symptoms, or baseline pain and disability [13]. Given that responders and nonresponders from the original study [13] were almost identical on key baseline characteristics, this offers some reassurance regarding the likelihood of nonresponse bias, although the possibility of unmeasured differences cannot be excluded.
We used electronic databases, which were likely to contain complete data regarding all prescribed opioids. However, opioids can be purchased in low doses without prescription in the UK, and a potential limitation of our study is that it did not capture the use of over-the counter opioid-containing medications. Inequality in the number of subjects in each opioid dose group and in particular the small numbers in the high-dose groups limited our ability to explore the effect of opioid dose on disability, nor could we explore the effect of prescribed opioid use before enrolment in the original study.
Our findings indicate that even after adjusting for a large number of covariates, receipt of an opioid prescription did not improve functional outcome from LBP. Furthermore, our findings suggest that those who are prescribed opioids differ not only in terms of the nature and intensity of reported pain but also in terms of how they respond to that pain, as assessed by self-reported distress, self-efficacy, and coping strategies. It is possible that these and other responses may influence not only disability associated with LBP, but also prescribing behavior of clinicians [31]. It is also possible that patient preference for or against treatment with prescribed opioid analgesics is associated with other differences in patient behavior in the presence of LBP, and that such differences may in turn influence disability.
This study found that opioids were commonly prescribed for patients with LBP, and yet our findings and the previously published literature in this field provide little evidence that this is a useful therapeutic strategy. Although individual randomized controlled trials of opioid analgesics in LBP have demonstrated evidence of short-term pain relief and modest functional improvement in highly selected subjects with LBP, reviews [9], [19] have highlighted the lack of evidence for longer-term pain relief or clinically significant functional improvement. A further recent review [37] concluded that in terms of efficacy in LBP, opioids could not be recommended as a first-line treatment for LBP in view of their side effect profile, potential for tolerance with long-term use, and in the absence of any evidence of superior efficacy compared with NSAIDs. However, it is likely that in recent years, concerns about cardiovascular risk with certain NSAIDs have led to changes in prescribing practice, including increased prescribing of opioid analgesics [2]. It is important that clinicians are aware that prescribing opioids for LBP may not improve patient function. This may encourage closer monitoring of the response to opioid analgesics in terms of both pain and function, providing the opportunity to discontinue where there is no evidence of benefit, and may encourage consideration of alternative therapeutic strategies, including nonpharmacological and psychologically based approaches.
Our findings raise a number of questions for future research. Patients with LBP represent a heterogeneous population, and it is likely that response to opioids and other analgesic medications may differ between subgroups [37]. It is possible that although on average opioids do not improve function in LBP, this conceals a range of responses, including some subjects who obtain functional benefits and others who have a poor response to opioids. An important objective for future studies would be to identify the subgroups of patients who might benefit. Furthermore, identifying the factors that influence a clinician’s decision to prescribe opioids and a patient’s decision to take them might identify potential causal mechanisms for our findings. Pragmatic clinical trials in real-life settings with long-term follow-up are required to inform clinical decisions regarding the management of LBP. The incorporation of subgroup analysis into such trials may further aid the development of clinical recommendations for the management of this heterogeneous patient group.
5. Conflict of interest
There are no conflicts of interest to declare.
Acknowledgements
The authors thank Danielle van der Windt for her advice and feedback on the manuscript. The authors also thank Milisa Blagojevic-Bucknall and John Bedson for their advice. The BeBack study was supported by Arthritis Research UK, and the North Staffordshire Primary Care Research Consortium. Kate Dunn is supported by a Wellcome Trust Research Career Development Fellowship [083572]. Daniel Green is funded by a National Institute for Health Research School for Primary Care Research studentship. The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Adams N.J., Plane M.B., Fleming M.F., Mundt M.P., Saunders L.A., Stauffacher E.A. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage. 2001;22:791–796. doi: 10.1016/s0885-3924(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 2.Bedson J., Belcher J., Martino O.I., Ndlovu M., Rathod T., Walters K., Dunn K.M., Jordan K.P. The effectiveness of national guidance in changing analgesic prescribing in primary care from 2002 to 2009: an observational database study. Eur J Pain. 2002;2012 doi: 10.1002/j.1532-2149.2012.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 4.Breckenridge J., Clark J.D. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–350. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 5.Burton A.K., McClune T.D., Clarke R.D., Main C.J. Long-term follow-up of patients with low back pain attending for manipulative care: outcomes and predictors. Man Ther. 2004;9:30–35. doi: 10.1016/s1356-689x(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 6.Caudill-Slosberg M.A., Schwartz L.M., Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. PAIN®. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Chou R., Ballantyne J.C., Fanciullo G.J., Fine P.G., Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Chou R., Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande A., Furlan A., Mailis-Gagnon A., Atlas S., Turk D. Opioids for chronic low-back pain. Cochrane Database Syst Rev. 2007:CD004959. doi: 10.1002/14651858.CD004959.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Dunn K.M., Saunders K.W., Rutter C.M., Banta-Green C.J., Merrill J.O., Sullivan M.D., Weisner C.M., Silverberg M.J., Campbell C.I., Psaty B.M., Von K.M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksen J., Sjogren P., Bruera E., Ekholm O., Rasmussen N.K. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. PAIN®. 2006;125:172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Fanciullo G.J., Ball P.A., Girault G., Rose R.J., Hanscom B., Weinstein J.N. An observational study on the prevalence and pattern of opioid use in 25,479 patients with spine and radicular pain. Spine (Phila Pa 1976) 2002;27:201–205. doi: 10.1097/00007632-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 13.Foster N.E., Bishop A., Thomas E., Main C., Horne R., Weinman J., Hay E. Illness perceptions of low back pain patients in primary care: what are they, do they change and are they associated with outcome? PAIN®. 2008;136:177–187. doi: 10.1016/j.pain.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Franklin G.M., Stover B.D., Turner J.A., Fulton-Kehoe D., Wickizer T.M. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976) 2008;33:199–204. doi: 10.1097/BRS.0b013e318160455c. [DOI] [PubMed] [Google Scholar]
- 15.Gross D.P., Stephens B., Bhambhani Y., Haykowsky M., Bostick G.P., Rashiq S. Opioid prescriptions in Canadian workers’ compensation claimants: prescription trends and associations between early prescription and future recovery. Spine (Phila Pa 1976) 2009;34:525–531. doi: 10.1097/BRS.0b013e3181971dea. [DOI] [PubMed] [Google Scholar]
- 16.Harland N.J., Georgieff K. Development of the coping strategies questionnaire 24, a clinically utilitarian version of the coping strategies questionnaire. Rehabil Psychol. 2003;48:296. [Google Scholar]
- 17.Kidner C.L., Mayer T.G., Gatchel R.J. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuijpers T., van Middelkoop M., Rubinstein S.M., Ostelo R., Verhagen A., Koes B.W., van Tulder M.W. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J. 2011;20:40–50. doi: 10.1007/s00586-010-1541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martell B.A., O’Connor P.G., Kerns R.D., Becker W.C., Morales K.H., Kosten T.R., Fiellin D.A. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas M.K. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11:153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Office for National Statistics . vol. 2. The Stationary Office; London: 2000. (Standard occupational classification. The coding index). [Google Scholar]
- 22.Office for National Statistics. The National Statistics socioeconomic classification user manual. Version 2. London: ONS; 2002.
- 23.Patrick D.L., Deyo R.A., Atlas S.J., Singer D.E., Chapin A., Keller R.B. Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976) 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Perkins A.J., Kroenke K., Unutzer J., Katon W., Williams J.W., Hope C., Callahan C.M. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Porcheret M., Hughes R., Evans D., Jordan K., Whitehurst T., Ogden H., Croft P. North Staffordshire General Practice Research Network. Data quality of general practice electronic health records: the impact of a program of assessments, feedback, and training. J Am Med Inform Assoc. 2004;11:78–86. doi: 10.1197/jamia.M1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid M.C., Engles-Horton L.L., Weber M.B., Kerns R.D., Rogers E.L., O’Connor P.G. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roland M., Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum P.R., Rubin D.B. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 29.Saunders K.W., Dunn K.M., Merrill J.O., Sullivan M., Weisner C., Braden J.B., Psaty B.M., Von K.M. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stover B.D., Turner J.A., Franklin G., Gluck J.V., Fulton-Kehoe D., Sheppard L., Wickizer T.M., Kaufman J., Egan K. Factors associated with early opioid prescription among workers with low back injuries. J Pain. 2006;7:718–725. doi: 10.1016/j.jpain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Turk D.C., Okifuji A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clin J Pain. 1997;13:330–336. doi: 10.1097/00002508-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Turner J.A., Franklin G., Fulton-Kehoe D., Sheppard L., Stover B., Wu R., Gluck J.V., Wickizer T.M. ISSLS prize winner: early predictors of chronic work disability: a prospective, population-based study of workers with back injuries. Spine (Phila Pa 1976) 2008;33:2809–2818. doi: 10.1097/BRS.0b013e31817df7a7. [DOI] [PubMed] [Google Scholar]
- 33.Vickers A.J. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlaeyen J.W., Kole-Snijders A.M., Boeren R.G., van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. PAIN®. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 35.Von Korff M., Saunders K., Thomas R.G., Boudreau D., Campbell C., Merrill J., Sullivan M.D., Rutter C.M., Silverberg M.J., Banta-Green C., Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster B.S., Verma S.K., Gatchel R.J. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32:2127–2132. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- 37.White A.P., Arnold P.M., Norvell D.C., Ecker E., Fehlings M.G. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976) 2011;36:S131–S143. doi: 10.1097/BRS.0b013e31822f178f. [DOI] [PubMed] [Google Scholar]
- 38.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]