Abstract
AIM: To investigate the effects of gastrin and cholecystokinin (CCK) and their specific antagonists on the growth of pancreatic and biliary tract cancer cell lines.
METHODS: Five pancreatic and 6 biliary cancer cell lines with 2 control cells were used in this study. Cell proliferation study was done using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) test and direct cell count method. Reverse transcription-polymerase chain reaction (RT-PCR) and slot blot hybridization were performed to examine and quantify the expression of hormonal receptors in these cell lines.
RESULTS: SNU-308 showed a growth stimulating effect by gastrin-17, as did SNU-478 by both gastrin-17 and CCK-8. The trophic effect of these two hormones was completely blocked by specific antagonists (L-365, 260 for gastrin and L-364, 718 for CCK). Other cell lines did not respond to gastrin or CCK. In RT-PCR, the presence of CCK-A receptor and CCK-B/gastrin receptor mRNA was detected in all biliary and pancreatic cancer cell lines. In slot blot hybridization, compared to the cell lines which did not respond to hormones, those that responded to hormones showed high expression of receptor mRNA.
CONCLUSION: Gastrin and CCK exert a trophic action on some of the biliary tract cancers.
Keywords: Bile duct cancer, Gallbladder cancer, Pancreatic cancer, Gastrin, Cholecystokinin
INTRODUCTION
Carcinomas of the pancreas and biliary tract remain a challenge to clinicians. The anatomical complexity and late diagnosis of such carcinomas have led to a disappointingly low resectability rate of around 10-20% especially in pancreatic cancer[1]. Moreover, even if it is possible to resect the tumor with clear margin, early recurrence and metastasis are frequently observed. The overall 5-year survival rates are reported as below 15% in pancreatic cancer and 15-50% in other biliary and periampullary cancers[2,3].
No adjuvant treatments have shown success in improving survival in periampullary cancers including pancreatic cancer until now. So with the progress of surgical treatment and early detection methods, new approaches including gene therapy and new chemotherapeutic drugs are definitely required to improve treatment results[4,5]. One such approach might be hormonal manipulation, which is currently accepted as the standard treatment modality for breast, prostate and thyroid cancers.
The gastrointestinal hormones gastrin and cholecystokinin (CCK) are structurally related. The former is known to be a stimulant of acid secretion by gastric mucosa, the latter a stimulant of enzyme secretion by the pancreas and contraction of the gallbladder. While exerting these classical actions, gastrin and CCK are also considered to act as growth stimulants for gastrointestinal malignancies such as gastric and colon cancer[6-9].
However, studies on the trophic effect of these hormones on pancreatic cancer have provided conflicting results. Although some researchers have documented that gastrin and CCK stimulate the growth of pancreatic cancer cells[10,11] and are involved in the early carcinogenesis, others have refuted these effects[12,13]. Few investigations have been performed in the bile duct cancer due to the shortage of cancer cell lines and tumor models in the biliary tract.
In the present study, we investigated the effects of gastrin and CCK on the growth of pancreatic and biliary tract cancer cell lines established at the Cancer Research Institute of Seoul National University College of Medicine, and the expression of hormonal receptors.
MATERIALS AND METHODS
Cell lines
The human cancer cell lines in the biliary tract and pancreas used in this experiment were SNU-245, SNU-308, SNU-478, SNU-869, SNU-1079, SNU-1196, SNU-213, SNU-324, SNU-410, PANC-1, Mia-PaCa. The SNU series were established at the Korean Cell Line Bank in the Cancer Research Institute of Seoul National University College of Medicine, Seoul, Korea[14,15]. Cell lines PANC-1, Mia-PaCa, LoVo and HT-1080 were obtained from the American Type Culture Collection (Rockville, MD).
SNU-245 was established from distal common bile duct cancer. SNU-478 and SNU-869 were obtained from ampulla of Vater cancer, SNU-1079 and SNU-1196 from upper bile duct cancer, and SNU-308 from gallbladder cancer. SNU-213, SNU-324 and SNU-410 were acquired from pancreatic head cancer. The characteristics of the cell lines are summarized in Table 1.
Table 1.
Characteristics of pancreatic cancer and periampullary cancer cell lines.
| Cell line |
In vivo |
In vitro |
|||||
| Origin | Degree ofdifferentiation | Nodal status | Growthcharacteristics | Viability | Doubling time | Cellmorphology | |
| SNU-245 | CBD | Well | 0/9 | Ad | 85 | 54 | P |
| SNU-308 | GB | Moderately | - | Ad | 88 | 4848 | P |
| SNU-478 | AoV | Poorly | 0/8 | Ad | 83 | 52 | P/S |
| SNU-869 | AoV | Well | 5/10 | Ad | 91 | 48 | P |
| SNU-1079 | IHD | Moderately | - | Ad | 89 | 72 | Ple |
| SNU-1196 | HDB | Moderately | 0/1 | Ad | 94 | 48 | Spindle to P |
| SNU-213 | Pancreas | Moderately | 1/8 | Ad | 89 | 48 | P |
| SNU-324 | Pancreas | Poorly | 0/15 | Ad+Fl | 95 | 52 | P/S |
| SNU-410 | Pancreas | Poorly | - | Ad | 92 | 72 | P |
CBD: common bile duct, GB: gallbladder, AoV: ampulla of Vater, Ad: adherent, Fl: floating, P: polygonal, S: spherical, Ple: pleomorphic.
Cell lines were grown in RPMI 1640 (GIBCO-BRL, Rockville, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in a humidified incubator at 37 °C in an atmosphere of 50 mL/L CO2.
Cell proliferation assay
MTT assay A colorimetric assay using tetrazolium salt, 3-[4,5 dimethyl thiazole-2-yl]-2,5 diphenyl tetrazolium bromide (MTT), was used to determine the hormonal stimulation effect on the cancer cell lines and the optimal stimulation concentration of the hormones. A single cell suspension was prepared and the cell number was calculated. MTT assay was performed as previously described[16].
An equal number of cells were inoculated into each well in 100 μL of serum free culture medium to which gastrin-17 (Sigma, St. Louis, MO) or CCK-8 (Sigma, St. Louis, MO) was added in a range from 10-6 mol/L to 10-12 mol/L. Twenty μL of gastrin-17 or CCK-8 was added daily to each well in order to maintain the determined hormonal concentration. After five days of culture, 0.1 mg MTT was added to each well and incubated at 37 °C for 4 more hours. Plates were centrifuged at 450 g for 5 min at room temperature and the medium was then aspirated. Dimethyl sulfoxide (150 μL) was added to each well to dissolve the crystals. The plates were read immediately at 540 nm on a scanning multi-well spectrophotometer. All experiments were performed 4 times.
We also performed another MTT assay to find the optimal inhibitory concentration of specific antagonists and to observe whether autocrine or paracrine effect of gastrin or CCK could affect the tumor cell growth by application of hormone receptor antagonists in the absence of exogenous ligand. L-364, 718 (antagonist for CCK-A receptor) and L-365, 260 (antagonist for CCK-B/gastrin receptor) were used in a range from 10-6 mol/L to 10-12 mol/L (10-9 mol/L), which were kindly supplied by ML laboratory in UK. Test condition, except for antagonists, was same as above.
Test for optimal antagonist concentration was performed in cell lines, which showed hormone-dependent growth stimulation. Under the optimal growth stimulation concentration of hormones, various concentrations of specific antagonists (range: 10-6-10-12 mol/L) were added (data not shown).
Direct cell count Cell lines showing a hormonal growth-stimulating effect in the MTT assay were selected for the direct cell count test to confirm the effect of hormones and whether the hormonal trophic effect on cancer cells could be blocked by specific antagonists with the concentration determined by MTT test.
Cells (30×104 for SNU-308; 5×104 for SNU-478) were plated onto 25 cm2 flasks (Falcon, Franklin Lake, NJ) in serum free RPMI 1640 medium. After twenty-four hours, cells were treated with hormone (CCK-8 or gastrin-17), hormonal antagonists (L-364, 718 (10-9 mol/L), antagonist for CCK-A receptors; L-365, 260 (10-9 mol/L), antagonist for CCK-B/gastrin receptors), combinations of hormones with their antagonist, or medium alone (control). Medium and reagents were added daily. Every third day, we harvested the cells and counted the cell number using a hemocytometer. Each experiment was performed in triplicate.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted according to the manufacturer’s instructions (TRIzol®, GIBCO-BRL, Rockville, MD). RNA concentration was measured spectrophotometrically at 260 nm and the integrity of mRNA was controlled by analyzing the ribosomal RNA content by electrophoresis on agarose gel.
After quantification of the extracted RNA, first strand complementary DNA (cDNA) of each cancer cell line was synthesized from 2 μg of total RNA using Molony murine leukemia virus reverse transcriptase (GIBCO-BRL, Rockville, MD). PCR reactions were performed in a total of 20 μg, composed of 2 μL cDNA, 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.25 mmol/L dNTPs, 1 μmol/L of each oligonucleotide primer, and 1 U of Taq DNA polymerase (Takara, Shiga, Japan). We used the primers described by Mandair et al[17] for CCK-A receptor, CCK-B/gastrin receptor, and β-actin synthesized in Bioneer Co. (Chungbuk, Korea). The forward primers were 5’-CCTACGACACCGCCT-CCGC-3’, 5’-ACCCCAACGACAGGAAAAGGT-3’, and 5’-CACTGTGTTGGCGTACAGGT-3’; the reverse primers were 5’-TCCGTTCTTTCTTCTCTGCCTCCT-3’, 5’-TTTGGGAAGGAAGGAGAGGGC-3’, and 5’-TCATCACCATTGGCAATGAG-3’ for the CCK-A receptor, CCK-B/gastrin receptor, and β-actin respectively. The amplification reaction involved denaturation at 94 °C for 5 min followed by the following cycling: for CCK-A receptor, 40 cycles of denaturation at 94 °C for 1 min, annealing for 1 min at 55 °C and extension for 1 min 30 s at 97 °C; for CCK-B/gastrin receptor, 40 cycles of denaturation at 94 °C for 1 min, annealing for 1 min at 58 °C and extension for 1 min 30 s at 97 °C; for β-actin, 35 cycles of denaturation at 94 °C for 1 min, annealing for 1 min at 55 °C and extension for 1 min at 97 °C.
The reaction proceeded in a DNA thermal cycler (Hybaid, Middlesex, U.K.), and the products of amplification were submitted to electrophoresis on 1.5% agarose gel and visualized with ethidium bromide staining.
Slot blot hybridization
Quantitative gene expression was determined by slot blot analysis. RNA samples were denatured at 68 °C for 15 min. Four different concentrations (10, 3, 1, and 0.3 µg) of RNA were loaded on per slot. Blots were prehybridized in buffer (5×SSPE, 30% formamide, 5×Denhardt’s solution, 1% SDS and 100 µg/mL salmon sperm DNA) for 2 h at 42 °C. Overnight hybridization was performed at 42 °C with a 32P-radiolabeled probe. The complementary riboprobes were prepared by ligating PCR products for CCK-A receptor, CCK-B/ gastrin receptor and β-actin gene cDNA into the pCR® II-TOPO® vector, using a TA cloning kit (Invitrogen, Carlsbad, CA, USA). The appropriate templates for each RNA probe were generated by linearization with a restriction endonuclease EcoRI. Radiolabeling of the probes was performed with [α-32P] deoxycytidine triphosphate using a Prime-It® II random primer labeling kit (Stratagene, La Jolla, CA, USA). After hybridization, the blots were washed twice in 2× SSPE plus 0.2% SDS at 42 °C for 20 min, then once with 0.1×SSPE at 42 °C for 15 min, and finally subjected to autoradiography with Fuji Super RX® film at -70 °C. Hybridization with a β-actin RNA probe was used to correct RNA loading. The autoradiograms were analyzed and quantitated by densitometry (BIPS ®, Biomedlab, Seoul, Korea).
Statistical analysis
Data were expressed as mean±SE. Comparison between groups was made using Mann-Whitney U test, P<0.05 was considered statistically significant.
RESULTS
MTT assay
The SNU-308 cell line originating from gallbladder cancer responded to the addition of gastrin-17, and in particular showed a maximum growth stimulating effect at the gastrin-17 concentration of 10-9 mol/L. At this concentration 27% of the growth stimulating effect could be seen compared to the control (Figure 1A). SNU-478 from ampulla of Vater cancer exhibited similar stimulating effects to both gastrin-17 (21%) and CCK-8 (23%) (Figure 1B). However, no significant differences between control and hormone treated cultures were observed at any concentration in any of the other cell lines. MIAPaCa-2 and PANC-1 showed similar results to those of the other pancreatic cell lines (Tables 2, 3).
Figure 1.

Effects of gastrin and CCK on SNU-308 from gallbladder cancer (A), SNU-478 from ampulla of Vater cancer (B). aP<0.05 vs control.
Table 2.
Effect of gastrin and L-365,260 on periampullary cancer cell lines.
| Cell line | Origin |
Maximum response to hormones and hormone concentration |
|||||
|
Gastrin |
L-365, 260 |
||||||
| Response (%) | Concentration (mol/L) | P-value | Response (%) | Concentration (mol/L) | P-value | ||
| SNU-245 | CBD | 108 | 10-7 | NS | 98 | 10-8 | NS |
| SNU-308 | GB | 127 | 10-9 | 0.038 | 96 | 10-9 | NS |
| SNU-478 | AoV | 121 | 10-9 | 0.047 | 94 | 10-9 | NS |
| SNU-869 | AoV | 107 | 10-6 | NS | 103 | 10-7 | NS |
| SNU-1079 | IHD | 107 | 10-9 | NS | 104 | 10-11 | NS |
| SNU-1196 | HDB | 104 | 10-8 | NS | 93 | 10-7 | NS |
| SNU-213 | Pancreas | 105 | 10-10 | NS | 97 | 10-8 | NS |
| SNU-324 | Pancreas | 112 | 10-11 | NS | 97 | 10-9 | NS |
| SNU-410 | Pancreas | 115 | 10-12 | NS | 98 | 10-8 | NS |
| MIAPaCa-2 | Pancreas | 106 | 10-10 | NS | 95 | 10-9 | NS |
| PANC-1 | Pancreas | 101 | 10-7 | NS | 94 | 10-8 | NS |
Table 3.
Effect of CCK and L-364,718 on periampullary cancer cell lines.
| Cell line | Origin |
Maximum response to hormones and hormone concentration |
|||||
|
CCK |
L-364, 718 |
||||||
| Response (%) | Concentration (mol/L) | P-value | Response (%) | Concentration (mol/L) | P-value | ||
| SNU-245 | CBD | 110 | 10-12 | NS | 96 | 10-10 | NS |
| SNU-308 | GB | 109 | 10-7 | NS | 94 | 10-8 | NS |
| SNU-478 | AoV | 123 | 10-9 | 0.043 | 93 | 10-9 | NS |
| SNU-869 | AoV | 107 | 10-10 | NS | 97 | 10-10 | NS |
| SNU-1079 | IHD | 113 | 10-9 | NS | 101 | 10-8 | NS |
| SNU-1196 | HDB | 109 | 10-8 | NS | 94 | 10-8 | NS |
| SNU-213 | Pancreas | 106 | 10-7 | NS | 98 | 10-6 | NS |
| SNU-324 | Pancreas | 116 | 10-12 | NS | 96 | 10-10 | NS |
| SNU-410 | Pancreas | 113 | 10-9 | NS | 94 | 10-8 | NS |
| MIAPaCa-2 | Pancreas | 108 | 10-10 | NS | 97 | 10-9 | NS |
| PANC-1 | Pancreas | 105 | 10-8 | NS | 98 | 10-8 | NS |
Suppression of cell growth by hormonal receptor antagonists (without exogenous hormone) was not definite in all pancreatobiliary cancer cell lines (Tables 2 , 3).
Effects of hormonal antagonists
The stimulatory effect of gastrin-17 exposure on the growth rate of SNU-308 cells was blocked by the specific antagonist L-365, 260. The growth stimulation effects of both the hormones on SNU-478 were blocked by their respective specific antagonists (Figure 2).
Figure 2.
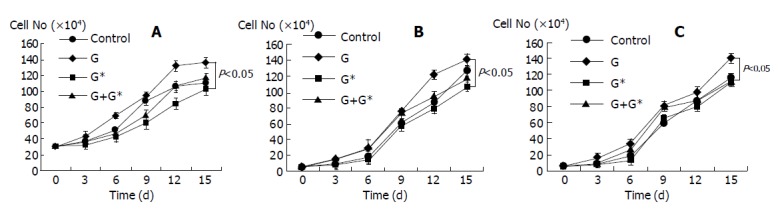
Growth curves for SNU-308 (A), and SNU-478 (B, C) cells grown with either hormone or hormone combined with antagonist or antagonist, or medium only. G: gastrin-17, G*: antagonist for CCK-B/gastrin receptor (L-365,260), C: CCK-8, C*: antagonist for CCK-A receptor (L-364,718).
RT-PCR for CCK-A and CCK-B/gastrin receptors
Amplification of cDNA yielded an approximately 340 bp fragment from the CCK-A receptor and 430 bp fragment from the CCK-B/gastrin receptor (Figure 3). Sequence analysis confirmed that each kind of PCR products was identical to that of the expected sequence[18,19]. CCK-A receptor and CCK-B/gastrin receptor mRNA were detected in all biliary and pancreatic cancer cell lines. We utilized the Mia PaCa-2 cell line as a positive control for the expression of CCK-A receptor[17] and the LoVo cell line for the CCK-B/gastrin receptor[20].
Figure 3.
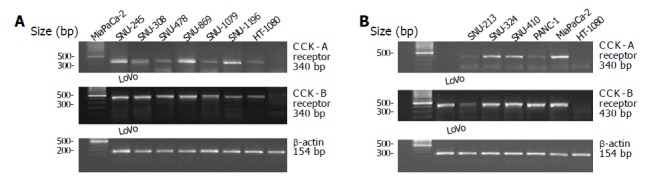
Amplification of CCK-A and CCK-B receptor mRNA in human biliary tract cancer cell lines (A) and pancreatic cancer cell lines (B) by RT-PCR.
Slot blot assay for CCK-A and CCK-B/gastrin receptors
CCK-A and CCK-B/gastrin receptor mRNA levels were measured by slot blot hybridization. The autoradiographic signals were compared with those of Mia PaCa-2 (for CCK-A receptor) and LoVo (for CCK-B/gastrin receptor). Figure 4 presents the slot blot assay results. SNU-308 and SNU-478 demonstrated a high expression of receptor mRNA compared with other cell lines that did not respond to hormones.
Figure 4.
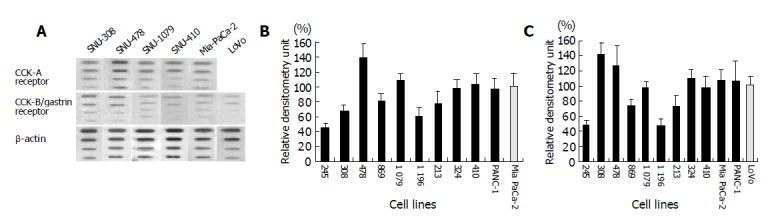
Representative slot blot hybridization. (A) Data from SNU-308, 478, 1079, 410, Mia PaCa-2, and LoVo cell lines. (B, C) Densitometrically quantified slot blots.
DISCUSSION
Gastrin and CCK, which share a peptide structure including C-terminal pentapeptide amide (Gly-Trp-Met-Asp-PheNH2), exert a trophic effect on the normal gastrointestinal organs, including the stomach, gallbladder and duodenum, in addition to the stimulation of secretion in the stomach and pancreas[21-23]. Except for these classical actions, gastrin and CCK also appear to stimulate growth of gastrointestinal cancers. Chronic endogenous hypergastrinemia and exogenous pentagastrin administration are known to significantly increase tumor cell number and the concentration of DNA, RNA, and protein in colon and gastric cancers[6,24,25].
Similar trophic effects of gastrin and CCK have been reported in pancreatic cancer. Smith et al[10] reported that the growth responses of 6 human pancreatic cancer cell lines (SW-1990, PANC-1, MIA PaCa-2, BxPC-3, RWP-2 and CAPAN-2) are stimulated by CCK in serum-free medium, and its trophic effect can be blocked by a specific antagonist[26]. They also demonstrated that gastrin exerts a growth-stimulating effect on pancreatic cancer in vivo and in vitro[27]. Furthermore many reports about the trophic effect of CCK and gastrin on pancreatic cancer have been published[11,28-30] and some studies have demonstrated that these hormones play a role in gastrointestinal cancer carcinogenesis[31,32]. On the contrary, other studies have suggested that gastrin and CCK may have no trophic effect on pancreatic cancer, and even exert an inhibitory effect on pancreatic growth and carcinogenesis.
Liehr et al[12] reported that CCK cannot affect the growth of PANC-1 and MIA PaCa-2 at the concentration of 10-12-10-6 mol/L. Robertson et al[13] showed that gastrin also has no trophic effect on the same pancreatic cancer cell lines. Recently, Detjen et al[33] demonstrated that CCK-A and CCK-B/gastrin receptors mediate growth inhibitory responses in pancreatic cancer in their experiment on the transfection of hormone receptors into the PANC-1 and MIA PaCa-2 lines.
In the experiment with cholangiocarcinoma cells (SLU 132), the growth of cancer xenografted in nude mice is significantly retarded by CCK[34]. Evers et al[35] demonstrated that CR-1409, a CCK receptor antagonist, prevents caerulein-mediated inhibition of cancer growth when combined with caerulein administration in SLU 132. Though a few cancer cell lines have been established in the biliary tract, there are limitations in the inhibitory effect of CCK on bile duct cancer as described above. In our study, cancer cell lines originating from the biliary tract exhibited a growth stimulating effect by gastrin or CCK. Therefore, further study using well-established cancer cells is mandatory to determine the hormonal trophic effect on biliary tract cancer.
We found that the trophic action of hormones was influenced by the degree of expression of its receptors, suggesting that biological response to peptide hormones is modulated by the amount of receptors in target cells. Some investigators explained that the trophic response of cancer to gastrin and CCK is determined by the presence of specific hormonal receptors, so that the responsiveness of tumor to hormone treatment can be predicted by the presence or absence of the receptor[7]. However, this explanation does not account for the conflicting results of stimulatory or inhibitory effect on the same cancer cells according to the investigators. In our study, most pancreatic and biliary cancer cell lines have CCK-A and CCK-B receptors. However, only 2 cell lines demonstrated tumor growth-stimulating effect by gastrin (SNU-308, SNU-478) and only one cell line exhibited tumor growth-stimulating effect by CCK (SNU-478). PANC-1 and Mia PaCa-2, the most prevalently studied cell lines in similar experiments, did not show any growth-stimulating effects by gastrin and CCK though both CCK-A and CCK-B/gastrin receptors presented. This non-response to exogenous hormone can be explained that endogenous hormonal stimulation is enough for the trophic action. However, we could not find sufficient endogenous growth stimulation, which is contrary to some reports[36]. We think endogenous stimulation effect is minimal even if it exists.
Another possibility is the diversity or mutation of specific hormonal receptors. Peptide hormones express their biological activity by binding to specific hormone receptors. Receptors for CCK have been pharmacologically classified on the basis of their affinity for both the peptide agonists CCK and gastrin, which share the same COOH-terminal pentapeptide amide sequence but differ in sulfation at the 6th (gastrin) and 7th (CCK) tyrosyl residue, and the recently developed subtype-specific antagonists.
Although two types of CCK receptors (CCK-A and CCK-B/gastrin) are well known, the possibility of new non-A non-B receptor types has recently been reported. Imdahl et al[37] reported that the expression of low affinity binding gastrin/CCK-C receptor is found in 75% of human colorectal carcinomas. Smith et al[38] recently showed that the new CCK receptor is expressed in pancreatic cancer specimens and they therefore designated it as CCK-C (cancer) receptor.
Recent advances in molecular biology reveal a broader distribution of CCK receptors in the gastrointestinal and central nervous systems than previously recognized, thereby suggesting additional physiological roles for these receptors. Although CCK receptors feature homology in structure among different species and even intra-species, slight differences in receptor structure and distribution result in significant pharmacological and physiological differences[39,40]. Minor peptide changes, even the difference of a single amino acid by mutation or polymorphism of the CCK receptor, can profoundly affect the binding affinities of hormones and antagonists, enabling antagonists to act as agonists and vice versa[41,42]. Actually, the functional effect and clinical significance of receptor mutations have been studied in some diseases[43].
Based on the present study, it may be concluded that specific receptors for gastrin and CCK exert a trophic action on some of the biliary tract cancers. However, many cancer cell lines cannot be affected by hormones despite the presence of CCK-A and CCK-B/gastrin receptors, suggesting the possibility of hormonal manipulation in limited cases of pancreatic and biliary tract cancer. An accurate method for the identification of hormonally responsive cancers is therefore required before adjunctive hormonal or antihormonal therapy can be recommended. Therefore, further investigations are required to elucidate the mechanism of the secondary signal pathway linked to the CCK receptor family, and to determine the functional and structural differences among the receptors. Furthermore, mutation or polymorphism studies of CCK receptors may be needed to ascertain the trophic or inhibitory effect of gut hormones.
Footnotes
Supported by a grant from Seoul National University Research Fund (03-99-080 and 082)
Edited by Wang XL
References
- 1.Janes RH, Niederhuber JE, Chmiel JS, Winchester DP, Ocwieja KC, Karnell JH, Clive RE, Menck HR. National patterns of care for pancreatic cancer. Results of a survey by the Commission on Cancer. Ann Surg. 1996;223:261–272. doi: 10.1097/00000658-199603000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet M, Gamagami RA, Gilpin EA, Romeo O, Sasson A, Easter DW, Moossa AR. Factors influencing survival after resection for periampullary neoplasms. Am J Surg. 2000;180:13–17. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- 3.Wade TP, el-Ghazzawy AG, Virgo KS, Johnson FE. The Whipple resection for cancer in U.S. Department of Veterans Affairs Hospitals. Ann Surg. 1995;221:241–248. doi: 10.1097/00000658-199503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiernik PH. Current status of and future prospects for the medical management of adenocarcinoma of the exocrine pancreas. J Clin Gastroenterol. 2000;30:357–363. doi: 10.1097/00004836-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg L, Lipsett M. Biotherapeutic approaches to pancreatic cancer. Expert Opin Biol Ther. 2003;3:319–337. doi: 10.1517/14712598.3.2.319. [DOI] [PubMed] [Google Scholar]
- 6.Watson SA, Durrant LG, Morris DL. Growth-promoting action of gastrin on human colonic and gastric tumour cells cultured in vitro. Br J Surg. 1988;75:342–345. doi: 10.1002/bjs.1800750416. [DOI] [PubMed] [Google Scholar]
- 7.Upp JR, Singh P, Townsend CM, Thompson JC. Clinical significance of gastrin receptors in human colon cancers. Cancer Res. 1989;49:488–492. [PubMed] [Google Scholar]
- 8.Zhou JJ, Chen ML, Zhang QZ, Hu JK, Wang WL. Coexpression of cholecystokinin-B/gastrin receptor and gastrin gene in human gastric tissues and gastric cancer cell line. World J Gastroenterol. 2004;10:791–794. doi: 10.3748/wjg.v10.i6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reubi JC, Schaer JC, Waser B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997;57:1377–1386. [PubMed] [Google Scholar]
- 10.Smith JP, Kramer ST, Solomon TE. CCK stimulates growth of six human pancreatic cancer cell lines in serum-free medium. Regul Pept. 1991;32:341–349. doi: 10.1016/0167-0115(91)90027-e. [DOI] [PubMed] [Google Scholar]
- 11.Blackmore M, Hirst BH. Autocrine stimulation of growth of AR4-2J rat pancreatic tumour cells by gastrin. Br J Cancer. 1992;66:32–38. doi: 10.1038/bjc.1992.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liehr RM, Melnykovych G, Solomon TE. Growth effects of regulatory peptides on human pancreatic cancer lines PANC-1 and MIA PaCa-2. Gastroenterology. 1990;98:1666–1674. doi: 10.1016/0016-5085(90)91105-f. [DOI] [PubMed] [Google Scholar]
- 13.Robertson JF, Watson SA, Hardcastle JD. Effect of gastrointestinal hormones and synthetic analogues on the growth of pancreatic cancer. Int J Cancer. 1995;63:69–75. doi: 10.1002/ijc.2910630114. [DOI] [PubMed] [Google Scholar]
- 14.Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, Kim SW, Park YH, Hwang JH, Yoon YB, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87:187–193. doi: 10.1038/sj.bjc.6600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku JL, Yoon KA, Kim WH, Jang Y, Suh KS, Kim SW, Park YH, Park JG. Establishment and characterization of four human pancreatic carcinoma cell lines. Genetic alterations in the TGFBR2 gene but not in the MADH4 gene. Cell Tissue Res. 2002;308:205–214. doi: 10.1007/s00441-001-0510-y. [DOI] [PubMed] [Google Scholar]
- 16.Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987;47:5875–5879. [PubMed] [Google Scholar]
- 17.Mandair KK, Towner P, Stamford IF, Morris JD, Harper E, Benjamin IS, Tavares IA. Cholecystokinin receptors in human pancreatic cancer cell lines. Eur J Cancer. 1998;34:1455–1459. doi: 10.1016/s0959-8049(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 18.de Weerth A, Pisegna JR, Huppi K, Wank SA. Molecular cloning, functional expression and chromosomal localization of the human cholecystokinin type A receptor. Biochem Biophys Res Commun. 1993;194:811–818. doi: 10.1006/bbrc.1993.1894. [DOI] [PubMed] [Google Scholar]
- 19.Pisegna JR, de Weerth A, Huppi K, Wank SA. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun. 1992;189:296–303. doi: 10.1016/0006-291x(92)91557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biagini P, Monges G, Vuaroqueaux V, Parriaux D, Cantaloube JF, De Micco P. The human gastrin/cholecystokinin receptors: type B and type C expression in colonic tumors and cell lines. Life Sci. 1997;61:1009–1018. doi: 10.1016/s0024-3205(97)00605-x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LR. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977;72:788–792. [PubMed] [Google Scholar]
- 22.Lamote J, Putz P, Willems G. Effect of cholecystokinin-octapeptide, caerulein, and pentagastrin on epithelial cell proliferation in the murine gallbladder. Gastroenterology. 1982;83:371–377. [PubMed] [Google Scholar]
- 23.Morisset J, Génik P. Effects of acute and chronic administration of secretin and caerulein on rat duodenal and gastric growth. Regul Pept. 1983;5:111–123. doi: 10.1016/0167-0115(83)90119-2. [DOI] [PubMed] [Google Scholar]
- 24.McGregor DB, Jones RD, Karlin DA, Romsdahl MM. Trophic effects of gastrin on colorectal neoplasms in the rat. Ann Surg. 1982;195:219–223. doi: 10.1097/00000658-198202000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumiyoshi H, Yasui W, Ochiai A, Tahara E. Effects of gastrin on tumor growth and cyclic nucleotide metabolism in xenotransplantable human gastric and colonic carcinomas in nude mice. Cancer Res. 1984;44:4276–4280. [PubMed] [Google Scholar]
- 26.Smith JP, Rickabaugh CA, McLaughlin PJ, Zagon IS. Cholecystokinin receptors and PANC-1 human pancreatic cancer cells. Am J Physiol. 1993;265:G149–G155. doi: 10.1152/ajpgi.1993.265.1.G149. [DOI] [PubMed] [Google Scholar]
- 27.Smith JP, Fantaskey AP, Liu G, Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 28.Frazier ML, Pathak S, Wang ZW, Cleary K, Singletary SE, Olive M, Mackay B, Steck PA, Levin B. Establishment of a new human pancreatic adenocarcinoma cell line, MDAPanc-3. Pancreas. 1990;5:8–16. doi: 10.1097/00006676-199001000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Townsend CM, Bold RJ, Ishizuka J. Gastrointestinal hormones and cell proliferation. Surg Today. 1994;24:772–777. doi: 10.1007/BF01636304. [DOI] [PubMed] [Google Scholar]
- 30.Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- 31.Tahara E, Shimamoto F, Taniyama K, Ito H, Kosako Y, Sumiyoshi H. Enhanced effect of gastrin on rat stomach carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1982;42:1781–1787. [PubMed] [Google Scholar]
- 32.Howatson AG, Carter DC. Pancreatic carcinogenesis-enhancement by cholecystokinin in the hamster-nitrosamine model. Br J Cancer. 1985;51:107–114. doi: 10.1038/bjc.1985.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detjen K, Fenrich MC, Logsdon CD. Transfected cholecystokinin receptors mediate growth inhibitory effects on human pancreatic cancer cell lines. Gastroenterology. 1997;112:952–959. doi: 10.1053/gast.1997.v112.pm9041258. [DOI] [PubMed] [Google Scholar]
- 34.Hudd C, LaRegina MC, Devine JE, Palmer DC, Herbold DR, Beinfeld MC, Gelder FB, Johnson FE. Response to exogenous cholecystokinin of six human gastrointestinal cancers xenografted in nude mice. Am J Surg. 1989;157:386–394. doi: 10.1016/0002-9610(89)90582-5. [DOI] [PubMed] [Google Scholar]
- 35.Evers BM, Gomez G, Townsend CM, Rajaraman S, Thompson JC. Endogenous cholecystokinin regulates growth of human cholangiocarcinoma. Ann Surg. 1989;210:317–322; discussion 322-323. doi: 10.1097/00000658-198909000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JP, Shih A, Wu Y, McLaughlin PJ, Zagon IS. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am J Physiol. 1996;270:R1078–R1084. doi: 10.1152/ajpregu.1996.270.5.R1078. [DOI] [PubMed] [Google Scholar]
- 37.Imdahl A, Mantamadiotis T, Eggstein S, Farthmann EH, Baldwin GS. Expression of gastrin, gastrin/CCK-B and gastrin/CCK-C receptors in human colorectal carcinomas. J Cancer Res Clin Oncol. 1995;121:661–666. doi: 10.1007/BF01218524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JP, Verderame MF, McLaughlin P, Martenis M, Ballard E, Zagon IS. Characterization of the CCK-C (cancer) receptor in human pancreatic cancer. Int J Mol Med. 2002;10:689–694. [PubMed] [Google Scholar]
- 39.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 40.Monstein HJ, Nylander AG, Salehi A, Chen D, Lundquist I, Håkanson R. Cholecystokinin-A and cholecystokinin-B/gastrin receptor mRNA expression in the gastrointestinal tract and pancreas of the rat and man. A polymerase chain reaction study. Scand J Gastroenterol. 1996;31:383–390. doi: 10.3109/00365529609006415. [DOI] [PubMed] [Google Scholar]
- 41.Beinborn M, Quinn SM, Kopin AS. Minor modifications of a cholecystokinin-B/gastrin receptor non-peptide antagonist confer a broad spectrum of functional properties. J Biol Chem. 1998;273:14146–14151. doi: 10.1074/jbc.273.23.14146. [DOI] [PubMed] [Google Scholar]
- 42.Bläker M, Ren Y, Gordon MC, Hsu JE, Beinborn M, Kopin AS. Mutations within the cholecystokinin-B/gastrin receptor ligand 'pocket' interconvert the functions of nonpeptide agonists and antagonists. Mol Pharmacol. 1998;54:857–863. doi: 10.1124/mol.54.5.857. [DOI] [PubMed] [Google Scholar]
- 43.Inoue H, Iannotti CA, Welling CM, Veile R, Donis-Keller H, Permutt MA. Human cholecystokinin type A receptor gene: cytogenetic localization, physical mapping, and identification of two missense variants in patients with obesity and non-insulin-dependent diabetes mellitus (NIDDM) Genomics. 1997;42:331–335. doi: 10.1006/geno.1997.4749. [DOI] [PubMed] [Google Scholar]


