Abstract
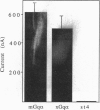
We cloned the Xenopus laevis form of Gq alpha subunit to study its effects on oocyte maturation. Injection of Xenopus Gq alpha mRNA into stage 6 oocytes activated the phospholipase C/phosphatidylinositol pathway. The oocyte membrane became permeable to calcium ions and was able to generate transient inward currents (T(in)), due to the opening of Ca(2+)-dependent Cl- channels. The T(in) amplitude developed over several hours and disappeared by 24 hr. Diacylglycerol levels were found to parallel the appearance and disappearance of the T(in). The concurrent decline of T(in) values and diacylglycerol was not due to a failure in the synthesis of Gq alpha protein, which was produced continuously for > 24 hr. After Xenopus Gq alpha mRNA injection, germinal vesicle breakdown (GVBD) was variable (0-100%) in stage 6 oocytes, whereas none of the stage 4 oocytes underwent GVBD. In contrast, stage 6 oocytes injected with mRNA encoding the Go alpha G protein consistently underwent GVBD but did not acquire T(in). Our results show that activation of phospholipase C is not an absolute requisite for the induction of maturation, although in oocytes of some frogs phospholipase C activation can trigger a pathway to GVBD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. L., Graber S. G., Waldo G. L., Harden T. K., Garrison J. C. Selective activation of phospholipase C by recombinant G-protein alpha- and beta gamma-subunits. J Biol Chem. 1994 Jan 28;269(4):2814–2819. [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Galili G., Kawata E. E., Smith L. D., Larkins B. A. Role of the 3'-poly(A) sequence in translational regulation of mRNAs in Xenopus laevis oocytes. J Biol Chem. 1988 Apr 25;263(12):5764–5770. [PubMed] [Google Scholar]
- Johnson A. D., Cork R. J., Williams M. A., Robinson K. R., Smith L. D. H-ras(val12) induces cytoplasmic but not nuclear events of the cell cycle in small Xenopus oocytes. Cell Regul. 1990 Jun;1(7):543–554. doi: 10.1091/mbc.1.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll S. D., Omri G., Landau E. M., Iyengar R. Activated alpha subunit of Go protein induces oocyte maturation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5182–5186. doi: 10.1073/pnas.88.12.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Park D., Wu D., Rhee S. G., Simon M. I. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J Biol Chem. 1992 Aug 15;267(23):16044–16047. [PubMed] [Google Scholar]
- Linder M. E., Middleton P., Hepler J. R., Taussig R., Gilman A. G., Mumby S. M. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Park D., Jhon D. Y., Kriz R., Knopf J., Rhee S. G. Cloning, sequencing, expression, and Gq-independent activation of phospholipase C-beta 2. J Biol Chem. 1992 Aug 15;267(23):16048–16055. [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):279–292. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Inositol trisphosphate activates a voltage-dependent calcium influx in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):27–36. doi: 10.1098/rspb.1987.0033. [DOI] [PubMed] [Google Scholar]
- Preiss J. E., Loomis C. R., Bell R. M., Niedel J. E. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol. 1987;141:294–300. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Ryba N. J., Findlay J. B., Reid J. D. The molecular cloning of the squid (Loligo forbesi) visual Gq-alpha subunit and its expression in Saccharomyces cerevisiae. Biochem J. 1993 Jun 1;292(Pt 2):333–341. doi: 10.1042/bj2920333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Kim U. H., Wahl M. I., Brown A. B., Carpenter G., Huang K. P., Rhee S. G. Feedback regulation of phospholipase C-beta by protein kinase C. J Biol Chem. 1990 Oct 15;265(29):17941–17945. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982 Jan 10;257(1):355–361. [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Stith B. J., Kirkwood A. J., Wohnlich E. Insulin-like growth factor 1, insulin, and progesterone induce early and late increases in Xenopus oocyte sn-1,2-diacylglycerol levels before meiotic cell division. J Cell Physiol. 1991 Nov;149(2):252–259. doi: 10.1002/jcp.1041490211. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Simon M. I. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Tigyi G., Dyer D., Matute C., Miledi R. A serum factor that activates the phosphatidylinositol phosphate signaling system in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1521–1525. doi: 10.1073/pnas.87.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992 Oct 25;267(30):21360–21367. [PubMed] [Google Scholar]
- Varnold R. L., Smith L. D. Protein kinase C and progesterone-induced maturation in Xenopus oocytes. Development. 1990 Jul;109(3):597–604. doi: 10.1242/dev.109.3.597. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Freedman A. B., LaBella J. J. sn-1,2-diacylglycerol levels increase in progesterone-stimulated Xenopus laevis oocytes. J Exp Zool. 1990 Jul;255(1):63–71. doi: 10.1002/jez.1402550109. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Richter J. D., Smith L. D. Protein synthesis during maturation promoting factor- and progesterone-induced maturation in Xenopus oocytes. Dev Biol. 1982 Jan;89(1):152–158. doi: 10.1016/0012-1606(82)90303-7. [DOI] [PubMed] [Google Scholar]
- Wu D. Q., Lee C. H., Rhee S. G., Simon M. I. Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem. 1992 Jan 25;267(3):1811–1817. [PubMed] [Google Scholar]