Abstract
We report a case of undifferentiated (embryonal) sarcoma of the liver (UESL), which showed cystic formation in a 20-year-old man with no prior history of any hepatitis or liver cirrhosis. He was admitted with abdominal pain and a palpable epigastric mass. The physical examination findings were unremarkable except for a tenderness mass and the results of routine laboratory studies were all within normal limits. Abdominal ultrasound and computed tomography (CT) both showed a cystic mass in the left hepatic lobe. Subsequently, the patient underwent a tumor excision and another two times of hepatectomy because of tumor recurrence. Immunohistochemical study results showed that the tumor cells were positive for vimentin, alpha-1-antichymotrypsin (AACT) and desmin staining, and negative for alpha-fetoprotein (AFP), and eosinophilic hyaline globules in the cytoplasm of some giant cells were strongly positive for periodic acid-Schiff (PAS) staining. The pathological diagnosis was UESL. The patient is still alive with no tumor recurrence for four months.
Keywords: Liver undifferentiated(embryonal) sarcoma
INTRODUCTION
Undifferentiated (embryonal) sarcoma of the liver (UESL) is a rare malignant hepatic tumor with poor prognosis that often presents in the pediatric population between the ages of 6 and 10 years, and rarely occurs in adults. UESL, first documented by Stocker and Ishak[1] in 1978, has been recognized as a unique clinicopathologic entity. This tumor usually appears on CT and ultrasound as a predominantly solid mass with or without cystic areas. We report a case of primary UESL, which showed cystic formation in an adult.
CASE REPORT
A 20-year-old male was presented with a palpable epigastric mass and upper abdominal pain and of 5 d’ duration. Five days ago, he abruptly suffered from intermittent sharp pain located at slightly-left side below the xiphoid process, which was aggravated on deep breath or raise of abdominal pressure but relieved at quiet rest, with no predisposing cause, and a fist-like mass was palpated in the epigastrium. He was admitted due to a cystic mass in the left lobe of the liver, revealed by ultrasonography on October 16, 2000. He was previously in good health. His family and past histories were not contributory. Physical examination on admission disclosed that his body temperature was 36.4 °C, pulse rate was 84 beats/min, respiratory rate was 16 breaths/min and blood pressure was 130/80 mmHg. He was in his right senses and well-nourished. Yellow skin, icteric sclera, spider naevi and palmar erythema were not noted. Cardiopulmonary examination was unremarkable. The upper abdomen was slightly swollen, but the abdomen was soft. A tender, smooth-faced mass, measuring 6 cm×6 cm, was palpable at slightly left side of the mid-epigastric abdomen. He felt a little pain when we percussed the right hypochondrium. Laboratory data showed liver function test was normal, hepatitis markers of HAV, HBV, HCV, etc. were negative and AFP was 55 ng/mL. The chest radiograph showed no pulmonary abnormality. Abdominal sonography showed that an 8.4 cm×5.9 cm multiloculated cystic lesion with a few internal septations, a peripheral integrated capsule and papillary protrusion extending inward the cystic cavity was located in the left lobe of the liver. The thickest portion of the wall of the cyst was 1.28 cm. These findings supported the diagnosis of a cystic occupied lesion of the left lobe of the liver. Abdominal unenhanced computed tomography (CT) showed that a 6 cm×7 cm mass in the left hepatic lobe with a few internal septations projected to the anterior abdominal wall, and the mass was predominantly hypodense with an attenuation value of 9-25 HU. On dynamic contrast CT, there was a thick-walled mass with marked enhancement of its septations and capsule with an attenuation value of 9-44 HU (Figure 1A). No other abnormality was seen in the rest of the liver or in other abdominal viscera. On account of these features, a cystic occupied lesion of the left lobe of the liver was suspected. So the initial diagnosis was hepatic cyst.
Figure 1.
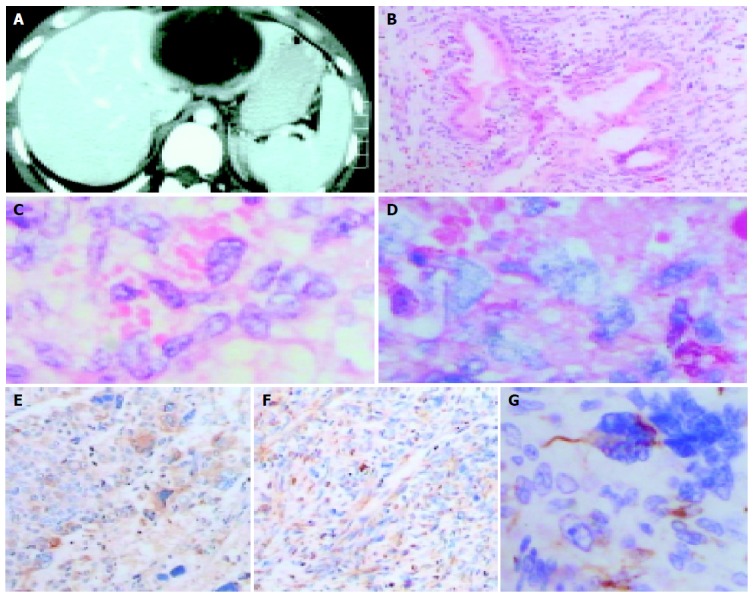
The appearance of UESL in enhanced CT, H&E stain, PAS staining and immunohistochemical assay. A: Enhanced CT scan of primary tumor. It shows a well encapsulated, multiloculated cystic mass in left hepatic lobe containing internal septations and mastoid protuberances in the intracavity, and the mass projects to the anterior abdominal wall; B: Microphotograph of primary tumor showing adeniform structure surrounded by predominantly atypical, pleomorphic sarcomatous cells (H&E stain, ×100); C: Eosinophilic hyaline globules seen in the cytoplasm of some tumor cells (H&E stain, ×400); D: Eosinophilic hyaline globules showing strongly positive for periodic acid-Schiff (PAS) staining (×400); E: Immunohistochemical assay revealing spindle shaped, polygonal, multinucleated or macronuclear cells with positive staining for vimentin (SP, ×100); F: Immunohistochemical feature presenting some anaplastic tumor cells with positive staining for alpha-1-antichymotrypsin (AACT) (SP, ×100); G: Immunohistochemical assay showing many pleomorphic tumor cells with positive staining for desmin (SP, ×400).
After preoperative preparation, the patient underwent an exploratory laparotomy under general anesthesia. During operation, a 10 cm×9 cm×6 cm cystic mass with ruptured capsule and abdominal invasion, which was located at the left exterior lobe of the liver, was noted, and there were also large quantities of blood clots and about 100 mL of bloody fluid near the mass. The right hepatic lobe was normal and no other tumor was found in the abdominal cavity. Bloody fluid was aspirated with fine-needle from the mass that contained not only fluid, but also a lower proportion of solid ingredients. A portion of neoplastic wall of biopsy was sent to pathology laboratory for frozen section, and the pathological report suggested the mass was hepatic cyst and microscopically some cells were atypia. Subsequently, hepatic segmentectomy of S2, S3 was performed because we considered the mass might be malignant. The pathological gross specimen showed the tumor was a multilocular cystic mass with mastoid protuberances in the intracavity on cut section. On microscopic examination postoperatively, the tumor consisted of inhomogeneous glandular cavities with some secretion. The tumor cells with giant nuclei deeply stained were spindle shaped, polygonal and predominantly atypical. The cells were distributed diffusely (Figure 1B). Pathological diagnosis was malignant tumor of the liver (adenofibrosarcoma).
The epigastric pain recurred in July 2002. Abdominal sonography and CT both showed the mass was a cystic tumor, and was close to the previous resection margin of the left hepatic lobe. Subsequently, the patient underwent an intervention of hepatic segmentectomy of S4. At surgery, the cystic cavity of the tumor with inhomogeneous wall was full of bloody fluid and its anterior part adhered to abdominal wall. Postoperative pathology revealed that most of cystic wall was organized, and there were only a few spindle shaped, polygonal atypical cells and no adenoid structure.
During follow-up, a tumor was discovered in the right upper abdomen in July 2003. Abdominal ultrasonography and CT both showed a solid-to-cystic lesion followed by surgical resection of the tumor. Gross appearance of the lesion on section was reddish white fish-meat-like and the cystic cavity with inhomogeneous wall was filled with bloody fluid. Postoperative histopathology on microscopic examination showed that the cells with frequent abnormal mitosis were spindle shaped, polygonal, multinucleated or macronucleus, and variable numbers of eosinophilic hyaline globules in the cytoplasm of some giant cells were seen (Figure 1C), which were strongly positive for periodic acid-Schiff (PAS) staining (Figure 1D). There were mucinous degeneration of mesenchyme and no adenoid structure. Immunohistochemical study (SP method) results showed that the tumor cells were positive for vimentin (Figure 1E), alpha-1-antichymotrypsin (AACT) (Figure 1F) and desmin staining (Figure 1G), and negative for alpha-fetoprotein. The pathological diagnosis was UESL.
The postoperative course was uneventful with no adjuvant chemotherapy and then the patient was discharged. He has been receiving regular follow-up examinations at the outpatient department and is still alive with no tumor recurrence for four months.
DISCUSSION
Cystic-occupied lesions of liver were classified according to pathogenesis by Henson[2] in 1956 into four types: (1) congenital cyst of liver, including solitary (single) and diffuse forms; (2) traumatic cyst; (3) inflammatory hepatic cyst, including specific and nonspecific ones; and (4) tumorous hepatic cyst, including benign and malignant forms. Cystadenoma and cystadenocarcinoma are the main forms of tumorous hepatic cyst. Moreover, ischemic necrosis followed by cystic change, attributed to rapid growth of the tumor, may occur at the central part of the mass of primary hepatocarcinoma. Hepatic sarcoma is of low incidence in malignant tumors of the liver, and those that present cystic change are especially rare.
UESL, also called malignant mesenchymoma of the liver, is a rare tumor that most often presents in late childhood (6-10 years old) but infrequently in adults. Less than 50 cases of UESL in adults have been reported within 40 years up to 2003 in the world-wide literature[3-8]. Grossly, it is usually a large, solitary and well-circumscribed mass with variable areas of hemorrhage, necrosis and cystic degeneration[1]. Some lesions may be with a capsule or pseudocapsule. Microscopically, the tumor is predominantly composed of a mixture of highly atypical spindle-shaped and giant cells[8]. The presence of malignant fibrous histiocytoma-like, rhabdomyosarcoma-like or fibrosarcoma-like areas can be seen. Tumor cells arrange loosely or densely within myxoid matrix. The larger cells often contain numerous intracytoplasmic eosinophilic hyaline globules that are strongly positive for PAS staining, and caryomitosis can be seen. Scattered hyperplastic or degenerating bile duct-like structures surrounded by tumor cells are seen in most cases, and considered to the residue of normal bile duct or, much more likely, be the constituent of the tumor[9-11]. But the pathohistologic feature in this case suggested that duct-like structure might be present in primary tumor, but not in secondary tumor. We drew a conclusion that the duct-like structure in this case might be the residue of normal bile duct. On immunohistochemical analysis of documentary reports, the tumor cells were positive for vimentin, alpha-1-antichymotrypsin (AACT), alpha-1-antitrypsin (α1AT), lysozyme, desmin, smooth muscle actin and cytokeratin[8-12]. The peculiar combination of phenotypical features has led a couple of authors to postulate a histogenetic relationship with primary hepatic embryonal rhabdomyosarcoma[11], and others to suggest that the tumor could originate from mesenchymal hamartoma[13,14].
UESL has no specific clinical features. The most common clinical presentation includes hepatomegaly, an upper abdominal mass, which was accompanied by abdominalgia or not, and weight loss. Occasionally, tumoral spontaneous rupture may result in intraperitoneal hemorrhage due to rapid growth of the tumor. Different from primary carcinoma of the liver, UESL has no relation to hepatitis or liver cirrhosis, no disturbance of hepatic function or elevation of AFP. CT and magnetic resonance image (MRI) features of cystic change are usually different from that of solid-to-cystic change revealed by sonography or upon macroscopy[15]. The cystic change may be the result of hemorrhage or necrosis during tumor growth. In our case, both the primary and the first recurrent tumor mainly displayed cystic change, but the second recurrent mass presented with solid-to-cystic change and solid type predominance. Bloody fluid in the cystic cavity of the primary or recurrent tumor mass suggested that cystic degeneration had relation to the presence of hemorrhage or necrosis in the tumor. But, on the other hand, the inner wall of the cystic cavity was rather smooth, and we could deduce that the tumor had a tendency to develop a cyst spontaneously. The clinical diagnosis of UESL is rather difficult, so is the differential diagnosis, such as malignant fibrous histiocytoma. The ultimate diagnosis relies on pathological examination. Immunohistochemical and electron microscopic examinations are supposed to be carried out if it is difficult to evaluate tumoral histogenesis. In our case, the definite diagnosis was not made until PAS staining and immunohistochemical examination were applied to the second recurrent tumor mass. Radical resection of the tumor is the optimal treatment of choice[16]. The prognosis for UESL has been poor to date and majority of the patients died of tumor recurrence or metastasis within two years after operation[1,17,18]. Recent researches have shown that pre- and/or post-operative systemic chemotherapy (with cisplatin, adriamycin, cyclopho-sphamide) and/or radiotherapy, when necessary, can remarkably improve patients survival[19,20]. It has been reported that a patient has been free from tumor for 20 years postoperatively.
In conclusion, through the diagnosis and treatment of the case, several points are worth recommending. First, we need to extend our understanding of cystic change of malignant tumor of liver, such as sarcoma. Hepatic cystic mass with abrupt abdominalgia, which should be paid special attention to, usually suggests spontaneous rupture or intra-tumor hemorrhage. Second, we should study imaging features of hepatic cystic lesion comprehensively in differential diagnosis of malignant mass or not. In our case, CT and sonography both revealed the primary tumor with complete capsule, heterogeneous cystic wall with thickness, a few internal septations of cystic cavity, papillary protrusion extending inward the cavities and especially prominent protrusion of parenchyma of the tumor attributed to the outward growth. These imaging features are seldom seen in benign cysts, such as congenital solitary hepatic cyst. Third, we should carry out timely excision of non-traumatic cyst suspected of malignancy when bloody fluid was drawn out with fine needle under the direction of ultrasound or revealed inside cyst during operation. Fourth, frozen section, during operation, is of great value for ascertaining the nature of tumor. But for fear of false-negative results, the material should be precisely harvested from the proper tissue. And last, post-operative follow-up, is necessary for the patients with hepatosarcoma as well as for those with primary carcinoma of liver. Once there is an evidence of recurrence, resection of the tumor wherever feasible should be performed in most cases.
Footnotes
Edited by Kumar M and Zhu LH
References
- 1.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336–348. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Henson SW, Gray HK, Dockerty MB. Benign tumors of the liver. I. Adenomas. Surg Gynecol Obstet. 1956;103:23–30. [PubMed] [Google Scholar]
- 3.Tokunaga Y, Ryo J, Hoppou T, Kitaoka A, Tokuka A, Osumi K, Tanaka T. Hepatic undifferentiated (embryonal) sarcoma in an adult: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2000;12:1247–1251. doi: 10.1097/00042737-200012110-00014. [DOI] [PubMed] [Google Scholar]
- 4.Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Guleria S, Dinda AK, Kumar L, Tarique S. Embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Trop Gastroenterol. 2001;22:141–142. [PubMed] [Google Scholar]
- 6.Shah SR, Joshi P, Bhaduri AS, Bhalerao RA. Cystic variant of embryonal sarcoma of liver. Indian J Gastroenterol. 2002;21:35–36. [PubMed] [Google Scholar]
- 7.Shufaro Y, Uzieli B, Pappo O, Abramov Y. Pregnancy and delivery in a patient with metastatic embryonal sarcoma of the liver. Obstet Gynecol. 2002;99:951–953. doi: 10.1016/s0029-7844(02)01965-8. [DOI] [PubMed] [Google Scholar]
- 8.Nishio J, Iwasaki H, Sakashita N, Haraoka S, Isayama T, Naito M, Miyayama H, Yamashita Y, Kikuchi M. Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol. 2003;34:246–252. doi: 10.1053/hupa.2003.44. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama C, Hachitanda Y, Sato JK, Said JW, Shimada H. Undifferentiated (embryonal) sarcoma of the liver. A tumor of uncertain histogenesis showing divergent differentiation. Am J Surg Pathol. 1991;15:615–624. doi: 10.1097/00000478-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Keating S, Taylor GP. Undifferentiated (embryonal) sarcoma of the liver: ultrastructural and immunohistochemical similarities with malignant fibrous histiocytoma. Hum Pathol. 1985;16:693–699. doi: 10.1016/s0046-8177(85)80154-4. [DOI] [PubMed] [Google Scholar]
- 11.Parham DM, Kelly DR, Donnelly WH, Douglass EC. Immunohistochemical and ultrastructural spectrum of hepatic sarcomas of childhood: evidence for a common histogenesis. Mod Pathol. 1991;4:648–653. [PubMed] [Google Scholar]
- 12.Joshi SW, Merchant NH, Jambhekar NA. Primary multilocular cystic undifferentiated (embryonal) sarcoma of the liver in childhood resembling hydatid cyst of the liver. Br J Radiol. 1997;70:314–316. doi: 10.1259/bjr.70.831.9166061. [DOI] [PubMed] [Google Scholar]
- 13.Begueret H, Trouette H, Vielh P, Laurent C, MacGrogan G, Delsol M, Belleannee G, Masson B, De Mascarel A. Hepatic undifferentiated embryonal sarcoma: malignant evolution of mesenchymal hamartoma? Study of one case with immunohistochemical and flow cytometric emphasis. J Hepatol. 2001;34:178–179. doi: 10.1016/s0168-8278(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 14.Lauwers GY, Grant LD, Donnelly WH, Meloni AM, Foss RM, Sanberg AA, Langham MR. Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol. 1997;21:1248–1254. doi: 10.1097/00000478-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, Levine MS, Goodman ZD. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology. 1997;203:779–783. doi: 10.1148/radiology.203.3.9169704. [DOI] [PubMed] [Google Scholar]
- 16.Grazi GL, Gallucci A, Masetti M, Jovine E, Fiorentino M, Mazziotti A, Gozzetti G. Surgical therapy for undifferentiated (embryonal) sarcomas of the liver in adults. Am Surg. 1996;62:901–906. [PubMed] [Google Scholar]
- 17.Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol. 1991;15:1–16. [PubMed] [Google Scholar]
- 18.Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol. 1990;21:68–76. doi: 10.1016/0046-8177(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 19.Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, Treuner J, Carli M. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002;94:252–257. doi: 10.1002/cncr.10191. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37:1419–1423. doi: 10.1053/jpsu.2002.35404. [DOI] [PubMed] [Google Scholar]


