Abstract
AIM: To evaluate the association between obesity and colorectal cancer risk.
METHODS: We searched PubMed, EMBASE, and the Cochrane Library up to January 1, 2007. Cohort studies permitting the assessment of causal association between obesity and colorectal cancer, with clear definition of obesity and well-defined outcome of colorectal cancer were eligible. Study design, sample size at baseline, mean follow-up time, co-activators and study results were extracted. Pooled standardized effect sizes were calculated.
RESULTS: The pooled relative risk (RR) of colorectal cancer was 1.37 (95% CI: 1.21-1.56) for overweight and obese men, 1.07 (95% CI: 0.97-1.18) for women measured by body mass index (BMI). The pooled RR for the highest vs the lowest quantiles of BMI was 1.59 (95% CI: 1.35-1.86) for men and 1.22 (95% CI: 1.08-1.39) for women at risk of colon cancer, 1.16 (95% CI: 0.93-1.46) for men and 1.23 (95% CI: 0.98-1.54) for women at risk of rectal cancer. The pooled RR for the highest vs the lowest quantiles of waist circumference was 1.68 (95% CI: 1.36-2.08) for men and 1.48 (95% CI: 1.19-1.84) for women at risk of colon cancer, 1.26 (95% CI: 0.90-1.77) for men and 1.23 (95% CI: 0.81-1.86) for women at risk of rectal cancer. The pooled RR for the highest quantiles vs the lowest quantiles of waist-to-hip ratio was 1.91 (95% CI: 1.46-2.49) for men and 1.49 (95% CI 1.23-1.81) for women at risk of colon cancer, 1.93 (95% CI: 1.19-3.13) for men and 1.20 (95% CI: 0.81-1.78) for women at risk of rectal cancer. Compared with 'normal range', the pooled RR for proximal colon cancer was 1.14 (95% CI : 0.88-1.47) for the overweight and 1.41 (95% CI: 0.66-3.01) for the obese. The pooled RR for the highest quantiles vs the lowest quantiles was 2.05 (95% CI: 1.23-3.41) with waist circumference, 1.66 (95% CI: 0.69-3.99) with waist-to-hip ratio. Compared with 'normal range', the pooled RR for distal colon cancer was 1.38 (95% CI: 1.02-1.87) for the overweight and 1.23 (95% CI: 0.80-1.90) for the obese. The pooled RR for the highest quantiles vs the lowest quantiles was 1.86 (95% CI: 1.05-3.30) with waist circumference, and 1.79 (95% CI: 0.82-3.90) with waist-to-hip ratio.
CONCLUSION: Obesity is a statistically significant risk factor for colorectal cancer and the relationship is more significant in men than in women among different cancer subsites. Indexes of abdominal obesity are more sensitive than those of overall obesity.
Keywords: Obesity, Colorectal cancer, Waist-to-hip ratio, Waist circumference, Weight, Cohort study, Meta analysis
INTRODUCTION
The prevalence of overweight and obesity is increasing dramatically in most parts of the world, and can lead to obesity-related diseases. In 1940, it was first hypothesized that excess body weight could contribute to the development of some cancers[1]. Colorectal cancer, the second most common malignancy in developed countries and the fourth most common cancer throughout the world[2], has been suggested to have a certain relationship with obesity in some epidemiological studies in the past decades[3-5].
Interpretation of the results of epidemiological studies on the relation between obesity and colorectal cancer has been hampered due to differences in methodology and inconsistent approaches in defining obesity, and the epidemiological evidence linking obesity to colorectal cancer has not been critically reviewed. To provide a quantitative assessment of the association between obesity and risk of colorectal cancer, we conducted a meta-analysis of published cohort studies. We also evaluated whether the association varies with sex and cancer subsites (colon versus rectum and proximal colon versus distal colon).
MATERIALS AND METHODS
Search strategy
We identified English and Chinese language studies by a literature search of the PubMed, EMBASE, and the Cochrane Library up to January 1, 2007 with the following medical subject headings and/or key words and/or text words: “obesity”, “overweight”, “body mass index”, “waist circumference”, “waist-to-hip ratio”, “colorectal cancer”, “colorectal neoplasm”, “colon cancer”, “rectal cancer”, “colon neoplasm” and “rectal neoplasm”. We also reviewed the references of the additional pertinent studies.
Inclusion and exclusion criteria
The inclusion criteria were (1) cohort study permitting assessment of causal association between overweight or obesity and colorectal cancer, (2) clear definition of obesity defined by body mass index (BMI) in kg/m2 or waist-to-hip ratio or waist circumference, and (3) outcome of colorectal cancer by clinical and pathological criteria. We excluded case reports and case series, studies on less than 50 cases, and studies not reporting risk estimates or raw data to allow independent calculation of these estimates.
Data extration
The following data were recorded for each study, including study type, duration of follow-up, age-range of participants, country, variables for which statistical adjustment was performed, number of cases and controls or person years, timing and categories of BMI measurement, point estimates (RR, OR, or SIR) and 95% CI. When several risk estimates were presented, we used those adjusted for the greatest number of potential confounders.
Statistical analysis
We calculated the weighted pooled risk estimates of two categories of adult BMI [BMI = weight [in kilograms]/height (in meters)2] defined by the World Health Organization: overweight (BMI = 25-29.9) and obesity (BMI ≥ 30) compared to ‘normal’ weight (BMI = 18.5-24.9) as the reference category. When the non-standard categories of BMI were used, we selected the category most similar to those defined by WHO. We also included studies using a reference BMI category that was less than the WHO defined ‘normal BMI’, if the RR estimate for the ‘normal BMI’ category was 1.0. We included more than one estimate from some studies (e.g., if a study reported and the odds ratio for persons with BMI = 25-28 or BMI ≥ 28, both odds ratios were included in the summary estimate for BMI ≥ 25). Because waist circumference and WHR are different among races and studies, and have no unified criteria around the world, we combined the different outcomes between the smallest waist or WHR quantile and the largest waist or WHR quantile in studies using relative risks in meta-analysis of random effects. We assessed the heterogeneity for each pooled estimate with a Cochran’s Q test. Finally, we conducted sensitivity analyses omitting each study in turn to determine whether the results were influenced excessively by a single study.
RESULTS
Study characteristics
The primary computerized literature search identified 196 potential relevant studies. The excluded studies included reviews, animal experiments, case controlled studies, studies not reporting the subject of interest, or duplicate publications. If more than one publication was found to be a duplicate, we used the most recent or relevant paper. After primary search, we retrieved 43 manuscripts for further review, of which 15 cohort studies met the inclusion criteria for the systematic review[6-20].
Six of the cohort studies were performed in the USA[6,12,13,15,16,20], 3 in Sweden[8,9,19], 2 in Australia[11,18], 1 in Austria[7], 1 in Canada[17], 1 in Japan[14], and 1 in 10 European countries[10]. The characteristics of enrolled studies are summarized in Table 1. There were 6458 colorectal cancer cases among 1 058 883 participants. Among the studies, 4 concerned about men[8,13,14,18], 6 about women[11,15-17,19,20], and 5 about both genders[6,7,9,10,12]. Fifteen[6-20] studies used BMI as measurement of obesity, 7 of which[6,8,10,11,13,15,18] used waist circumference and 6[10,11,13,15,16,18] WHR. Types of quantile and the respective cut-off points of the highest and lowest quantiles for the waist and WHR measurements are reported in Table 2. One study also examined the predictive role of skin folds[12], and another study demonstrated the measurement of weight and height as the risk of colorectal cancer[18].
Table 1.
Characteristics of cohort studies investigating the association between obesity and colorectal cancer
| Author, publication yr, country, cohort | Study period | Age range | Sex | Cases/Cohort size | Body size measurement | Data collection | Cancer sites |
| Moore (2004)[6] USA | 1948-1999 | 30-79 | M/F | 306/7566 | BMI, WC | Measured | CC |
| Rapp (2005)[7] Austria | 1985-2001 | 18-93 | M/F | 802/145 000 | BMI | Measured | CC/RC |
| Larsson (2006)[8] Sweden | Began in 1997, Mean follow-up 7.1 yr | 45-79 | M | 496/45 906 | BMI, WC | Self-report | CC/RC |
| Lukanova (2006)[9] Sweden | 1985-2003 | 30-60 | M/F | 244/68 786 | BMI | Self-report | CC/RC |
| Pischon (2006)[10] | 1992-2000 | 25-70 | M/F | 1570/368 277 | BMI, WC, WHR | Measured | CC/RC |
| 10 European countries | |||||||
| Maclnnis (2006)[11] Australia | Mean follow-up 10.4 yr | 27-75 | F | 212/24 072 | BMI, WC, WHR | Measured | CC |
| Ford (1999)[12] USA | 1975-1992 | 25-74 | M/F | 222/13 420 | BMI | Measured | CC |
| Giovannucci (1995)[13] USA | 1986-1992 | 40-75 | M | 203/47 723 | BMI, WC, WHR | Self-report | CC |
| Chyou (1996)[14] Japan | 1965-1995 | Born in 1900-1919 | M | 453/7945 | BMI | Measured | CC/RC |
| Martinez (1997)[15] USA | 1980-1992 | 30-55 | F | 396/89 448 | BMI, WC, WHR | Self-report | CC |
| Bostick (1994)[16] USA | 1986-1990 | 55-69 | F | 212/35 215 | BMI, WHR | Self-report | CC |
| Terry (2002)[17] Canada | 1982-1993 | 40-59 | F | 527/89 835 | BMI | Self-report | CC/RC |
| MacInnis (2004)[18] Australia | 1994-2002 | 27-75 99.3% 40-69 | M | 153/16 556 | BMI, WC, WHR | Measured | CC |
| Terry (2001)[19] Sweden | 1990-1998 | 40-76 | F | 460/61 463 | BMI | Self-report | CC/RC |
| Lin (2004)[20] USA | average of 8.7 yr follow-up | ≥ 45 | F | 202/37 671 | BMI | Self-report | CC RC |
M: male; F: female; BMI: body mass index; WC: waist circumference; WHR: waist-to-hip ratio; CC: colon cancer; RC: rectal cancer.
Table 2.
Types of quantile and cut-off points for upper and lower quantiles for waist and waist-to-hip ratio (WHR) used in the studies
| Study | Type of quantiles used |
Waist (cm) |
WHR |
||
| Upper limit of lower quantile | Lower limit of upper quantile | Upper limit of lower quantile | Lower limit of upper quantile | ||
| Moore (2004)[6] | Quartiles | 83.8 (M)/81.3 (F) | 101.6 (M)/99.1 (F) | ND | ND |
| Pischon (2006)[10] | Quintiles | 86 (M)/70.2 (F) | 103 (M)/89 (F) | 0.887 (M)/0.734 (F) | 0.990 (M)/0.846 (F) |
| Maclnnis (2006)[11] | Tertiles | 80 (F) | 88 (F) | 0.75 (F) | 0.80 (F) |
| Giovannucci (1995)[13] | Quintiles | 35in (M) | 43 in (M) | 0.90 (M) | 0.99 (M) |
| Martinez (1997)[15] | Quintiles | 27.5in (F) | 34in (F) | 0.728 (F) | 0.833 (F) |
| Bostick (1994)[16] | Quintiles | ND | ND | 0.764 (F) | 0.906 (F) |
| MacInnis (2004)[18] | Quartiles | 87 (M) | 99.3 (M) | 0.88 (M) | 0.96 (M) |
M: male; F: female; ND: no data.
BMI and risk of colorectal cancer
Compared with those in ‘normal’ range, the pooled RR from colon cancer was 1.26 (95% CI = 1.04-1.52) and 1.71 (95% CI = 1.33-2.19) with no significant heterogeneity (P = 0.61) and 1.71 (95% CI = 1.33-2.19) for overweight and obese men respectively with no significant heterogeneity (P = 0.61, P = 0.73). The pooled RR for rectal cancer of overweight and obese men was 1.07 (95% CI = 0.78-1.46) and 1.75 (95% CI = 1.17-2.621), respectively with no significant heterogeneity (P = 0.26) (Figure 1A). The pooled RR for colorectal cancer of overweight and obese men was 1.37 (95% CI = 1.21-1.56) with no significant heterogeneity (P = 0.42).
Figure 1.
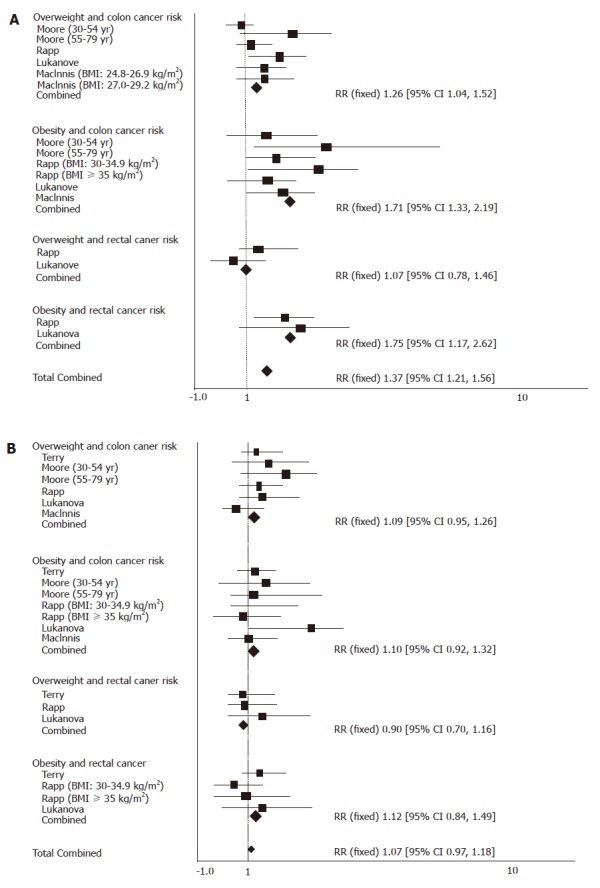
Overweight/obesity and colorectal cancer risk in men (A), and in women (B).
Compared with those in the ‘normal’ range, the pooled RR for colon cancer was 1.09 (95% CI 0.95-1.26) and 1.10 (95% CI = 0.92-1.32) for overweight and obese women (P = 0.24) with no significant heterogeneity (P = 0.24). The pooled RR for rectal cancer of overweight women was 0.90 (95% CI = 0.70-1.16, P = 0.59) and 1.12 (95% CI = 0.84-1.49) for obese women with no significant heterogeneity (P = 0.26) (Figure 1B). The pooled RR for colorectal cancer of overweight and obese women was 1.07 (95% CI = 0.97-1.18) with no significant heterogeneity (P = 0.29).
Waist circumference and risk of colorectal cancer
Compared with those in the lowest quantile, the pooled RR for colon cancer was 1.68 (95% CI = 1.36-2.08) for men and 1.48 (95% CI = 1.19-1.84) for women with no significant heterogeneity (P = 0.31). The pooled RR for rectal cancer was 1.26 (95% CI = 0.90-1.77) for men (P = 0.95) and 1.23 (95% CI = 0.81-1.86) for women with the highest quantile of waist circumference (P = 0.95, Figure 2A).
Figure 2.
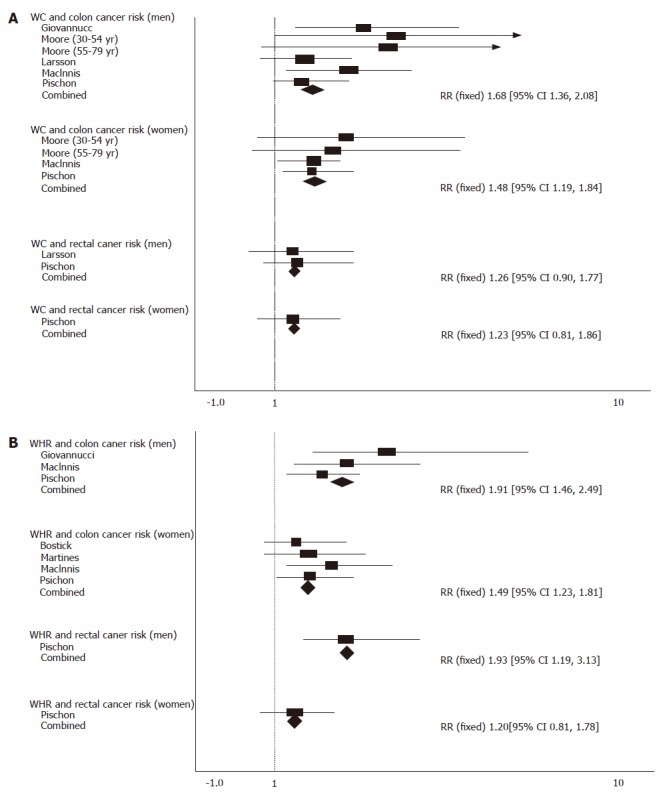
Waist circumference and colorectal cancer risk (A), and waist-to-hip ratio and colorectal cancer risk (B).
WHR and risk of colorectal cancer
Compared with those in the lowest quantile, the pooled RR for colon cancer was 1.91 (95% CI = 1.46-2.49) for men (P = 0.23) with the highest quantile of WHR, and 1.49 (95% CI = 1.23-1.81) for women with no significant heterogeneity (P = 0.76). The pooled RR for rectal cancer was 1.93 (95% CI = 1.19-3.13) for men and 1.20 (95% CI = 0.81-1.78) for women with the highest quantile of WHR (Figure 2B).
Different anthropometric indexes of obesity and risk of colorectal cancer
To further analyze obesity and the risk of colorectal cancer, we calculated the pooled RR for colorectal cancer in the highest quantiles of different anthropometric indexes of obesity compared with the lowest quantiles (Table 3). Compared with those in the lowest quantile, the pooled RR for colon cancer was 1.59 (95% CI = 1.35-1.86) for men with no significant heterogeneity (P = 0.82) but with the highest quantile of BMI, and 1.22 (95% CI =1 .08-1.39) for women with no significant heterogeneity (P = 0.15). The pooled RR for rectal cancer was 1.16 (95% CI = 0.93-1.46) for men with significant heterogeneity (P = 0.03) and the highest quantile of BMI, and 1.23 (95% CI = 0.98-1.54) for women with no significant heterogeneity (P = 0.93).
Table 3.
Comparison of the highest-to-lowest quantiles of BMI, WC and WHR to predict colorectal cancer risk
| Body size measurement | Category | Pooled effect estimate (95% CI) | Pheterogeneity | Number of studies |
| BMI | Male (colon cancer) | 1.59 (1.35-1.86) | 0.82 | 10 |
| Female (colon cancer) | 1.22 (1.08-1.39) | 0.15 | 12 | |
| Male (rectal cancer) | 1.16 (0.93-1.46) | 0.03 | 5 | |
| Female (rectal cancer) | 1.23 (0.98-1.54) | 0.93 | 6 | |
| Waist circumference | Male (colon cancer) | 1.68 (1.36-2.08) | 0.31 | 6 |
| Female (colon cancer) | 1.48 (1.19-1.84) | 0.79 | 4 | |
| Male (rectal cancer) | 1.26 (0.90-1.77) | 0.95 | 2 | |
| Female (rectal cancer) | 1.23 (0.81-1.86) | - | 1 | |
| Waist-to-hip ratio | Male (colon cancer) | 1.91 (1.46-2.49) | 0.23 | 3 |
| Female (colon cancer) | 1.49 (1.23-1.81) | 0.76 | 4 | |
| Male (rectal cancer) | 1.93 (1.19-3.13) | - | 1 | |
| Female (rectal cancer) | 1.20 (0.81-1.78) | - | 1 |
Obesity and risk of different cancer subsites
Compared with those in the ‘normal’ range, the pooled RR for proximal colon cancer was 1.14 (95% CI = 0.88-1.47) for the overweigh with no significant heterogeneity (P = 0.39) and 1.41 (95% CI = 0.66-3.01) for the obese with significant heterogeneity (P = 0.04). Compared with those in the lowest quantile, the pooled RR for proximal colon cancer was 2.05 (95% CI = 1.23-3.41)) for those with the highest quantile of waist circumference and no significant heterogeneity (P = 0.63) and 1.66 (95% CI = 0.69-3.99) for those with the highest quantile of WHR (Figure 3A).
Figure 3.

BMI/waist circumference/WHR and proximal colon cancer risk (A), and BMI/waist circumference/WHR and distal colon cancer risk (B).
Compared with those in the ‘normal’ range, the pooled RR for distal colon cancer was 1.38 (95% CI = 1.02-1.87) for the overweight with no significant heterogeneity (P = 0.59) and was 1.23 (95% CI = 0.80-1.90) for the obese with significant heterogeneity (P = 0.76). Compared with those in the lowest quantile, the pooled RR for proximal colon cancer was 1.86 (95% CI = 1.05-3.30) for those with the highest quantile of waist circumference with no significant heterogeneity (P = 0.80) and 1.79 (95% CI= 0.82-3.90) for those with the highest quantile of WHR (Figure 3B).
Menopausal status
There are studies stratifying their analyses by menopausal status[6,17,19]. Menopause was clearly defined only in one study[17], and obesity was found to be associated with approximately twofold increased risk of colorectal cancer among women who were pre-menopausal at baseline, but no positive association was found among postmenopausal women. In the other two studies, one found a stronger relationship between older women and BMI as well as between waist circumference and WHR[6], the other found no association between BMI and colorectal cancer risk among older women[19]. Two studies further demonstrated the effect of postmenopausal hormone therapy (PMH)[10,20] and no change in the positive relationship between obesity and colorectal cancer after estrogen exposure among postmenopausal women.
Publication bias
The funnel plot of effect estimates for the risk of colorectal cancer related to obesity was close to symmetry, suggesting that there is no appreciable publication bias.
DISCUSSION
Our meta-analysis quantitatively assessed the relation between obesity and colorectal cancer risk. The risk of colorectal cancer increases with increasing BMI in men. A positive association was found between proximal and distal colon cancerand rectal cancer. However, the relationship is inconsistent in women[6,7,11,19]. Based on our survey of the published literature, the risk of overweight is lower in the obesity and is of marginal statistical significance. It is worth noting that, when waist circumference and WHR were used as anthropometric measures of obesity, the relationship was consistent in women and men and in subsites of colorectal cancer. Further analyses showed that cancer risk of the highest quantiles was higher in waist circumference and WHR than in BMI. It was reported that adjustment for waist circumference or WHR can diminish the relationship between BMI and colorectal cancer risk[6].
It was reported that obesity is related to the development and prognosis of colorectal cancer[22,23]. Following mechanisms have been suggested for the effect of obesity on colorectal cancer. First, from the view of metabolic syndrome, obesity is a major determinant of insulin resistance, and insulin is an important growth factor for colonic epithelial cells and a mitogen of tumor cell growth in vitro[21]. C peptide, insulin-like growth factorIand insulin-like growth factor binding proteins which are elevated in obesity are related to colorectal cancer risk[24]. It was also reported that insulin-like growth factor polymorphism is related to colorectal cancer risk[33]. Second, the fat itself can also influence colorectal cancer risk[25]. Adipocytes and preadipocytes can promote proliferation of colon cancer cells[26]. Fatty acid synthase over-expression has been shown to be associated with colorectal cancer phenotype[27]. Third, adipokines such as leptin[28], adiponectin[29] are also associated with colorectal cancer risk. Fourth, limited data implicate obesity-related inflammation in pathogenesis of colorectal cancer[26,27]. Elevated physical activity[8,13,32] and low-caloric diet can reduce cancer risk. The etiologic factors for cancer of the proximal and distal colon may differ because there are various molecular and clinical differences between the two subsites that may influence the susceptibility to environmental factors[34]. The causes for disparities in relationship between BMI and colorectal cancer risk related to genders have not been well understood yet. The higher prevalence of visceral obesity in men and the protective role of estrogen in women may play an important role. Different factors in men and women such as self-esteem and body image may also contribute to explaining the differential associations.
Our study has several advantages. First, we only included prospective studies which are less vulnerable to selection and recall biases that may affect case-control studies on associations between obesity and colorectal cancer. The inclusion criteria also minimized variation in studies due to their design or quality. Second, we separated meta-analysis of different anthropomatric measures of body fat, removing potential sources of heterogeneity that may occur in a mata-analysis of the published literature. Different kinds of fat disposition may have different effects on colorectal cancer risk.
Our study also has some limitations. First, anthropomatric measurement at baseline could not investigate the changes during follow-up. Second, the age range was different am, leading to some heterogeneity of the analysis. However, all the individual results were adjusted for this factor, and the funnel plot analysis indicated no appreciable publication bias, demonstrating that this deficiency is not important. Third, some sub-analysis was based on the data of one study, which should be re-evaluated in further studies. Finally, measurement error in different indexes may bias the results.
In conclusion, the findings of the meta-analysis suggest that obesity is a statistically significant risk factor for colorectal cancer and is of public health significance since obesity is a potentially modifiable risk factor, in contrast to almost all other known risk factors for colorectal cancer. Body fat indexes according to abdominal obesity are more sensitive than overall obesity. The mechanism should be further evaluated.
COMMENTS
Background
Colorectal cancer has long been known to have some relationship with obesity in epidemiological studies. However, the interpretation of the results of epidemiological studies evaluating the relation between obesity and colorectal cancer has been hampered due to the differences in methodology and inconsistent approaches to defining obesity, and the epidemiological evidence linking obesity to colorectal cancer has not been critically reviewed.
Research frontiers
There are many epidemiological studies highlighting the important influences of obesity on colorectal cancer risk. The related mechanisms are concerned about metabolic syndrome, adipokines and growth-promoting function of related factors.
Innovations and breakthroughs
This study systematically analyzed the relationship between obesity and colorectal cancer risk, and compared three different indexes of body fat in different cancer subsites and both genders.
Applications
The meta-analysis suggests that obesity is a statistically significant risk factor for colorectal cancer. Body fat indexes according to abdominal obesity are more sensitive than overall obesity. This finding is of public health significance since obesity is a potentially modifiable risk factor, in contrast to almost all other known risk factors for colorectal cancer. It is valuable for prevention and prediction of colorectal cancer in high-risk populations.
Terminology
Meta-analysis is a statistical method of combining the results of different studies in order to provide a larger sample size for evaluation and to produce a stronger conclusion than that by any single study.
Peer review
Differences in colon and rectal cancer and colon cancers were evaluated between males and females, showing that obesity is a significant risk factor for colorectal cancer which is more significant in men than in women. The body fat indexes, waist circumference and waist to hip ratio, were found to be more sensitive than overall obesity in determining RR for colon cancer, and more consistent RR between men and women was observed when these indices were used. Overall this manuscript is reasonably well written and contains interesting information. Comments from other reviewers are considered.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Wang HF
References
- 1.Tannenbaum A. Relationship of body weight to cancer indidence. Arch Pathol. 1940;30:509–517. [Google Scholar]
- 2.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 3.Caan BJ, Coates AO, Slattery ML, Potter JD, Quesenberry CP, Edwards SM. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord. 1998;22:178–184. doi: 10.1038/sj.ijo.0800561. [DOI] [PubMed] [Google Scholar]
- 4.Hou L, Ji BT, Blair A, Dai Q, Gao YT, Potter JD, Chow WH. Body mass index and colon cancer risk in Chinese people: menopause as an effect modifier. Eur J Cancer. 2006;42:84–90. doi: 10.1016/j.ejca.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slattery ML, Ballard-Barbash R, Edwards S, Caan BJ, Potter JD. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control. 2003;14:75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 6.Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, Kreger BE. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28:559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 7.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson SC, Rutegård J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–2597. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Lukanova A, Björ O, Kaaks R, Lenner P, Lindahl B, Hallmans G, Stattin P. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118:458–466. doi: 10.1002/ijc.21354. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 11.MacInnis RJ, English DR, Hopper JL, Gertig DM, Haydon AM, Giles GG. Body size and composition and colon cancer risk in women. Int J Cancer. 2006;118:1496–1500. doi: 10.1002/ijc.21508. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol. 1999;150:390–398. doi: 10.1093/oxfordjournals.aje.a010018. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of colon and rectal cancer among Hawaii Japanese men. Ann Epidemiol. 1996;6:276–282. doi: 10.1016/s1047-2797(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 15.Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 16.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, Gapstur SM, Folsom AR. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States) Cancer Causes Control. 1994;5:38–52. doi: 10.1007/BF01830725. [DOI] [PubMed] [Google Scholar]
- 17.Terry PD, Miller AB, Rohan TE. Obesity and colorectal cancer risk in women. Gut. 2002;51:191–194. doi: 10.1136/gut.51.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13:553–559. [PubMed] [Google Scholar]
- 19.Terry P, Giovannucci E, Bergkvist L, Holmberg L, Wolk A. Body weight and colorectal cancer risk in a cohort of Swedish women: relation varies by age and cancer site. Br J Cancer. 2001;85:346–349. doi: 10.1054/bjoc.2001.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Zhang SM, Cook NR, Rexrode KM, Lee IM, Buring JE. Body mass index and risk of colorectal cancer in women (United States) Cancer Causes Control. 2004;15:581–589. doi: 10.1023/B:CACO.0000036168.23351.f1. [DOI] [PubMed] [Google Scholar]
- 21.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 22.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almendingen K, Hofstad B, Vatn MH. Does high body fatness increase the risk of presence and growth of colorectal adenomas followed up in situ for 3 years? Am J Gastroenterol. 2001;96:2238–2246. doi: 10.1111/j.1572-0241.2001.03942.x. [DOI] [PubMed] [Google Scholar]
- 24.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int J Cancer. 2007;120:2007–2012. doi: 10.1002/ijc.22556. [DOI] [PubMed] [Google Scholar]
- 25.Chung YW, Han DS, Park YK, Son BK, Paik CH, Lee HL, Jeon YC, Sohn JH. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Dig Liver Dis. 2006;38:668–672. doi: 10.1016/j.dld.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ogino S, Kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. Fatty acid synthase overexpression in colorectal cancer is associated with microsatellite instability, independent of CpG island methylator phenotype. Hum Pathol. 2007;38:842–849. doi: 10.1016/j.humpath.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Slattery ML, Wolff RK. Leptin and colorectal cancer: an undefined link. Nat Clin Pract Gastroenterol Hepatol. 2007;4:118–119. doi: 10.1038/ncpgasthep0743. [DOI] [PubMed] [Google Scholar]
- 29.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 30.Slattery ML, Curtin K, Wolff R, Ma KN, Sweeney C, Murtaugh M, Potter JD, Levin TR, Samowitz W. PPARgamma and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States) Cancer Causes Control. 2006;17:239–249. doi: 10.1007/s10552-005-0411-6. [DOI] [PubMed] [Google Scholar]
- 31.Sandler RS. Aspirin and other nonsteroidal anti-inflammatory agents in the prevention of colorectal cancer. Important Adv Oncol. 1996:123–137. [PubMed] [Google Scholar]
- 32.Isomura K, Kono S, Moore MA, Toyomura K, Nagano J, Mizoue T, Mibu R, Tanaka M, Kakeji Y, Maehara Y, et al. Physical activity and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2006;97:1099–1104. doi: 10.1111/j.1349-7006.2006.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Insulin-like growth factor polymorphisms and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1204–1211. doi: 10.1158/1055-9965.EPI-04-0695. [DOI] [PubMed] [Google Scholar]
- 34.Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004;7:187–200. doi: 10.1079/phn2003588. [DOI] [PubMed] [Google Scholar]


