Abstract
AIM: To study the effect of the toxic secondary bile acid lithocholic acid (LCA) on the expression of fibroblast growth factor 19 (FGF19) in intestinal cells and to characterize the pregnane-X-receptor (PXR) response of the FGF19 promoter region.
METHODS: The intestinal cell line LS174T was stimulated with various concentrations of chenodeoxy-cholic acid and lithocholic acid for several time points. FGF19 mRNA levels were determined with quantitative realtime RT-PCR. FGF19 deletion promoter constructs were generated and the LCA response was analzyed in reporter assays. Co-transfections with PXR and RXR were carried out to study FGF19 regulation by these factors.
RESULTS: LCA and CDCA strongly up-regulate FGF19 mRNA expression in LS174T cells in a time and dose dependent manner. Using reporter gene assays with several deletion constructs we found that the LCA responsive element in the human FGF19 promoter maps to the proximal regulatory region containing two potential binding sites for PXR. Overexpression of PXR and its dimerization partner retinoid X receptor (RXR) and stimulation with LCA or the potent PXR ligand rifampicin leads to a significant induction of FGF19 promoter activity in intestinal cells.
CONCLUSION: LCA induced feedback inhibition of bile acid synthesis in the liver is likely to be regulated by PXR inducing intestinal FGF19 expression.
Keywords: Pregnane X receptor, Detoxification, Fibroblast growth factor 19, Lithocholic acid
INTRODUCTION
Bile acid metabolism is primarily regulated by feed back regulation where high levels of bile acids inhibit biosynthesis in the liver via the function of the ligand-activated nuclear receptor farnesoid X receptor (FXR)[1-3]. FXR activation in hepatocytes increases the expression of the nuclear receptor small heterodimer partner (SHP), which associates with liver receptor homolog 1 (LRH1) to block transcription from the CYP7A1 promoter[4,5]. The CYP7A1 gene encodes cholesterol 7α-hydroxylase, the rate- limiting enzyme in bile acid synthesis.
A second regulatory loop of bile acid homeostasis is a feed forward regulation, where potentially toxic bile acid precursors and secondary bile acids activate PXR and consecutively force their own metabolism, detoxification and excretion[6,7]. Likewise, the sterol metabolites 5β-Cholestan-3α, 7α, 12α-triol and 7α-hydroxy-4-cholesten-3-one, which accumulate in CYP27 deficient livers, and lithocholic acid are direct agonistic ligands for human and murine PXR and thereby induce expression of phase 1 and phase 2 metabolic enzymes as well as sterol transporters[4].
Whereas the molecular mechanisms of FXR and PXR regulated bile acid metabolism in the liver are well characterized, the functions and target genes of both nuclear receptors in the intestine are poorly understood. However, recent evidence suggests that both receptors and their bile acid ligands fulfill an important homeostatic function in the gut. Inagaki et al[8] could show in a mouse model that antibacterial defense in the small intestine requires the presence of FXR. Moreover, data from our group[9] and recent reports from other laboratories[10,11] demonstrated that loss of PXR expression as well as dysfunction is associated with human and rodent inflammatory bowel disease (IBD). PXR regulated genes may therefore be novel candidates as genetic susceptibility markers for IBD[12,13].
Fibroblast growth factor 19 (FGF19), the human ortholog of the murine Fgf15 gene has been recently identified as a FXR target gene in the liver[14] and most importantly in the intestine[15,16]. Gut secreted FGF19 binds to FGF receptor 4 (FGFR4) on hepatocytes initiating a c-jun N-terminal kinase (JNK) signaling pathway, causing an inhibition of CYP7A1 and thereby hepatic bile acid synthesis. Moreover, FGF19 seems to be an important factor in the postprandial feedback loop to stimulate gallbladder filling[17], indicating that investigating the transcriptional regulation of FGF19 is of major importance for a better understanding of bile acid metabolism and enterohepatic signaling.
In this paper we followed our hypothesis that FGF19 could also be a novel target gene of PXR and that the toxic secondary bile acid LCA might upregulate FGF19 expression in intestinal cells as an enterohepatic feedback mechanism to limit the supply of LCA precursors.
MATERIALS AND METHODS
Cell culture and bile acid stimulation experiments
The human adenocarcinoma cell line LS174T (ATCC, Manassas, USA) was used for bile acid stimulation experiments. Cells were grown in Dulbecco’s Modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L glutamine, and 1% (wt/vol) penicillin/streptomycin (Gibco BRL, Gaithersburg, USA). Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. Confluent monolayers were stimulated for 3, 6, and 24 h with three different concentrations (50, 100, and 250 μmol/L) of chenodeoxcholic acid (CDCA) and lithocholic acid (LCA) sodium salts (Sigma, St. Louis, USA) or 10 μmol/L rifampicin sodium salt (Eremfat®, Fatol Arzneimittel GmbH, Schiffweiler, Germany) as known activator of human PXR in the presence of 10% FCS.
RNA isolation and reverse transcription
Total RNA was extracted from cultured cells according to the manufacturer’s instructions using the RNeasy Midi Kit (Qiagen, Hilden, Germany). Purity and integrity of the RNA was assessed on the Agilent 2100 bioanalyzer with the RNA 6000 Nano LabChip® reagent set (Agilent Technologie, Santa Clara, USA). The RNA was quantified spectrophotometrically and then stored at -80°C. Subsequently, cDNAs were generated by reverse transcriptase reactions. The reaction was performed in 20 μL reaction volume containing 2 μg of total cellular RNA, 4 μL 5 × first strand buffer (Invitrogen, Karlsruhe, Germany), 2 μL 0.1 mol/L DTT, 1 μL dN6 primer (10 mmol/L), 1 μL dNTPs (10 mmol/L), and DEPC water. The reaction mix was incubated for 10 min at 70°C. Then 1μL of Superscript II RT (Invitrogen, Karlsruhe, Germany) was added and RNAs were transcribed for 1 h at 37°C. RT was inactivated at 70°C for 10 min and RNA was degraded by digestion with 1 μL RNase A (10 mg/mL) at 37°C for 30 min.
Realtime quantitative RT-PCR (TaqMan) analysis
Realtime quantitative RT-PCR analysis was performed with an ABI7900HT machine (Applied Biosystems, Foster City, USA). All reagents necessary for running a TaqMan RT-PCR assay including primers and probes were purchased from Applied Biosystems and used according to the manufacturer’s instructions. TaqMan analysis of FGF19 transcripts was carried out with pre-designed and optimized Assays on Demand (Assay Hs00 192 780_m1). The reaction parameters were: 2 min 50°C hold, 30 min 60°C hold, 5 min 95°C hold; followed by 35 cycles of 20 s 94°C melt and 1 min 60°C anneal/extend. Measurements were carried out in triplicates. Results were analyzed with an ABI sequence detector software version 2.0 and relative quantitation was carried out as described earlier[18].
Plasmid constructs, transfections and reporter assays
Primers for genomic PCR amplification of the proximal human CYP3A4 promoter and four FGF19 promoter sequences were as follows: CYP3A4-Luc-390F 5'-CCCCTCGAGGGCACAGGCACACTCCAGGCATAGG-3', CYP3A4-Luc+191R 5'-CCCAAGCTTCTCTATCTGTGAGTAACTGTTCAGG-3', FGF19-Luc-1954F 5'-CCCCTCGAGTCAACACCTTCATGAGTGCTACATC-3', FGF19-Luc-882F 5'-CCCCTCGAGTATTGGCAGGAACCGCTTCATGGAG-3', FGF19-Luc-532F 5'-CCCCTCGAGCTTTCAGGTTGCATCTCGCGCACAG-3', FGF19-Luc-301F 5'-CCCCTCGAGAAGAACCTGAGACTGTCGGAACTGC-3', FGF19-Luc+244R 5'-CCCAAGCTTGATGCAATCCCGATAAGAAATGCTC-3'. Human genomic DNA isolated from leukocytes using the Qiamp blood kit (Qiagen, Hilden, Germany) served as a template for the amplification of the promoter sequences with the High Fidelity PCR System (Roche, Mannheim, Germany). Reporter constructs were cloned by ligation of PCR fragments into the Xho I and Hind III restriction sites of the pGL3-basic vector and subsequently transformed into competent E.coli-DH5alpha. Plasmid purification was achieved by using the Qiagen Plasmid Maxi kit (Qiagen, Hilden, Germany). The identity of the subcloned DNA fragments was confirmed by DNA sequencing. An empty pGL3-basic vector served as negative control, while a pGL3-CMV promoter plasmid was used as positive control. LS174T cells in 6-well plates were transfected with Fugene6 (Roche, Mannheim, Germany) as described previously[19] with 2 μg of reporter plasmids and stimulated with LCA or rifampicin as described above. For cotransfection experiments 2 μg of reporter plasmids and 100 ng of each pSG5-hPXR and pSG5-hRXR[20] or empty expression vector was used. Cells were harvested 24 h after transfection and lysed in reporter lysis buffer (Promega, Madison, USA). Luciferase assay reagent containing luciferyl-CoA was added after centrifugation. Luciferase activity was determined in a LUMAT LB9501 (Berthold Technologies, Bad Wildbad, Germany) and was subsequently normalized to the protein content. Each experiment was repeated three times and measurements were done in triplicates.
Analysis of PXR receptor binding motifs
CYP3A4 and FGF19 promoter sequences and transcriptional start sites have been retrieved by an in silico approach using the programs DBTSS (database of transcription start sites, http://elmo.ims.u_tokyo.ac.jp/dbtss)[21] and Gene to promoter (Genomatix GmbH, Munich, Germany). The computer algorithm NUBIScan[22] and a self defined matrix based on known PXR target genes[9] has been used to predict DNA recognition sites for PXR in the regulatory region of FGF19.
RESULTS
FGF19 is induced by the PXR agonist lithocholic acid in intestinal cells
FGF19 was previously shown to be upregulated in primary human hepatocytes as well as in the intestinal cell line Caco2 by the natural and synthetic FXR ligands chenodeoxycholic acid (CDCA) and GW4064, respectively[14-16]. Our aim was to analyze whether FGF19 is also induced by the secondary bile acid LCA, which acts as a natural agonist of human PXR. Therefore, FGF19 transcript levels were determined in intestinal LS174T cells stimulated with various concentrations of CDCA and LCA in a time course experiment. Figure 1A shows the induction of FGF19 mRNA by the FXR ligand CDCA, which was used as a positive control for the experimental setup. All analyzed concentrations of CDCA could increase the amount of FGF19 mRNA and a time dependency was noticed for the highest concentration (250 μmol/L), indicating that LS174T cells are a suitable cell model to study FGF19 induction by bile acids. In contrast to CaCo2 and HT-29, LS174T cells also have a high expression of detoxification enzymes and PXR target genes as was shown in our previous studies[9]. Stimulation of LS174T cells with LCA resulted in a significant induction of FGF19 transcripts, starting at lower concentrations but most prominently after 24 h at a concentration of 250 μmol/L (Figure 1B). Higher concentrations of bile acids (500 μmol/L) had toxic effects and were not used for further studies (data not shown). These data clearly demonstrate that the FGF19 gene is activated by the PXR ligand LCA in a similar manner as CDCA.
Figure 1.
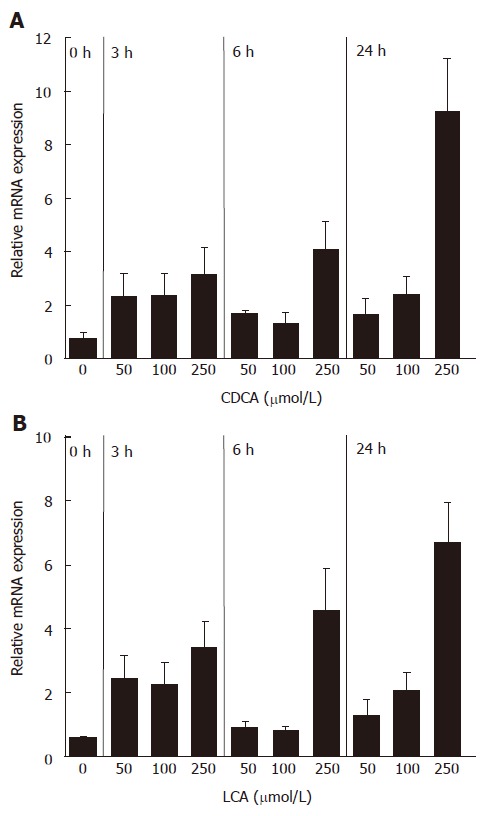
mRNA induction of FGF19 in LS174T cells by CDCA (A) and LCA (B) (mean ± SD, Student’s t-test). P < 0.05 between 3 h, 6 h and 24 h treatment groups and 0 h group in both A and B.
The proximal FGF19 promoter is activated by LCA and Rifampicin
To study whether upregulation of FGF19 mRNA levels were a direct effect of LCA on the promoter, several reporter constructs were amplified from genomic DNA and cloned into luciferase reporter vectors. As depicted in Figure 2A, four FGF19 deletion constructs ranging from positions -1954 to -301 relative to the major transcription start site were created. In addition, a short proximal promoter region of the CYP3A4 gene (-390/+191), which has been previously shown to contain a PXR responsive element (ER6) was generated as a positive control[20].
Figure 2.
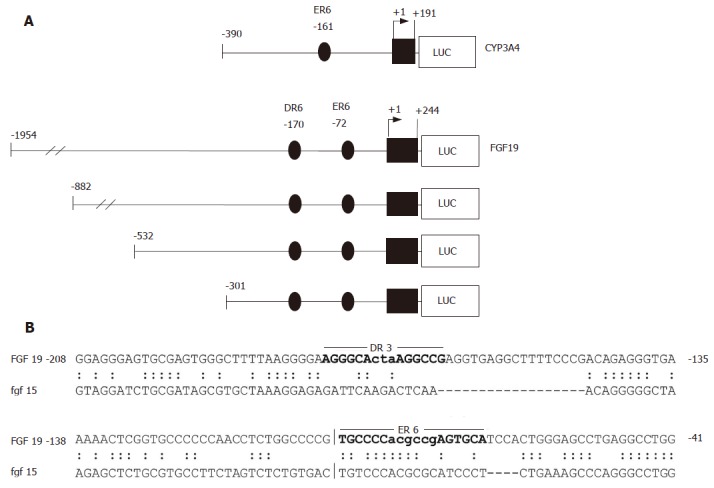
CYP3A4 and FGF19 promoter constructs and sequence alignment of the human and murine FGF19/Fgf15 regulatory regions. A: CYP3A4 and FGF19 promoter constructs used for transient transfection assays. The positions are calculated relative to the major transcription start site; B: Sequence comparison of homologous regions in the human FGF19 and murine Fgf15 promoters. The locations of the DR3 and ER6 elements identified in the human FGF19 promoter by NUBIScan analysis are indictated by bold letters.
In contrast to FXR/RXR heterodimers, which mainly bind to inverted repeats (AG(G/T)TCA) separated by one nucleotide, PXR is a very promiscuous receptor. Thus, PXR/RXR complexes can bind several direct repeats (DR3, DR4, DR5), extroverted repeats (ER6, ER8), and inverted repeats (IR0)[23]. Although the location for the FXR responsive element in the FGF19 gene has been previously mapped to the second intron[14], we concentrated our search for PXR regulatory elements on the 2000 bp upstream region of the FGF19 gene. Since LCA has been described as an FXR antagonist[24], the possibility that LCA acts activating on FGF19 via the known FXR responsive element is very unlikely. Using a self defined optimized matrix for putative PXR DNA binding motifs derived from 17 known PXR target genes[9] and the NUBIScan program[22], we were able to identify two high score PXR matrices in the FGF19 promoter. Figure 2B displays the DR3 and ER6 element at positions -170 and -72, respectively. The ER6 element is conserved between the human FGF19 promoter (upper sequence) and the corresponding region in the murine Fgf15 homolog (lower sequence) and may thus be the most likely candidate for PXR binding. However, species specific differences in PXR regulated gene expression should be taken into account[25], limiting the information gained from this homology alignment.
As the next step to analyze the effects of LCA and the strong PXR agonist rifampicin (Rif) on the FGF19 promoter, LS174T cells were transfected with several 5′-deletion constructs of FGF19 and the CYP3A4 reporter as a known PXR target gene and luciferase activity was determined. CYP3A4 promoter activity was low but significantly above background (data not shown) and simultaneous Rif stimulation for 24 h caused moderate upregulation of promoter activity, whereas LCA had no significant effect (Figure 3A). This finding is most likely explained by the lack of the 8kb distal enhancer element[26] in our CYP3A4 promoter construct. The -1954/+244 FGF19 promoter construct showed highest basal activity, which was reduced when shortening the promoter length down to -301/+244 (Figure 3A, white bars). Despite this drop of overall luciferase activity, which could be explained by the lack of factors required for high basal activity, a significant promoter activating effect could be observed for LCA and Rif with all four analyzed constructs. These data clearly indicate that the LCA/Rif responsive element is localized within the first 301 bp of the FGF19 proximal promoter region.
Figure 3.
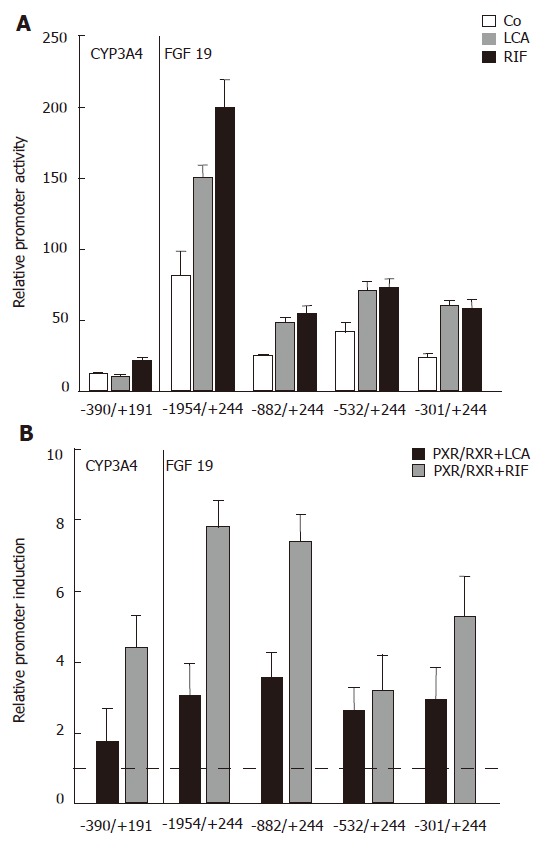
Localization of the LCA/Rif/PXR responsive element in the human FGF19 promoter. A: Relative CYP3A4 and FGF19 promoter activities of LS174T cells treated with LCA (250 μmol/L) and Rif (10 μmol/L) compared to control treated cells for 24 h (P < 0.05 between LCA and Rif treatment groups and control treated groups); B: Reporter constructs were cotransfected with PXR/RXR or mock plasmids and stimulated with LCA (250 μmol/L) and Rif (10 μmol/L) as indicated for 24 h. Mock transfected and solely LCA/Rif stimulated cells were used for normalization and calculation of relative promoter induction (mean ± SD, Student’s t-test). The dotted line indicates the threshold for significant promoter induction. P < 0.05 between PXR/RXR cotransfected and LCA/Rif treated groups and mock transfected and LCA/Rif treated groups. The used reporter constructs are indicated on the x-axis.
PXR/RXR heterodimers induce the proximal FGF19 promoter
To directly show that PXR/RXR heterodimers can activate the FGF19 promoter, we then performed transient cotransfection experiments of all luciferase reporters with human PXR and RXR expression plasmids. Relative promoter induction levels were determined by calculating the promoter activity of PXR/RXR cotransfected and LCA/Rif stimulated intestinal cells relative to mock transfected and solely LCA/Rif stimulated cells. Figure 3B shows that PXR/RXR overexpression lead to a significant increase of CYP3A4 and FGF19 promoter activities. As expected, the stimulatory effect was higher when cells were stimulated with Rif compared to LCA. Interestingly, a high inducible effect of both PXR ligands was retained in the smallest FGF19 promoter construct -301/+244. These results confirm the promoter assays from LCA/Rif stimulated cells (Figure 3A) and furthermore provide evidence that PXR/RXR heterodimers bind to the proximal FGF19 promoter region that harbors the DR3 and ER6 elements as candidate binding sites.
DISCUSSION
Our results reveal for the first time that the toxic secondary bile acid LCA and its receptor PXR act as positive regulators of FGF19 gene expression in intestinal cells by directly activating the proximal promoter region. The physiological consequences of FGF19 upregulation by PXR-bound LCA is very likely an enterohepatic feedback inhibition of bile acid synthesis in the liver. Thus, in addition to stimulating enhanced enterocyte metabolism of LCA by CYP3A4, intestinal PXR seems to control liver bile acid metabolism.
Our group and others have shown that PXR levels were significantly downregulated in the colon of patients with ulcerative colitis[9] and that genetic variants in PXR are associated with IBD[12]. Therefore, it will be interesting to see whether intestinal FGF19 mRNA levels are compromised in IBD patients. However, since FGF19 is also regulated by FXR, a functional redundancy in bile acid regulation of FGF19 cannot be excluded.
Recently, PXR-mediated repression of NF-kappaB target genes in the colon has been identified as a critical mechanism by which PXR activators such as rifampicin decrease the susceptibility of mice to DSS-induced IBD[10]. Furthermore, NF-kappaB activation reciprocally inhibits PXR and inhibition of NF-kappaB enhances PXR activity, demonstrating a direct relationship between PXR and the intestinal inflammatory system[11]. It is thus tempting to speculate that NF-kappaB-mediated inflammatory processes could also affect FGF19 expression and function.
In conclusion, our results implicate that PXR agonists may have a novel therapeutic potential to control bile acid metabolism and detoxification via regulating enterohepatic signaling involving FGF19.
ACKNOWLEDGMENTS
Expression plasmids for pSG5-hPXR and pSG5-hRXR were kindly provided by Dr. Bryan Goodwin (GlaxoSmithKline Research and Development). We also thank Manfred Haas for excellent technical assistance. This work was supported by grants form the Deutsche Forschungsgemeinschaft (SFB585/A1) and the Stiftung für Pathobiochemie und Molekulare Diagnostik.
COMMENTS
Background
The pregnane-X-receptor controls liver and intestinal detoxification of xenobiotics and endobiotics including bile acids. Identification of tissue-specific molecular targets and effects of PXR will help to understand the coordinate regulation of enterohepatic detoxification and bile acid metabolism.
Research frontiers
The article deals with the molecular mechanisms of bile-acid dependent regulation of the fibroblast growth factor 19 gene (FGF19). The findings are related to bile acid metabolism and the nuclear receptor system including PXR.
Innovations and breakthroughs
Applying a combined mRNA expression study and comprehensive promoter analysis of the FGF19 gene, we have identified lithocholic acid (LCA)-dependent regulation of the human FGF19 gene. The induction of FGF19 mRNA by LCA is critically dependent on PXR/RXR heterodimers stimulating FGF19 promoter activity. We conclude that intestinal LCA-levels regulate FGF19 expression and thereby control enterohepatic bile acid metabolism.
Applications
Correlating bile acid levels with the expression profiles of PXR-regulated genes such as FGF19 may allow physicians to estimate the bile acid detoxification potential of an individual. A potential modulation of PXR function by pharmacological ligands may improve detoxification of xenobiotics and endobiotics in individuals with inflammatory bowel disease (IBD).
Terminology
FGF19 is a growth factor secreted in the intestine that signals through the liver fibroblast growth factor receptor 4. Thereby, FGF19 inhibits the expression of hepatic CYP7A1 in the liver and elicits a feedback mechanism on bile acid synthesis. PXR belongs to the nuclear receptor superfamily and controls the expression of a variety of detoxification genes in the liver and the intestine. In addition to xenobiotics, PXR is activated by endobiotics including toxic secondary bile acids.
Peer review
The topic of the research is modern and attractive and the contents of the article are significant. The research is novel and innovative. The manuscript is well written, readable and nicely documented by enclosed figures. Presented work meets ethical standards for in vitro studies.
Footnotes
S- Editor Zhu LH L- Editor Alpini GD E- Editor Li JL
References
- 1.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 2.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 5.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 6.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci USA. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–G1122. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O'Donoghue D, O'Sullivan M, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006;130:341–348; quiz 592. doi: 10.1053/j.gastro.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Langmann T, Schmitz G. Loss of detoxification in inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:358–359. doi: 10.1038/ncpgasthep0545. [DOI] [PubMed] [Google Scholar]
- 14.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Pircher PC, Schulman IG, Westin SK. Regulation of complement C3 expression by the bile acid receptor FXR. J Biol Chem. 2005;280:7427–7434. doi: 10.1074/jbc.M411473200. [DOI] [PubMed] [Google Scholar]
- 17.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 18.Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, Schmitz G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 19.Langmann T, Porsch-Ozcürümez M, Heimerl S, Probst M, Moehle C, Taher M, Borsukova H, Kielar D, Kaminski WE, Dittrich-Wengenroth E, et al. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J Biol Chem. 2002;277:14443–14450. doi: 10.1074/jbc.M110270200. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki Y, Yamashita R, Nakai K, Sugano S. DBTSS: DataBase of human Transcriptional Start Sites and full-length cDNAs. Nucleic Acids Res. 2002;30:328–331. doi: 10.1093/nar/30.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podvinec M, Kaufmann MR, Handschin C, Meyer UA. NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol Endocrinol. 2002;16:1269–1279. doi: 10.1210/mend.16.6.0851. [DOI] [PubMed] [Google Scholar]
- 23.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277:31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- 25.Krasowski MD, Yasuda K, Hagey LR, Schuetz EG. Evolution of the pregnane x receptor: adaptation to cross-species differences in biliary bile salts. Mol Endocrinol. 2005;19:1720–1739. doi: 10.1210/me.2004-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]


