Abstract
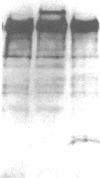
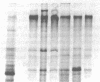
A human hepatocellular carcinoma line, HepG2, was found to secrete coagulation Factor V. Factor V activity in HepG2 culture fluid increased nearly linearly during a 20-h time course (5 ng Factor Va/h per 10(6) cells). Thrombin treatment increased Factor V activity in HepG2 culture medium six- to ninefold, indicating that the medium accumulates a mixture of Factors V and Va. To demonstrate de novo synthesis of Factor V, HepG2 cells were incubated in culture medium containing [35S]methionine. Labeled Factor V was immunoprecipitated from the medium and was shown to co-migrate with purified plasma Factor V upon sodium dodecyl sulfate polyacrylamide gel electrophoresis and fluorography. When medium was treated with thrombin before immunoprecipitation and fluorography, the 330,000-Mr [35S]methionine-labeled Factor V was converted to Factor Va. Factor Va coagulant activities from HepG2 cells and human plasma were inhibited in parallel by anti-Factor V antibody, indicating that HepG2 and plasma Factor Va have the same intrinsic activity. If normal hepatocytes synthesize Factor V at the same rate as HepG2 cells, then hepatocyte secretion can account for the total Factor V present in plasma. The production of Factor V by cultured human umbilical vein endothelial cells was also examined. Spent culture medium from endothelial cells contained only Factor Va and the amount was less than 1% of the activity found in medium from HepG2 cells under comparable conditions. The amount of Factor V activity in endothelial cell culture fluid did not change with time in culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowie E. J., Thompson J. H., Jr, Didisheim P., Owen C. A., Jr Disappearance rates of coagulation factors: transfusion studies in factor-deficient patients. Transfusion. 1967 May-Jun;7(3):174–184. doi: 10.1111/j.1537-2995.1967.tb05507.x. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981 Nov;68(5):1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coots M. C., Muhleman A. F., Glueck H. I. Hemorrhagic death associated with a high titer factor V inhibitor. Am J Hematol. 1978;4(2):193–206. doi: 10.1002/ajh.2830040212. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Giddings J. C., Shearn S. A., Bloom A. L. The immunological localization of factor V in human tissue. Br J Haematol. 1975 Jan;29(1):57–65. doi: 10.1111/j.1365-2141.1975.tb01799.x. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. Purification and characterization of human coagulation factor V. J Biol Chem. 1981 Jan 25;256(2):1002–1007. [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. The interaction of human coagulation factor Va with platelets. J Biol Chem. 1982 Apr 10;257(7):3963–3969. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laposata M., Prescott S. M., Bross T. E., Majerus P. W. Development and characterization of a tissue culture cell line with essential fatty acid deficiency. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7654–7658. doi: 10.1073/pnas.79.24.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner K., Niessner H., Thaler E. Coagulation abnormalities in liver disease. Semin Thromb Hemost. 1977 Summer;4(1):40–56. doi: 10.1055/s-0028-1087127. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Majerus D. W., Majerus P. W. Patients with congenital factor V deficiency have decreased factor Xa binding sites on their platelets. J Clin Invest. 1978 Oct;62(4):824–831. doi: 10.1172/JCI109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. M., Aden D. P., Knowles B. B., Colten H. R. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982 Oct;70(4):906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. P., Miller L. L., Troup S. B. Synthesis of clotting factors by the isolated perfused rat liver. J Clin Invest. 1966 May;45(5):690–701. doi: 10.1172/JCI105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers G. M., Shuman M. A. Prothrombin is activated on vascular endothelial cells by factor Xa and calcium. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7001–7005. doi: 10.1073/pnas.80.22.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H. H., Broze G. J., Miletich J. P., Majerus P. W. Human coagulation factor Va is a cofactor for the activation of protein C. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1584–1588. doi: 10.1073/pnas.80.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H. H., Broze G. J., Miletich J. P., Majerus P. W. The light chain of factor Va contains the activity of factor Va that accelerates protein C activation by thrombin. J Biol Chem. 1983 Jul 25;258(14):8531–8534. [PubMed] [Google Scholar]
- Suzuki K., Dahlbäck B., Stenflo J. Thrombin-catalyzed activation of human coagulation factor V. J Biol Chem. 1982 Jun 10;257(11):6556–6564. [PubMed] [Google Scholar]
- Tracy P. B., Rohrbach M. S., Mann K. G. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983 Jun 25;258(12):7264–7267. [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]