Abstract
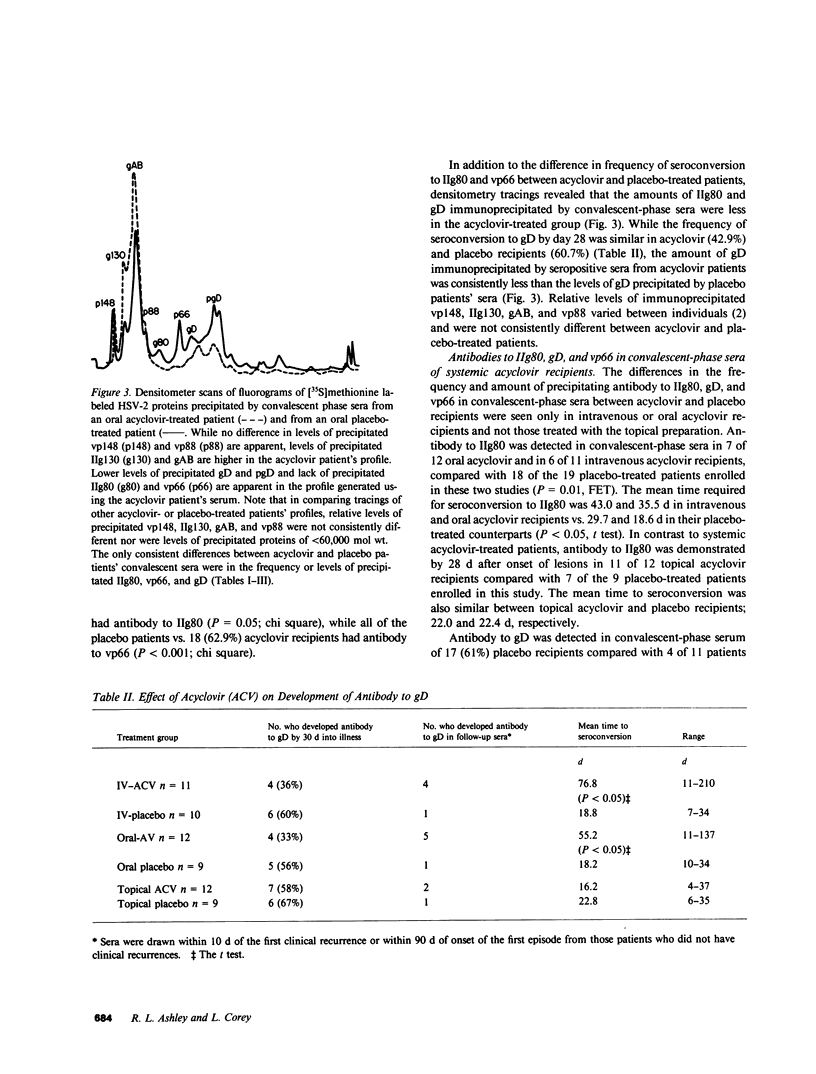
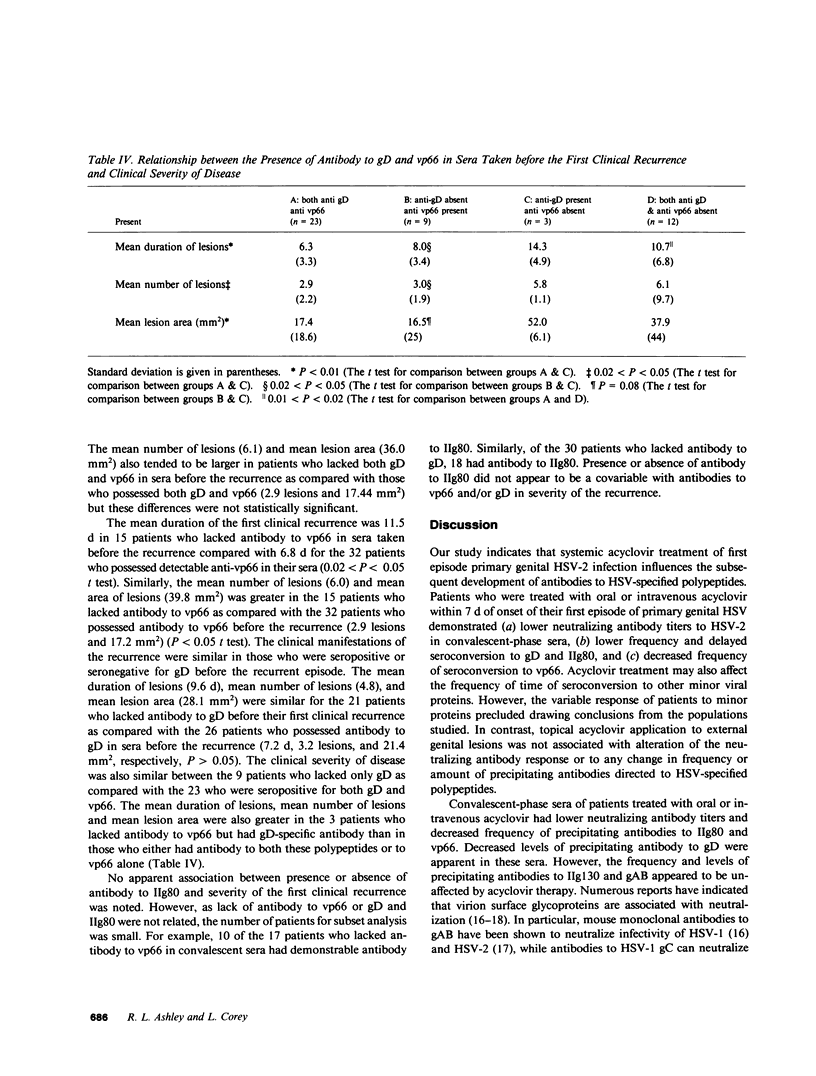
Sera from patients with first episode primary genital herpes infections who were treated with the antiviral drug acyclovir were studied to determine the effect of therapy on the immune response to herpes simplex virus (HSV) glycoproteins and polypeptides. 63 patients were evaluated, 35 patients received acyclovir: 11 intravenously, 12 orally, and 12 topically, while 28 received placebo. Topical application of acyclovir had no effect on the immune response to HSV infection. However, both oral and intravenous acyclovir were associated with later development of antibodies to two glycoproteins (of 80,000 and 60,000 mol wt [IIg80 and gD, respectively]) and one nonglycosylated polypeptide of 66,000 mol wt (vp66). Antibody to IIg80 was present in convalescent phase serum in 13/23 systemic acyclovir recipients vs. 18/19 placebo recipients (P = 0.01) and antibody to gD was detected in 8/23 oral or intravenous acyclovir recipients vs. 11/19 placebo recipients (P = 0.06). The mean time to seroconversion to IIg80 (39.0 d) and gD (55.5 d) was significantly longer for systemic acyclovir recipients than for the placebo controls, 23.4 and 18.5 d, respectively (P less than 0.05 for each comparison). 7 (30%) of 23 systemic acyclovir recipients compared with 100% of the placebo recipients had antibody to vp66 by 30 d after onset of the primary episode (P less than 0.001). Subsequent untreated recurrences of genital herpes were associated with seroconversion to gD, IIg80, and vp66. Patients who lacked antibody to both gD and vp66 in sera taken before their first clinical recurrence of disease experienced a longer duration of the recurrent episode (10.8 d) than those who possessed antibody to both vp66 and gD (6.3 d) (P less than 0.05). In addition, the mean duration of lesions, number of lesions, and mean lesion area were greater in patients who lacked antibody to vp66 but had anti gD, as compared with those who had anti-p66 but lacked anti-gD; suggesting that antibody to vp66 correlated more closely with subsequent disease severity than did antibody to gD. Acyclovir therapy appears to influence the frequency and time of development of antibody to a number of different HSV-specific polypeptides. Further studies of the effects of antiviral therapies on the immune response to these proteins may help clarify the role of these polypeptides in the pathogenesis of disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Benedetti J. K., Critchlow C. W., Remington M. R., Winter C. A., Fahnlander A. L., Smith K., Salter D. L., Keeney R. E., Davis L. G. Double-blind controlled trial of topical acyclovir in genital herpes simplex virus infections. Am J Med. 1982 Jul 20;73(1A):326–334. doi: 10.1016/0002-9343(82)90117-6. [DOI] [PubMed] [Google Scholar]
- Corey L., Fife K. H., Benedetti J. K., Winter C. A., Fahnlander A., Connor J. D., Hintz M. A., Holmes K. K. Intravenous acyclovir for the treatment of primary genital herpes. Ann Intern Med. 1983 Jun;98(6):914–921. doi: 10.7326/0003-4819-98-6-914. [DOI] [PubMed] [Google Scholar]
- Corey L., Nahmias A. J., Guinan M. E., Benedetti J. K., Critchlow C. W., Holmes K. K. A trial of topical acyclovir in genital herpes simplex virus infections. N Engl J Med. 1982 Jun 3;306(22):1313–1319. doi: 10.1056/NEJM198206033062201. [DOI] [PubMed] [Google Scholar]
- Corey L. The diagnosis and treatment of genital herpes. JAMA. 1982 Sep 3;248(9):1041–1049. [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindel A., Adler M. W., Sutherland S., Fiddian A. P. Intravenous acyclovir treatment for primary genital herpes. Lancet. 1982 Mar 27;1(8274):697–700. doi: 10.1016/s0140-6736(82)92618-6. [DOI] [PubMed] [Google Scholar]
- Nilsen A. E., Aasen T., Halsos A. M., Kinge B. R., Tjøtta E. A., Wikström K., Fiddian A. P. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet. 1982 Sep 11;2(8298):571–573. doi: 10.1016/s0140-6736(82)90658-4. [DOI] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Buchan A., Sim C., Watson D. H. Type-specific protein in herpes simplex virus envelope reacts with neutralising antibody. Nature. 1974 May 24;249(455):360–361. doi: 10.1038/249360a0. [DOI] [PubMed] [Google Scholar]
- Rattray M. C., Corey L., Reeves W. C., Vontver L. A., Holmes K. K. Recurrent genital herpes among women: symptomatic v. asymptomatic viral shedding. Br J Vener Dis. 1978 Aug;54(4):262–265. doi: 10.1136/sti.54.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontver L. A., Hickok D. E., Brown Z., Reid L., Corey L. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. Am J Obstet Gynecol. 1982 May 1;143(1):75–84. doi: 10.1016/0002-9378(82)90686-x. [DOI] [PubMed] [Google Scholar]