Abstract
AIM: To investigate the inhibitory effect of gefitinib combined with cytotoxic agent cisplatin (CDDP) on hepatocellular carcinoma (HCC).
METHODS: Female Kunming mice and H22 hepatocarcinoma cells were used. Gefitinib at daily dose of 100 mg/kg body weight (BW) or lecithin liquid was given by gastrogavage once a day for 5 or 10 successive days. CDDP or normal saline (NS) was administered intraperitoneally (i.p.) once a day for 5 successive days. Mice were randomly divided into control group (lecithin, or NS, i.p.), CDDP group (daily dose, 1.2 mg/kg BW; d1-5, or d6-10), Gefitinib (d1-5, or d6-10, or d1-10), and Gefitinib combined with CDDP groups. The inhibitory rate (IR) of tumor, net BW, spleen index (SI), thymus index (TI) and the amount of peripheral blood cells of mice were detected on the 12th experiment day.
RESULTS: The growth of HCC in mice was inhibited by Gefitinib alone (IR: 41% in d1-10 group and 30% in d1-5 group, respectively) or CDDP alone (IR: 32-54% in d1-5 group or d6-10 group). The highest inhibitory effect (IR: 56%) on HCC growth was observed in Gefitinib (d1-10) combined with CDDP (d1-5) group. Higher inhibition was also observed in CDDP (d1-5) followed by Gefitinib (d6-10) group than that in Gefitinib (d1-5) followed by CDDP (d6-10) group (IR: 61% vs 36%, P<0.01) in the independent study. Net BW, SI, TI and the amount of blood cells of mice in Gefitinib alone group were not significantly different from those in control groups.
CONCLUSION: Gefitinib can significantly inhibit the growth of murine H22 hepatocellular carcinoma. If Gefitinib is used after CDDP treatment in animal experiments, the inhibitory effect could be enhanced.
Keywords: Neoplasms Gefitinib, Therapy carcinoma, Hepatocellular cisplatin
INTRODUCTION
Hepatocellular carcinoma (HCC) is a malignant tumor with a poor prognosis and is not potentially curable by currently available therapeutic modalities, such as chemotherapy[1]. HCC is known to frequently overexpress the epidermal growth factor receptor (EGFR), which is associated with more aggressive diseases and a poor prognosis[2,3].
For these reasons, the blockade of EGFR activation and/or function might be proposed as a potential therapeutic strategy for HCC. The efficacy of Gefitinib on HCC is still unknown. The present study aimed to determine whether gefitinib (IRESSA, ZD1839) combined with cisplatin (CDDP) caused the inhibitory effects that were dependent on the sequence of administration in murine HCC. Part of the results in this study has been published in Chinese journal[4].
MATERIALS AND METHODS
Drugs, cells and animals
CDDP was produced by Haosheng Pharmaceutical Corporation (Lianyungong, China). Gefitinib was purchased from AstraZeneca Pharmaceutical Co. (No. po10019452, Macclesfield, UK). Mouse H22 hepatocarcinoma cell line was provided by the Beijing Institute for Cancer Research. Female Kunming mice (16.0-21.1 g) were supplied from the Medical Animal Center of Academy of Military Medical Sciences. All animals were fed on basic diet and tap water. The mice were dissected to measure body weight (BW), weight of tumor, spleen and thymus on the 12th experimented day. Blood samples were also collected at the same time.
Administration of animals
Cells (107) in 0.2 mL suspension were injected subcutaneously into mice over the rib cage for implanted tumors. From the second day after the implantation of H22 cells, Gefitinib at a daily dose of 100 mg/kg BW or lecithin liquid (taken as oral control group) was given by gastrogavage once a day for 5 or 10 successive days (d1-5, or d6-10, or d1-10). CDDP at a daily dose of 1.2 mg/kg (0.1 mL/10 g BW), or NS (0.1 mL/10 g BW, taken as NS control group) was administered intraperitoneally (i.p.) once a day for 5 successive days (d1-5 or d6-10).
Experimental design
From the second day after the implantation of H22 cells, mice were randomly divided into different groups (12-14/group). Two independent experiments were carried out at fixed daily doses of CDDP and Gefitinib, but at various beginning time and duration of CDDP and Gefitinib. In the first experiment, mice were randomly divided into control group (lecithin, d1-10, or NS, i.p. d1-10), CDDP (d1-5, or d6-10), Gefitinib group (d1-10), and Gefitinib combined with CDDP groups including Gefitinib d1-10+CDDP d1-5 group (Gefitinib plus CDDP for 5 d followed by Gefitinib for 5 d) or Gefitinib d1-10+CDDP d6-10 group (Gefitinib for 5 d followed by Gefitinib plus CDDP for 5 da). In the second experiment, mice were divided into control group (lecithin, d1-5, or NS, i.p. d1-5), CDDP group (d1-5, or d6-10), Gefitinib group (d1-5), and Gefitinib combined with CDDP groups including Gefitinib d1-5+CDDP d1-5 group (Gefitinib plus CDDP for 5 d), or Gefitinib d1-5→CDDP d6-10 group (Gefitinib from d 1 to d 5 followed by CDDP from d 6 to d 10), or CDDP d1-5→Gefitinib d6-10 group (CDDP from d 1 to d 5 followed by Gefitinib from d 6 to d 10).
Observation index
The growth inhibition of tumor was described by the inhibitory rate (IR). IR, net BW, spleen index (SI), thymus index (TI) and the amount of peripheral blood WBC, RBC, HB and PLT of mice were detected. IR was calculated according to the following formula: IR (%) = (mean weight of tumors in control group - mean weight of tumors in test groups/mean weight of tumors in control group). SI or TI = 10×weight of spleen or thymus (mg)/mouse BW. Net BW (g) = mouse BW - tumor weight at sacrifice.
Statistical analysis
Student’s t-test was used for statistical analysis. P value less than 0.05 was considered statistically significant.
RESULTS
Antitumor effect of Gefitinib on implanted H22 tumor
The growth of HCC in mice was inhibited by Gefitinib alone (IR: 41% in d1-10 group and 30% in d1-5 group, respectively, P>0.05) or CDDP alone (IR: 54% in d1-5 group and 46% in d6-10 group in the first experiment, or 43 and 32% in the second experiment, respectively) (Table 1 and Figures 1A and B). IR in Gefitinib group was not significantly different from that in CDDP group, P>0.05.
Table 1.
Inhibitory effect of Gefitinib or/and CDDP on mouse implanted H22 tumors (mean).
| Groups |
n |
Weight of tumor (g) |
IR (%) |
|||
| Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | |
| Lecithin control | 14 | 14 | 1.39±0.70 | 1.42±0.41 | 0 | 0 |
| NS control | 13 | 14 | 1.49±0.80 | 1.41±0.46 | 0 | 0 |
| CDDP d1-5 | 14 | 13 | 0.68±0.27 | 0.81±0.30 | 54 | 43 |
| CDDP d6-10 | 12 | 14 | 0.80±0.25 | 0.96±0.32 | 46 | 32 |
| Gefitinib d1-10 | 14 | 0.88±0.28 | 41 | |||
| Gefitinib d1-5 | 14 | 0.99±0.39 | 30 | |||
| Gefitinib d1-10+CDDP d1-5 | 13 | 0.66±0.24 | 56 | |||
| Gefitinib d1-10+CDDP d6-10 | 14 | 1.11±0.41b | 26b | |||
| Gefitinib d1-5+CDDP d1-5 | 14 | 0.63±0.35 | 56 | |||
| CDDP d1-5→Gefitinib d6-10 | 13 | 0.55±0.25b | 61b | |||
| Gefitinib d1-5→CDDP d6-10 | 13 | 0.90±0.30 | 36 | |||
P<0.01 vs Gefitinib d1-10+CDDP d1-5, or Gefitinib d1-5→CDDP d6-10 group, IR: inhibitory rate, CDDP: cisplatin, Exp 1: the first experiment, Exp 2: the second experiment.
Figure 1.
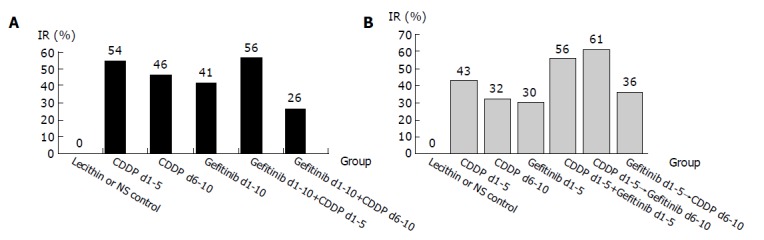
Inhibitory effect of Gefitinib or/and CDDP on mouse implanted H22 tumors in the first experiment “A” and in the second experiment “B”.
Evaluation of the combined effect of Gefitinib and cisplatin
The highest inhibitory effect on HCC growth (IR: 56%) was observed in Gefitinib (d1-10) combined with CDDP (d1-5) group in the first experiment. IR in Gefitinib d1-10+CDDP d6-10 group (IR: 26%) was significantly lower than that in Gefitinib group (IR: 41%) in the first experiment (P<0.01). Higher inhibition was also observed in CDDP (d1-5) followed by Gefitinib (d6-10) group than that in Gefitinib (d1-5) followed by CDDP (d6-10) group in the second experiment (IR: 61% vs 36%, P<0.01). Higher inhibition of tumor was also observed in gefitinib d1-5+CDDP d1-5 group than that in Gefitinib (d1-5) group in the second experiment (IR: 56% vs 30%, P<0.01) (Table 1 and Figures 1A and B).
Effect of gefitinib or/and CDDP on the amount of peripheral blood cells of mice with implanted H22 tumors
There was no significant difference in the amount of WBC, RBC, HB and PLT of peripheral blood among Gefitinib and control groups, P>0.05. The amount of RBC and PLT in CDDP (d1-5) group was remarkably less than that in Gefitinib group. The amount of WBC, PLT of mice in CDDP d1-5→Gefitinib d6-10 group was significantly different from that in Gefitinib d1-5→CDDP d6-10 group (Table 2).
Table 2.
Effect of Gefitinib or/and CDDP on the amount of peripheral blood cells of mice bearing H22 tumors (mean±SD).
| Groups |
n |
WBC (109/L) |
RBC (109/L) |
HB (g/L) |
PLT(109/L) |
|||||
| Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | |
| Lecithin control | 14 | 14 | 7.6±1.8 | 8.5±1.8 | 6.8±2.3 | 7.5±1.1 | 110.2±14.8 | 126.6±16.5 | 1259.1±336.4 | 1051.4±208.7 |
| NS control | 13 | 14 | 7.6±1.7 | 8.4±1.7 | 6.5±3.2 | 8.0±1.8 | 108.4±14.0 | 125.2±13.5 | 1149.2±304.4 | 1057.1±200.4 |
| CDDP d1-5 | 14 | 13 | 6.6±1.8 | 6.7±2.3a | 5.5±3.5a | 6.7±0.9a | 120.4±16.6 | 124.5±9.6 | 542.9±256.4b | 589.2±190.3b |
| CDDP d6-10 | 12 | 14 | 7.7±1.4 | 6.8±1.9 | 5.9±2.8 | 6.7±1.1 | 117.2±9.8 | 122.6±18.6 | 1318.7±829.8 | 1100.1±281.9 |
| Gefitinib d1-10 | 14 | 8.2±2.8 | 6.2±3.8 | 116.3±15.6 | 1203.3±310.5 | |||||
| Gefitinib d1-5 | 14 | 9.9±2.5 | 7.7±1.2 | 127.9±15.2 | 1100.3±170.6 | |||||
| Gefitinib d1-10 | 13 | 7.2±2.9 | 6.4±2.3 | 124.0±16.7 | 523.8±252.9b | |||||
| +CDDP d1-5 | ||||||||||
| Gefitinib d1-10 | 14 | 7.7±2.5 | 6.8±1.2 | 123.9±8.3 | 1307.5±474.4 | |||||
| +CDDP d6-10 | ||||||||||
| Gefitinib d1-5 | 14 | 8.2±2.4 | 6.9±1.3 | 122.9±18.6 | 589.9±168.4b | |||||
| +CDDP d1-5 | ||||||||||
| CDDPd1-5 | 13 | 7.8±1.7 | 7.3±1.1 | 126.1±19.3 | 514.4±204.3b | |||||
| →Gefitinibd6-10 | ||||||||||
| Gefitinib d1-5 | 13 | 6.1±1.3a | 7.1±1.2 | 126.5±20.4 | 1135.8±245.5 | |||||
| →CDDP d6-10 | ||||||||||
P<0.05,
P<0.01 vs Gefitinib (d1-5 or d1-10) group, Exp 1: first experiment, Exp 2: second experiment.
Effect of Gefitinib or/and CDDP on net body weight and weight of immune organs of mice with implanted H22 tumors
SI, TI and net BW of mice in Gefitinib group were not significantly different compared with control groups (P>0.05) SI and TI in groups of CDDP (d1-5, or d6-10), Gefitinib combined with CDDP were significantly lower than those in Gefitinib group. Net BW in groups of CDDP (d6-10), Gefitinib combined with CDDP was significantly lower than that in Gefitinib group (Table 3).
Table 3.
Effect of Gefitinib or/and CDDP on BW, TI, and SI of mice bearing H22 tumors (mean±SD).
| Groups |
n |
SI |
TI |
BW prior to treatment (g) |
BW at sacrifice (g) |
Net BW at sacrifice (g) |
||||||
| Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | |
| Lecithin control | 14 | 14 | 92.1±34.9 | 76.9±18.0 | 53.9±13.2 | 45.2±7.9 | 16.5±1.0 | 19.5±1.0 | 26.6±4.2 | 28.3±2.0 | 25.1±4.4 | 27.5±2.0 |
| NS control | 13 | 14 | 92.7±35.0 | 75.7±17.2 | 52.6±14.2 | 46.3±8.0 | 16.5±0.9 | 19.4±0.8 | 25.6±4.0 | 28.3±2.0 | 25.0±3.8 | 27.5±2.0 |
| CDDP d1-5 | 14 | 13 | 70.2±17.9b | 56.0±16.0b | 40.3±5.8a | 35.9±4.1a | 16.6±0.8 | 19.8±0.9 | 22.9±1.5 | 26.9±2.7 | 22.3±1.3 | 25.9±2.7 |
| CDDP d6-10 | 12 | 14 | 59.0±11.9b | 52.8±12.9b | 39.7±4.9a | 35.1±6.3a | 16.6±0.8 | 18.9±0.7 | 22.0±2.1a | 25.3±3.0b | 21.2±2.1a | 24.4±2.9b |
| Gefitinib d1-10 | 14 | 91.3±21.5 | 46.3±7.7 | 16.5±0.9 | 24.6±2.8 | 23.8±2.9 | ||||||
| Gefitinib d1-5 | 14 | 83.6±22.5 | 41.4±8.0 | 19.7±0.8 | 28.0±1.4 | 27.4±1.5 | ||||||
| Gefitinib d1-10 | 13 | 59.3±32.5b | 39.6±5.1a | 16.4±1.0 | 17.6±2.1b | 16.9±2.1b | ||||||
| +CDDP d1-5 | ||||||||||||
| Gefitinib d1-10 | 14 | 51.7±14.5b | 39.7±8.4a | 16.5±0.9 | 19.9±1.6b | 18.8±1.6b | ||||||
| +CDDP d6-10 | ||||||||||||
| Gefitinib d1-5 | 14 | 56.4±8.4b | 35.8±4.1a | 18.9±0.8 | 24.6±1.4b | 23.7±1.4b | ||||||
| +CDDP d1-5 | ||||||||||||
| CDDPd1-5 | 13 | 54.5±16.4b | 34.6±5.8a | 19.7±0.7 | 24.1±2.3b | 24.1±2.3b | ||||||
| →Gefitinib d6-10 | ||||||||||||
| Gefitinib d1-5 | 13 | 46.3±12.6b | 34.1±6.1a | 18.9±0.7 | 24.8±2.4b | 24.3±2.3b | ||||||
| →CDDP d6-10 | ||||||||||||
P<0.05,
P<0.01 vs gefitinib (d1-5 or d1-10) group, BW: body weight, SI: spleen index [10 × weight of spleen (mg)/BW(g)], TI: thymus index [10 × weight of thymus (mg)/BW (g)], Exp 1: first experiment, Exp 2: second experiment.
DISCUSSION
Gefitinib, an anilinoquinazoline, is an orally active EGFR tyrosine kinase inhibitor (EGFR-TKI) with inhibitory effects on the growth of a variety of tumors[5-8]. However, the inhibitory effect of Gefitinib on HCC remains unclear. Cisplatin, a usual platin analogue, has shown significant single-agent cytotoxic activity in advanced cancers.
The results in this study using implanted H22 HCC cells demonstrated that Gefitinib alone (100 mg/kg) had obviously inhibitory effects (IR: 41 or 30%) on mouse HCC growth. CDDP (1.2 mg/kg) was demonstrated as the suitable dose for this mouse model, which could achieve remarkable inhibitory effects (IR: 32-54%). IR of Gefitinib on tumor growth was not significantly different from that of CDDP, indicating that Gefitinib could produce powerful inhibitory effects on tumor growth as CDDP alone in both experiments. Gefitinib could also block EGFR, decrease angiogenesis, and induce apoptosis of tumor cells, resulting in antitumor activity[9,10]. In preclinical and clinical studies, Gefitinib has showed growth-inhibitory effects on a variety of cancer cell lines, tumor xenograft models, and cancer patients. The upregulating expression of EGFR was frequently found in HCC, and was associated with more aggressive diseases and poor prognosis[2,3]. The blockade of EGFR activation and/or function might be proposed as a potential therapeutic strategy for HCC.
We tried to enhance the antitumor efficacy by combining chemotherapeutic agent CDDP with Gefitinib and to determine whether the enhancement of inhibitory effect was dependent on the sequence of combination of Gefitinib and CDDP. In the experiment, CDDP and Gefitinib were given together at the fixed daily doses on the different schedules, and resulted in a significant difference in tumor regression (IR: 26% vs 56%). The higher inhibitory effect on tumor growth could be achieved following sequential exposure to CDDP+/-Gefitinib then to Gefitinib, which indicated that CDDP+/-Gefitinib followed by Gefitinib could improve the inhibitory effect on HCC growth. This result may be related to the possibility that Gefitinib enhances the cytotoxic action and irrepairable damage to cancer cells of CDDP by maintaining and potentiating apoptosis, decreasing the repair of DNA damage, and inhibiting removal of Pt-DNA adducts induced by CDDP. Chemotherapeutic agent CDDP could kill tumor cells rapidly and powerfully. Simultaneous or subsequent blockade of EGFR signaling by Gefitinib could significantly enhance the antitumor action of CDDP on H22 tumors. With these results, this study may provide some information for the rational design of a clinical protocol of Gefitinib combined with cytotoxic agents. In tumor cell lines and in several tumor xenograft models, Gefitinib produced an additive or a synergistic activity in combination with cytotoxic agents.
It has been demonstrated that the combination effect of Gefitinib and CDDP was impaired when sequential exposure to Gefitinib for 5 d followed by Gefitinib plus CDDP for 5 d in the first experiment (IR: 26%). A possible explanation for the effect may be that due to Gefitinib blockade or arrest of tumor cells in G0/G1 phases, growth of tumor cells were markedly inhibited by Gefitinib before CDDP. CDDP had a weaker effect on tumor cells at G0/G1 phases, as a result, cytotoxicity of CDDP was impaired and weakened. The result suggests that Gefitinib followed by CDDP is not an appropriate combination in hepatocellular cancer.
Although in preclinical experiments Gefitinib has shown a synergistic effect with chemotherapeutic drugs[11-14], results of two large clinical trials has demonstrated that there was no added clinical benefit when Gefitinib was added to chemotherapy simultaneously in patients with non-small-cell lung cancer[15,16]. These results suggest that the appropriate sequence or proper timing of Gefitinib in combination with cytotoxic agents should be studied further.
Gefitinib alone did not significantly decrease net BW, SI and TI of mice bearing implanted H22 tumors, suggesting that Gefitinib may not damage the function of the host immune system. Gefitinib did not produce significant effects on the amount of peripheral blood cells including WBC, RBC, HB, and PLT. The results in this study showed that CDDP alone decreased net BW and the weight of immune organs (spleen or thymus) of mice. Gefitinib did not decrease the weight of immune organs. When TI and SI decreased, the weight of immune organs decreased, the function of the host immune system was damaged, the tumors grew more rapidly. The hematological toxicity and the effect of Gefitinib on the weight of immune organs were not obvious in the study.
In summary, Gefitinib can remarkably inhibit the growth of H22 tumor without the loss of net BW, SI, and TI and hematological toxicity on WBC, HB, RBC, and PLT. The inhibitory effect could be further improved by combining CDDP with Gefitinib. Furthermore, sequential exposure to CDDP followed by Gefitinib can enhance the antineoplastic activity. For different chemotherapeutic drugs and different types of cancer, the pattern of CDDP combined with Gefitinib might be different.
Footnotes
Assistant Editor Guo SY Edited by Wang XL and Gabbe M
References
- 1.Nakakura EK, Choti MA. Management of hepatocellular carcinoma. Oncology (Williston Park) 2000;14:1085–1098; discussion 1098-1102. [PubMed] [Google Scholar]
- 2.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 3.Wu BW, Wu Y, Wang JL, Lin JS, Yuan SY, Li A, Cui WR. Study on the mechanism of epidermal growth factor-induced proliferation of hepatoma cells. World J Gastroenterol. 2003;9:271–275. doi: 10.3748/wjg.v9.i2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu BD, Yuan SJ, Xu JM, Zhao QC, Li X, Li Y, Hao LH. The effect of IRESSA on H22 mouse hepatocellular carcinoma. Zhonghua YiXue ZaZhi. 2004;84:684–686. [PubMed] [Google Scholar]
- 5.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 6.Herbst RS. ZD1839: targeting the epidermal growth factor receptor in cancer therapy. Expert Opin Investig Drugs. 2002;11:837–849. doi: 10.1517/13543784.11.6.837. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo M, Sakurai H, Saiki I. ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, shows antimetastatic activity using a hepatocellular carcinoma model. Mol Cancer Ther. 2003;2:557–561. [PubMed] [Google Scholar]
- 8.Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 9.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, De Placido S, Bianco AR, Tortora G. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 10.Hirata A, Ogawa S, Kometani T, Kuwano T, Naito S, Kuwano M, Ono M. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res. 2002;62:2554–2560. [PubMed] [Google Scholar]
- 11.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
- 12.Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- 13.Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 16.Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]


