Abstract
AIM: In nonresectable cholangiocellular carcinoma (CCC) therapeutic options are limited. Recently, systemic chemotherapy has shown response rates of up to 30%. Additional regional therapy of the arterially hyper vascularized hepatic tumors might represent a rational approach in an attempt to further improve response and palliation. Hence, a protocol combining transarterial chemoembolization and systemic chemotherapy was applied in patients with CCC limited to the liver.
METHODS: Eight patients (6 women, 2 men, mean age 62 years) with nonresectable CCC received systemic chemotherapy (gemcitabine 1000 mg/m2) and additional transarterial chemoembolization procedures (50 mg/m2 cisplatin, 50 mg/m2 doxorubicin, up to 600 mg degradable starch microspheres). Clinical follow-up of patients, tumor markers, CT and ultrasound were performed to evaluate maximum response and toxicity.
RESULTS: Both systemic and regional therapies were tolerated well; no severe toxicity (WHO III/IV) was encountered. Nausea and fever were the most commonly observed side effects. A progressive rarefication of the intrahepatic arteries limited the maximum number of chemoembolization procedures in 4 patients. A median of 2 chemoembolization cycles (range, 1-3) and a median of 6.5 gemcitabine cycles (range, 4-11) were administered. Complete responses were not achieved. As maximum response, partial responses were achieved in 3 cases, stable diseases in 5 cases. Two patients died from progressive disease after 9 and 10 mo. Six patients are still alive. The current median survival is 12 mo (range, 9-18); the median time to tumor progression is 7 mo (range, 3-18). Seven patients suffered from tumor-related symptoms prior to therapy, 3 of these experienced a treatment-related clinical relief. In one patient the tumor became resectable under therapy and was successfully removed after 10 mo.
CONCLUSION: The present results indicate that a combination of systemic gemcitabine therapy and repeated regional chemoembolizations is well tolerated and may enhance the effect of palliation in a selected group of patients with intrahepatic nonresectable CCC.
Keywords: Cholangiocellular carcinoma, Gemcitabine, Intraarterial chemoembolization
INTRODUCTION
Intrahepatic cholangiocellular carcinoma (CCC) is a rare entity associated with chronic inflammatory bile duct disorders. Diagnosis often occurs in patients having large tumors at nonresectable stages[1,2]. In the past, systemic chemotherapy has shown limited success in terms of objective responses or prolonged survival especially in single agent therapy using 5-FU. Response rates of about 25% were the most to be expected[3]. Gemcitabine (2, 2-dideoxy-fluorocytidine) has gained importance in the palliative treatment of solid tumors. The substance demonstrated activity against breast, bladder, ovarian, pancreatic and non-small cell lung cancer while having a low toxicity profile[4,5]. A large phase III trial on pancreatic cancer revealed a higher activity of gemcitabine compared to 5-FU in terms of patient survival and improvement of symptoms[6]. In a recent phase II study on nonresectable CCC of our institution systemic gemcitabine monotherapy achieved objective response rates of 30%[3]. Furthermore, a majority of the patients experienced a chemotherapy-related relief of symptoms.
Regional intraarterial therapies of the liver have shown efficacy and safety in hypervascularized hepatic tumors or metastases[7]. The rationale for this approach is further increased antitumoral activity in the liver by inducing a temporary local ischemia and increasing local chemotherapy concentrations while reducing the systemic side effects[8]. Hence, in an attempt to improve palliation a combination therapy of systemic gemcitabine with repeated intraarterial chemoembolization appears promising in those patients with CCC limited to the liver in order to further enhance response rates and symptom relief while maintaining low toxicity. Based on this hypothesis a selected group of patients with nonresectable intrahepatic CCC was included in a study to investigate this combination regime.
MATERIALS AND METHODS
From June 2002 to February 2004 eight patients (6 women, 2 men, mean age 62 years) with histologically proven nonresectable CCC were included to the study (Table 1). Inclusion criteria were (1) measurable, unresectable intrahepatic tumors; (2) >18 years of age, a life expectancy of >3 mo; (3) no prior chemotherapy; (4) technically feasible catheterization of the hepatic artery; (5) adequate functional, hematological and biochemical parameters (i.e., cardiac ejection fraction >50%, leukocytes >3000/μL, thrombocytes >90000/μL, creatinine-clearance >60 mL/min, bilirubin <3 mg/dL, cholinesterase >2 mU/mL (21 °C), bilirubin <3 mg/mL, prothrombin time >50%); and (6) informed consent. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics, maximum toxicity, maximum response, adverse events, and arterial hypovascularisation under therapy.
| Patient number | Age (yr) | Gender | Maximum number of gemcitabine cycles | Maximum number of chemo- occlusion cycles | Maximum response | Neutro- penia acc. To WHO | Thrombo- penia acc. To WHO | Anemia acc. To WHO | Choles- tasis acc. To WHO | Nausea acc. To WHO | Flush symptoms acc. To WHO | Tumor- related disease | Clinical therapy related benefit | Progressive arterial hypovascu- larization |
| 1 | 60 | M | 5 | 2 | SD | 2 | 2 | 2 | 2 | 2 | 1 | yes | - | - |
| 2 | 71 | F | 4 | 1 | SD | 2 | 2 | 1 | 2 | 2 | 1 | yes | - | - |
| 3 | 67 | F | 11 | 3 | PR | 1 | 1 | 1 | 2 | 0 | 1 | - | - | yes |
| 4 | 47 | F | 7 | 3 (+11) | PR | 1 | 0 | 1 | 1 | 2 | 2 | yes | - | yes |
| 5 | 63 | F | 9 | 2 (+11) | PR | 0 | 0 | 1 | 2 | 1 | 1 | yes | yes | yes |
| 6 | 76 | F | 6 | 2 (+11) | SD | 0 | 0 | 0 | 0 | 1 | 1 | yes | yes | yes |
| 7 | 53 | F | 9 | 2 | SD | 0 | 1 | 1 | 2 | 2 | 2 | yes | yes | - |
| 8 | 56 | M | 4 | 1 | SD | 0 | 0 | 2 | 2 | 1 | 2 | yes | - | - |
Single chemoperfusion without DSM because of early vascular stasis due to progressive arterial hypovascularization.
Systemic gemcitabine therapy
Systemic gemcitabine was administered on an out-patient basis. 1000 mg/m2 were given once a week intravenously over 30 min for 3 consecutive weeks followed by a pause of one week. One therapy cycle was defined as one 4-wk period. During the first cycle gemcitabine was also given during the fourth week. Prophylaxis against nausea consisted of metoclopramide orally.
Intraarterial chemoembolization
Intraarterial chemoembolization was performed on an in-patient basis after 2 systemic gemcitabine cycles had been given and was tolerated in terms of toxicity and clinical response. Chemoembolizations were repeated in case of stable disease or remission after an interval of at least 8 wk.
Antiemetics (5 mg tropisetron) and steroids (24 mg dexamethasone) were injected intravenously prior to each procedure. Also, intravenous antibiotic prophylaxis (3×200 mg ciprofloxacin/d, 3×500 mg metronidazole/d) against biliary infections was administered before and for 7 d after the procedure.
During arterial angiography using the femoral approach, an aortography and a selective mesenteric portography were performed to show vascular anatomy and the patency of the portal vein. Then, a selective hepatography was done using a standard diagnostic catheter or, if necessary, a coaxial catheter system. Intra-arterial analgesia (50-100 mg pethidine) was applied to control possible local pain. According to tumor vascularization a mixture of doxorubicin (50 mg/m2) and cisplatin (50 mg/m2) and 300-600 mg degradable starch microspheres (DSM, Spherex™, Pharmacia, Erlangen, Germany) was administered via hand injection under fluoroscopic control to check for stasis or reflux. In case of stasis, the injection was stopped until the arterial flow resumed. Finally, a control hepatography was performed. After the chemoembolization procedure the patient remained on the ward for at least five days. Systemic gemcitabine treatment was continued under laboratory controls for therapy-related toxicity after an interval of 3 to 4 wk.
Evaluation of response and toxicity
Pretreatment evaluation included physical examinations, evaluation of tumor markers, hematological and biochemical parameters. For tumor staging transabdominal ultrasound and a CT scan of thorax and abdomen were performed. The imaging modality and technique showing the best visibility at the start of treatment were used for baseline and follow-up tumor measurements (Figure 1). The sizes of measurable lesions were determined as the products of the two greatest perpendicular diameters. The following definitions were used: complete response (CR) for the disappearance of all clinical evidence of tumor for a minimum of 4 wk, partial response (PR) for a decrease of more than 50% in the measurable disease for a minimum of 4 wk, progressive disease (PD) for an increase in tumor size of more than 25%, the appearance of new lesions, or a deterioration of clinical status consistent with disease progression. Patients who did not meet criteria of CR, PR or PD were defined as having a stable disease (SD). Time to tumor progression was defined as the time between administration of the first chemotherapy and the time PD was diagnosed.
Figure 1.
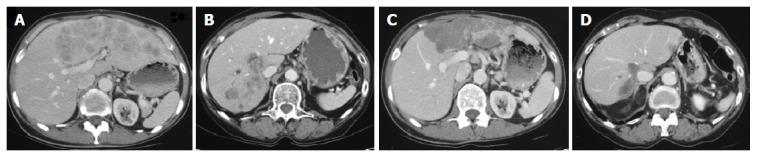
A: Axial CT shows a large hyper vascularized CCC in the left liver lobe of patient 4 before therapy; B: In patient 3 axial CT shows hyper vascularized CCC nodules in the lateral right liver; C: After 7 mo of therapy there is marked shrinkage and hypodense transformation of the tumor in PR in patient 4; D: In patient 3 the same changes are apparent in the right liver in PR after 7 mo of therapy.
Therapy-related toxicity was assessed once weekly during treatment by investigations of serum liver enzymes, bilirubin, creatinine, and complete blood counts. Clinical relief was defined as a chemotherapy-related relief of tumor symptoms or weight gain (including third-space fluid) of >7% from baseline for at least 4 wk without deterioration in any other parameters.
Evaluation of quality of life (QOL)
QOL was assessed prior to each chemoembolization procedure using the SF-36 Health Survey[9]. As components of QOL, the SF-36 questionnaire comprises the distinct aspects physical functioning, physical role, emotional role, social functioning, mental health index, general health, vitality, and bodily pain. A score for each category was calculated (0-100). Based on these scores a bodily sum score was determined. The sum scores before and during chemotherapy were compared.
RESULTS
A median of 2 chemoembolization cycles (range, 1-3) and a median of 6.5 gemcitabine cycles (range, 4-11) were applied (Table 1).
Response
In terms of maximum response, there were five patients with SD and 3 patients with PR; CR was not achieved (Table 1, 2). The median time to tumor progression was 7 mo (range, 3-18). Six patients are still alive, 2 patients died from tumor progression after 9 and 10 mo. The current median survival is 12 mo (range, 9-18).
Table 2.
Present status, chemotherapy-related response and changes of tumor markers.
| Patient number | Observervation period (mo) | Maximum response | Highest tumor markers before | Lowest tumor marker under therapy (%) | Time to tumor progression (mo) | Survival therapy (mo) |
| 1 | 10 | SD | None | - | 7 | Dead (10) |
| 2 | 14 | SD | Ca15-3:48 | 47 (-21) | 5 | Alive |
| 3 | 18 | PR | Ca19-9:1 442 | 34 (-981) | 18 | Alive |
| 4 | 12 | PR | Ca19-9:89 | 19 (-771) | NA (still PR) | Alive |
| AFP:35 | 6 (-831) | |||||
| 5 | 10 | PR | Ca19-9:786 | 139 (-821) | NA resected after 10 | Alive |
| 6 | 16 | SD | Ca15-3:54 | 43 (-201) | 7 | Alive |
| Ca19-9:88 | 115 (+311) | |||||
| 7 | 12 | SD | Ca19-9:845 200 | 435 (-991) | 7 | |
| CEA:285 | 2 (-991) | Alive | ||||
| 8 | 9 | SD | Ca15-3:435 | 204 (-531) | 3 | |
| Ca19-9:117 | 84 (-281) | Dead (9) |
Change of tumor marker in percent compared to highest value before therapy NA: not assessable.
Seven patients suffered from tumor-related symptoms, 3 of these experienced a treatment-related clinical relief (Table 1). The tumor of patient 5 became resectable during PR and was successfully removed by hemi-hepatectomy 10 mo after onset of therapy.
Six patients had elevated tumor marker Ca19-9 serum levels. Four patients showed a chemotherapy-related decrease of more than 75% compared to the baseline value further indicating response to therapy (Table 2).
Toxicity
Both systemic and regional therapies were tolerated well, no severe toxicity (WHO III/IV) was encountered (Table 1). Nausea and fever were the most commonly observed side effects. In patients 3, 4, 5, and 6 repeated chemoembolization led to a progressive rarefication of the intrahepatic arteries limiting the maximum applicable number of interventions (Table 1, Figure 2). In patient 3, the left hepatic artery remained permanently occluded after the second procedure. Further chemoembolization cycles were attempted in patients 4, 5, and 6. However, due to the progressive arterial hypovascularization the application of DSM had become impossible. The chemotherapeutics were finally administrated once in the course of a regional chemoperfusion before further regional therapy was considered impossible in these patients (Table 1).
Figure 2.

A: The arteriogram of patient 4 shows a beginning rarefication of the intrahepatic arteries after one chemoembolization; B: After three chemoembolizations the arteriogram of the same patient shows a progressive arterial rarefication with irregular stenoses and occlusions of peripheral branches.
Quality of life (QOL)
Patients 1, 2, 4, 5, and 6 were evaluable in terms of QOL follow-up using the SF-36; the remaining three patients did not provide follow-up data. The bodily sum scale remained stable under therapy in 4 patients (46/45, 39/31, 49/47, and 48/50). Patient 4 showed a gradual decrease from 53 to 38.
DISCUSSION
The overall prognosis of patients with nonresectable CCC remains dismal[10]. At present, only palliative therapeutical options are available. In addition to the improvement of the prognosis, chemotherapy has to be tolerable and should maintain the quality of life. The combination of 5-FU and leucovorin improved survival and QOL compared to best supportive care; however, the effect was limited[11]. In the search for improved systemic chemotherapy gemcitabine demonstrated tolerability and good QOL for patients with solid tumors[12]. In advanced pancreatic tumors, a phase III study revealed a better survival and symptom control compared to 5-FU[6]. Due to a common developmental origin of the pancreas and the biliary tract, CCC and pancreatic cancer share similarities in terms of tumor markers and resistance to chemotherapy. Hence, gemcitabine was administered in a phase II study to patients with unresectable CCC showing an objective response rate of 30%, a median time to progression of 27 wk and a median survival time of 9.3 mo[3].
Regional intraarterial therapies of the liver have shown efficacy in hyper vascularized hepatic malignancies[7,13,14]. While normal liver parenchyma receives more than two-thirds of its blood supply from the portal vein, hepatic tumors derive their blood supply almost completely from the hepatic artery[15]. The rationale for a regional intraarterial approach is a further increased anti tumoral activity induced by a temporary local ischemia and increasing local chemotherapy concentrations while reducing the systemic side effects[8].
Only limited data is available in terms of regional treatment of nonresectable CCC[16,17]. Cisplatin and doxorubicin have been applied successfully in the regional chemotherapy of hepatocellular carcinoma (HCC). The combination with lipiodol yielded anti-tumoral efficacy at a low systemic toxicity[18]. These observations led to the assumption that additional intraarterial chemoembolization of hypervascularized CCC might further enhance intrahepatic tumor response while maintaining the efficacy of systemic gemcitabine therapy.
In the course of the present study 8 patients with intrahepatic CCC received a combination of systemic chemotherapy and regional intraarterial chemoembolization. Maximum response was PR in 3 patients and SD in 5 patients. Six patients are still alive after a mean observation period of 14 mo. Three of the 7 patients who suffered from tumor-related disease symptoms experienced a clinical relief under therapy. Furthermore, from the 6 patients with elevated Ca19-9 levels, 4 patients showed a therapy-related decrease of more than 75%; among those the 3 patients with PR. QOL remained stable under therapy in those patients evaluable. These data are well in line and even exceed those from the gemcitabine monotherapy study from Kubicka et al showing objective responses in 30%, a therapy-related decrease in CA19-9 levels in 11 of 14 patients and a chemotherapy-related clinical relief in 7 of 11 patients with tumor symptoms[3].
One patient of our study showed a partial tumor remission under the combined therapy. The initially unresectable tumor became resectable after 10 mo. Whether a neoadjuvant intention might improve the prognosis in terms of secondary resectability will have to be determined by future observations. However, secondary resectability does not necessarily represent cure. Valverde and coworkers observed a 3-year-survival rate of only 22% after extensive surgical resection of intrahepatic CCC mainly related to the presence of intrahepatic satellite nodules and/or regional lymph node metastasis[2].
In spite of careful technique and the regular application of dexamethasone before the chemoembolization procedure, the number of cycles became limited to a maximum of three due to a progressive rarefication of the intrahepatic arteries. Incidents of regional therapy-related arteritis have been reported[19-21]. While a mechanical trauma may be responsible in some instances, toxic chemotherapy-related arteritis or ischemic reactions appear to be the more probable explanation in our situation for there is a progressive character of the changes. Interestingly, these phenomena occurred in 4 of our 8 patients with CCC in non-cirrhotic livers. In contrast, we rarely encountered it after regional therapies of our patients with HCC in cirrhotic livers. Demachi and colleagues suggested that hypertrophy of peribiliary capillary plexus in cirrhosis could act as a portoarterial shunt thereby acting protective during decreased arterial flow[22]. In CCC there is a constant risk of focal cholestasis. The biliary structures are mainly dependent on the arterial perfusion. Hence, arterial hypoperfusion during chemoembolization is associated with an increased risk of biliary complications and infections[23]. These vascular and biliary side effects of regional chemoembolization are also reported by other investigators to be more pronounced in the non-cirrhotic liver with a good organ function than in the cirrhotic liver[24]. In our set of patients we did not encounter a clinically obvious complication such as cholangitis or biloma; however, the administration of antibiotics during the chemoembolization therapy and careful catheter management seems mandatory to avoid these instances.
Our results suggest that in patients with nonresectable hepatic CCC being in a good physical condition regional chemoembolization in addition to systemic gemcitabine is well tolerated and may further enhance the palliative effect of systemic gemcitabine therapy. It is, however, too early to demonstrate a clear advantage towards systemic therapy alone. One should be aware of the potentially progressive arterial hypo vascularization after repeated chemoembolization cycles possibly limiting the maximum number of procedures.
ACKNOWLEDGEMENTS
The outstanding support and assistance of Sabine Tegtmeier, Frank Socko, Monique Horning, and Heike Steinlandt are greatfully acknowledged.
References
- 1.Pichlmayr R, Lamesch P, Weimann A, Tusch G, Ringe B. Surgical treatment of cholangiocellular carcinoma. World J Surg. 1995;19:83–88. doi: 10.1007/BF00316984. [DOI] [PubMed] [Google Scholar]
- 2.Valverde A, Bonhomme N, Farges O, Sauvanet A, Flejou JF, Belghiti J. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122–127. doi: 10.1007/s005340050094. [DOI] [PubMed] [Google Scholar]
- 3.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 4.Barton-Burke M. Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs. 1999;22:176–183. doi: 10.1097/00002820-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn R, Hribaschek A, Eichelmann K, Rudolph S, Fahlke J, Ridwelski K. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs. 2002;20:351–356. doi: 10.1023/a:1016209901417. [DOI] [PubMed] [Google Scholar]
- 6.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Lopez RR, Pan SH, Lois JF, McMonigle ME, Hoffman AL, Sher LS, Lugo D, Makowka L. Transarterial chemoembolization is a safe treatment for unresectable hepatic malignancies. Am Surg. 1997;63:923–926. [PubMed] [Google Scholar]
- 8.Chen HS, Gross JF. Intra-arterial infusion of anticancer drugs: theoretic aspects of drug delivery and review of responses. Cancer Treat Rep. 1980;64:31–40. [PubMed] [Google Scholar]
- 9.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kaczynski J, Hansson G, Wallerstedt S. Incidence, etiologic aspects and clinicopathologic features in intrahepatic cholangiocellular carcinoma--a study of 51 cases from a low-endemicity area. Acta Oncol. 1998;37:77–83. doi: 10.1080/028418698423212. [DOI] [PubMed] [Google Scholar]
- 11.Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, Linné T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 14.Tellez C, Benson AB, Lyster MT, Talamonti M, Shaw J, Braun MA, Nemcek AA, Vogelzang RL. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82:1250–1259. doi: 10.1002/(sici)1097-0142(19980401)82:7<1250::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 16.Melichar B, Cerman J, Dvorák J, Jandík P, Mergancová J, Melicharová K, Tousková M, Krajina A, Voboril Z. Regional chemotherapy in biliary tract cancers--a single institution experience. Hepatogastroenterology. 2002;49:900–906. [PubMed] [Google Scholar]
- 17.Tanaka N, Yamakado K, Nakatsuka A, Fujii A, Matsumura K, Takeda K. Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma--initial experience. Eur J Radiol. 2002;41:42–48. doi: 10.1016/s0720-048x(01)00414-4. [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Miyazono N, Hori A, Miyake S, Satake M, Kanetsuki I, Nishida H, Ikeda K, Nakajo M. Treatment of hepatocellular carcinoma by intraarterial injection of adriamycin/mitomycin C oil suspension (ADMOS) alone or combined with cis-diaminodichloroplatinum (CDDP) Acta Radiol. 1993;34:388–391. [PubMed] [Google Scholar]
- 19.Belli L, Magistretti G, Puricelli GP, Damiani G, Colombo E, Cornalba GP. Arteritis following intra-arterial chemotherapy for liver tumors. Eur Radiol. 1997;7:323–326. doi: 10.1007/s003300050159. [DOI] [PubMed] [Google Scholar]
- 20.Forsberg L, Hafstrom L, Lunderquist A, Sundqvist K. Arterial changes during treatment with intrahepatic arterial infusion of 5-fluorouracil. Radiology. 1978;126:49–52. doi: 10.1148/126.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Bledin AG, Kim EE, Chuang VP, Wallace S, Haynie TP. Changes of arterial blood flow patterns during infusion chemotherapy, as monitored by intra-arterially injected technetium 99m macroaggregated albumin. Br J Radiol. 1984;57:197–203. doi: 10.1259/0007-1285-57-675-197. [DOI] [PubMed] [Google Scholar]
- 22.Demachi H, Matsui O, Kawamori Y, Ueda K, Takashima T. The protective effect of portoarterial shunts after experimental hepatic artery embolization in rats with liver cirrhosis. Cardiovasc Intervent Radiol. 1995;18:97–101. doi: 10.1007/BF02807230. [DOI] [PubMed] [Google Scholar]
- 23.Kim HK, Chung YH, Song BC, Yang SH, Yoon HK, Yu E, Sung KB, Lee YS, Lee SG, Suh DJ. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32:423–427. doi: 10.1097/00004836-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Yu JS, Kim KW, Jeong MG, Lee DH, Park MS, Yoon SW. Predisposing factors of bile duct injury after transcatheter arterial chemoembolization (TACE) for hepatic malignancy. Cardiovasc Intervent Radiol. 2002;25:270–274. doi: 10.1007/s00270-001-0049-z. [DOI] [PubMed] [Google Scholar]


